Abstract
Background
Patients with schizophrenia suffer high rates of metabolic derangements on some antipsychotic medications that predispose them to cardiovascular diseases. Keeping this fact in mind, we planned this open-label study to see the effect on various metabolic parameters after switching stable schizophrenia subjects, who had developed metabolic syndrome on olanzapine, to aripiprazole.
Methods
Sixty-two patients with schizophrenia who were stable on olanzapine and were fulfilling modified National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP-III) criteria for the presence of metabolic syndrome were enrolled on the study. Patients were randomly assigned either to switch to aripiprazole or to stay on olanzapine, on a 1:1 basis. Cross-tapering over a period of 1 month was done while switching patients to aripiprazole. Laboratory assessment for metabolic parameters was done at baseline, 8 weeks, and 24 weeks after enrollment; efficacy assessment was done using the Positive and Negative Syndrome Scale (PANSS) at baseline and 24 weeks, the Clinical Global Impressions severity subscale (CGI-S) at baseline, and the Clinical Global Impressions improvement subscale (CGI-I) at 24 weeks.
Results
All parameters of metabolic syndrome (waist circumference, blood pressure, triglyceride level, fasting blood glucose, and high-density lipoprotein cholesterol) kept deteriorating in the stay group, compared with a continuous improvement in the switch group over time. At the end of the study, 26 patients (100%) from the stay group and 15 patients (42.8%) from switch group met the modified NCEP ATP-III criteria for presence of metabolic syndrome (P<0.001). There were no statistically significant differences between groups in psychopathology changes as measured by the PANSS total score and CGI-I scores.
Conclusion
Clinically stable patients with schizophrenia who are taking olanzapine and who have evidence of metabolic syndrome can be successfully switched to aripiprazole, with improvement in various parameters of metabolic syndrome and without any significant change in efficacy measures.
Introduction
Metabolic syndrome is a complex disorder comprising a set of cardiovascular risk factors, including obesity, dyslipidemia, deranged glucose metabolism, insulin insensitivity, and hypertension.Citation1 The clinical significance of metabolic syndrome comes from the fact that it is associated with development of coronary heart disease, cerebrovascular disease, and type 2 diabetes mellitus. Studies show that metabolic syndrome is associated with twofold to threefold increased risk of cardiovascular morbidity.Citation2
Metabolic syndrome is seen more frequently in persons with schizophrenia than in the general population.Citation3–Citation5 While it is possible that genetic factors play a role in the increased risk seen in such patients, it is likely that lifestyle issues such as poor nutrition and reduced exercise habits also play a key role. These metabolic abnormalities are further aggravated by antipsychotic medications. The first-generation antipsychotic medications are effective in treating positive symptoms but are not similarly effective for negative and cognitive symptoms; they are also less protective against relapses in the long-term.Citation6 In addition, extrapyramidal symptoms are more commonly seen in patients treated with first-generation antipsychotic medications.Citation7 In recent years, atypical antipsychotics, or second-generation antipsychotic medications, have been replacing first-generation antipsychotic medications as firstline treatment for schizophrenia with equal efficacy and fewer extrapyramidal side effects, and have thus ensured a better quality of life for the patient.Citation8–Citation11 However, other adverse effects, including weight gain, metabolic syndrome, and cardiovascular side effects, have been found after long-term use of some second-generation antipsychotic medications.Citation12
Second-generation antipsychotic medications can cause clinically significant weight gain, leading to insulin resistance and type 2 diabetes mellitus, and are thus a major risk factor for the development of metabolic syndrome.Citation13 A review of absolute weight gain in various placebo-controlled trials and head-to-head comparisons found that the magnitude of weight gain was not equal among antipsychotic medications.Citation12 Maximum weight gain was seen with olanzapine treatment, while it was least with aripiprazole and ziprasidone. Olanzapine was associated with a mean weight gain of >6 kg over 1 year,Citation14,Citation15 whereas aripiprazoleCitation16–Citation19 and ziprasidoneCitation20–Citation22 were associated with a mean weight gain of about 1 kg over 1 year. Aripiprazole has a unique mechanism of action among second-generation antipsychotic medications: it is a partial agonist at D2 dopamine and 5-HT1A serotonin receptors and an antagonist at 5-HT2A serotonin receptors.Citation23 It has the least chance of inducing weight gain and other metabolic side effectsCitation24 and is safe and effective during both the acute and maintenance phases of schizophrenia.Citation19,Citation25,Citation26
Switching antipsychotic medications in the treatment of schizophrenia is a common practice in clinical psychiatry.Citation27 Weight gain and metabolic syndrome are the two main reasons for switching antipsychotics.Citation28 It has been suggested that the ideal drugs for switching are aripiprazole or ziprasidone, with their low risk for metabolic syndrome.Citation29 We in our study chose aripiprazole because it was the newer option available and we expected it would be of most clinical interest when the study was completed. We hypothesized that switching to aripiprazole would result in an improvement in metabolic measures, compared with staying on olanzapine. We were also interested in determining if switching to aripiprazole would be accompanied by any clinical destabilization. Earlier, Fleischhacker et alCitation30 in their study comparing the efficacy and tolerability of aripiprazole with olanzapine in patients with schizophrenia found that olanzapine had a statistically significant efficacy advantage over aripiprazole, with more reduction in Positive and Negative Syndrome Scale (PANSS) total score. Pae et alCitation31 found that patients switched to aripiprazole, with sudden discontinuation of the previous antipsychotic medication, showed an increase in symptom severity during first week of switching. Moreover, few studies have been done in this field,Citation32,Citation33 and none has been from our Kashmir region.
Methods
Settings and subjects
The study was carried out at the outpatient unit of a tertiary care psychiatry hospital in North India (Kashmir) from June 2011 to May 2014, after seeking permission from the IEC of the government medical college, Srinagar. Participants were individuals with schizophrenia who had achieved clinical stability with olanzapine and who were assessed as having metabolic syndrome using modified National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP-III) criteria.Citation34,Citation35 Schizophrenia diagnoses were made using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Text Revision.Citation36 The patients were required to be on olanzapine for a minimum of 3 months without any other antipsychotic medication for 1 month prior to enrollment. The patients entered the study in order to improve their metabolic risk profile. Informed consent was obtained from each patient after fully explaining the study procedures.
Patients were randomly assigned either to switch to aripiprazole or to stay on olanzapine, on a 1:1 basis. Individuals assigned to stay on olanzapine remained on their prestudy dosage, with adjustments only as clinically indicated. Olanzapine was used in a dose range of 10–20 mg/day. Patients assigned to switch to aripiprazole began taking aripiprazole 5 mg/day while continuing olanzapine without dose reduction for 1 week. After 1 week, the aripiprazole dosage was increased to 10 mg/day and the dose of olanzapine was reduced 25%–50%. After 2 weeks, the aripiprazole dosage could be increased to 15 mg/day while the dose of olanzapine was reduced 50%–75% from the original dosage. After 3 weeks, the available range for aripiprazole was 10–20 mg/day, and olanzapine was stopped. After 4 weeks, aripiprazole was used in a dose range of 10–30 mg/day.
Patients were followed on a weekly basis during the first month of the treatment period and every 4 weeks thereafter; laboratory assessment was carried out at baseline, 8 weeks, and 24 weeks after enrollment. PANSS was used at baseline and 24 weeks, while the Clinical Global Impressions severity subscale (CGI-S) was used at baseline and the Clinical Global Impressions improvement subscale (CGI-I) was used at 24 weeks.
The addition of lithium, valproate, statins, or drugs prescribed for weight loss was not allowed during the study. Those who were taking these medications during the prestudy phase were allowed to continue without dose adjustments. All other medications except for nonstudy antipsychotic drugs were allowed.
Assessment
The waist circumference was measured in a horizontal plane, midway between the inferior margin of the ribs and the superior border of the iliac crest. The measurements were taken thrice and the mean was computed in all cases. Systolic and diastolic blood pressure were measured twice at an interval of 3 minutes in the sitting position after a 15-minute rest, and the mean was computed. Blood samples (3 mL) were drawn after 8–12 hours of overnight fasting for the measurement of high-density lipoprotein (HDL) cholesterol, triglycerides, and fasting plasma glucose levels. Plasma glucose was measured using the glucose oxidase–peroxidase method, triglycerides by standard enzymatic procedures, and HDL cholesterol by direct assay method.
Metabolic syndrome was diagnosed using modified NCEP ATP-III criteria for the Asian population.Citation35 Diagnosis was made when three or more of the following conditions were met:
Waist circumference >90 cm for males and >80 cm for females;
Triglycerides >150 mg/dL;
HDL levels <40 mg/dL for males and <50 mg/dL for females;
Systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg;
Fasting glucose ≥110 mg/dL.
A previous diagnosis of high blood pressure was considered sufficient to fulfill criterion 4 and a previous diagnosis of diabetes mellitus was considered as sufficient to fulfill criterion 5.
Assessment for any clinical destabilization was done using the PANSS and CGI scales. Efficacy failure was defined by psychiatric hospitalization, a 25% increase in the total PANSS score,Citation37 or ratings of much worse or very much worse on the CGI improvement subscale.Citation38
Statistical analysis
Data were entered into a Microsoft Excel spreadsheet. Continuous variables were summarized as mean and standard deviation. Categorical variables were summarized as percentages. CGI-I and CGI-S were summarized as median and interquartile range. Repeated-measures analysis of variance was used to analyze the difference in the values of a continuous variable over time. Comparisons between stay and switch groups were analyzed using independent samples Student’s t-test for continuous variables, Chi-square test for categorical variables, Fisher’s exact test for categorical variables when the Chi-square test did not meet Cochrane criterion,Citation39 and Mann–Whitney test for ordinal variables (CGI-I and CGI-S). The proportion of metabolic syndrome across time was tested using the Cochran Q-test. A P-value of <0.05 was taken as significant.
Results
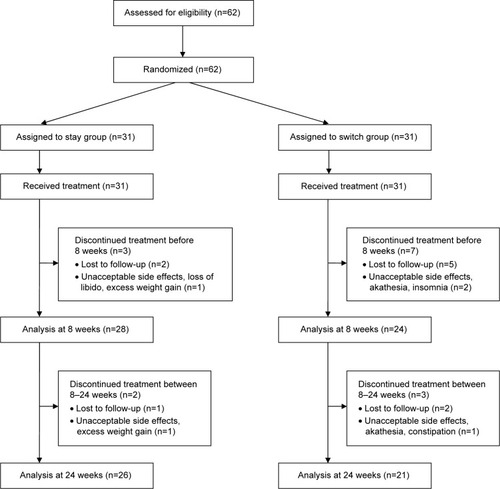
Our sample consisted of 62 patients who were randomly assigned to one of two groups, either the stay group (n=31) or the switch group (n=31). The distribution of other (nonstudy) allowed medications among the two groups was statistically insignificant and included lithium (P>0.999), sodium valproate (P>0.999), carbamazipine (P>0.999), lamotrigene (P>0.999), selective serotonin reuptake inhibitors (P>0.999), benzodiazepines (P=0.554), and beta blockers (P=0.011). The progression of the two groups through the study is shown in . Ten patients (32.2%) from the switch group and five (16.1%) from the stay group discontinued the treatment before the final assessment at 24 weeks (P=0.138). summarizes the sociodemographic characteristics of the participants. shows both intergroup and within-group trends in various metabolic and clinical variables. Last observation carried forward was employed for data imputation. Among the various parameters of metabolic syndrome, waist circumference, blood pressure, triglyceride level, and fasting blood glucose kept increasing in the stay group, while HDL level showed a decreasing trend. In the switch group, waist circumference, blood pressure, triglyceride level, and fasting blood glucose kept decreasing, while HDL level increased with time.
Table 1 Sociodemographic profiles of two groups
Table 2 Metabolic and clinical characteristics of stay group versus switch group
shows the analysis of completers versus noncompleters of the study. Twenty-six patients (100%) from stay group and 15 patients (42.8%) from the switch group met the modified NCEP ATP-III criteria for presence of metabolic syndrome (P<0.001) at the end of study.
Table 3 Analysis of completers versus noncompleters of study
Two patients from both groups experienced efficacy failure (ie, they were hospitalized). There were no differences between groups in psychopathology changes as measured by the PANSS total score at baseline and 24 weeks (P=0.911) and CGI-I scores at the end of study (P=0.375).
Discussion
Multiple trials over time have studied the metabolic derangements with second-generation antipsychotic medications. For example, the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Schizophrenia Trial found that, among the antipsychotic medications used, olanzapine was associated with highest risk for weight gain and dyslipidemia, especially elevated triglyceride levels.Citation40–Citation43 Various strategies have been adopted to counter these antipsychotic medication-induced metabolic derangements. Switching to antipsychotic medications with lesser metabolic side effects or adopting a lifestyle intervention focused on diet and exercise is the appropriate first step. Addition of statins (3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors) to the antipsychotic medication regimen tends to benefit low-density lipoprotein cholesterol and triglycerides rather than HDL or weight,Citation44 while adding metformin leads to weight reduction.Citation45,Citation46
Switching from olanzapine to aripiprazole in our study was effective in helping many patients improve the results of various parameters of metabolic syndrome, while those who continued on olanzapine experienced further deterioration in these parameters. These findings are in accordance with most of the studies conducted in this field.Citation32,Citation33,Citation47,Citation48 The fact that intergroup differences with respect to fasting levels of HDL cholesterol were statistically significant in our study as against the findings of Stroup et alCitation32 could be explained by the fact that we shifted only olanzapine-treated schizophrenia patients to aripiprazole, whereas in the Stroup et al study patients were switched from olanzapine, quetiapine, or risperidone to aripiprazole. Olanzapine causes more metabolic derangements than quetiapine and risperidone,Citation49 and this could be the reason that switching produced more beneficial effects in results of fasting levels of HDL cholesterol in our study.
At the end of our study there were no statistically significant differences between groups in psychopathology changes as measured by the PANSS total score (P=0.911) and CGI-I scores (P=0.375) in completers of the study. Fleischhacker et alCitation30 found that olanzapine had a statistically significant efficacy advantage over aripiprazole, with more reduction in PANSS total score. McCue et alCitation50 also found that aripiprazole was significantly less able to get patients out of hospital in 3 weeks (the primary outcome measure) compared with olanzapine. In these studies, acute relapsing patients with schizophrenia were shifted to aripiprazole, whereas in our study we switched only those patients with schizophrenia who were already stable on olanzapine. It is likely that patients with schizophrenia who are already stable on olanzapine maintain their remission even after switching to aripiprazole; even so, difference exists in acute psychotic episodes, where olanzapine is relatively superior in efficacy to aripiprazole.Citation50
Pae et alCitation31 found that patients switched to aripiprazole with sudden discontinuation of the previous antipsychotic medication showed an increase in symptom severity during the first week of switching. In our study, slow cross-titration of antipsychotic medication can explain the reason why there was no significant clinical destabilization in the switch group. Takeuchi and RemingtonCitation51 concluded in a recent systematic review that a small number of patients with schizophrenia or schizoaffective disorder risked an exacerbation of psychotic symptoms if aripiprazole was added to existing antipsychotic treatment. Many of the cases reported involved patients who were quite ill and had been exposed to long-term antipsychotic treatment; it is likely that aripiprazole was added to current treatment in patients with treatment-resistant schizophrenia, but in our study, patients with schizophrenia who were already stable on olanzapine were switched to aripiprazole. As our patients were already stable on olanzapine, this could be a reason for successful switching without much worsening in psychotic symptoms.
The difference in the metabolic derangements between the two groups can be explained by the differential receptor occupancy by aripiprazole and olanzapine. Aripiprazole is a partial agonist at D2 dopamine and 5HT1A serotonin receptors and an antagonist at 5HT2A serotonin receptors,Citation52 whereas olanzapine is an antagonist at D2 dopamine, 5HT2A and 5HT2C serotonin, M1 muscarinic, and histamine-1 receptors.Citation53 Histamine-1 and 5HT2C receptor antagonism closely correlates with the weight-gaining potential of some antipsychotics like olanzapineCitation54,Citation55 and may interfere with leptin-mediated appetite suppression.Citation55,Citation56 Adiposity alone does not explain the diabetogenic potential of some atypical antipsychotic medications.Citation57 Significant insulin resistance, even with a single dose of olanzapine, has also been documented in nonobese individuals receiving olanzapine versus those receiving risperidone.Citation58,Citation59 Blocking M3 (type 3) muscarinic and 5HT1A receptors may be a factor in diminished pancreatic beta-cell responsiveness, and blocking 5HT2A receptor may suppress glucose uptake in skeletal muscle.Citation53
Treatment with olanzapine in patients with schizophrenia offers the advantage of effectiveness for both positive and negative symptoms, with the fewest extrapyramidal side effects.Citation8–Citation11 However, because of growing evidence of olanzapine-induced metabolic derangements, as is evident from our study also, a prescribing choice must be based on an assessment of each drug’s efficacy as well as its potential to cause metabolic side effects. Because schizophrenia is a chronic illness that requires antipsychotic treatment for a prolonged period, an antipsychotic agent with fewer metabolic side effects, such as aripiprazole, can be used for maintenance, to prevent psychotic relapse and long-term deterioration. As is evident from our study, the dropout rate was higher in the switch group than in the stay group (P=0.138), which can be an impediment in stabilized schizophrenia patients while switching them to other antipsychotic medications and is something that clinicians will want to address. This problem can partially be addressed by slow cross-titration of drugs and close follow-up of such patients. Monitoring blood levels of aripiprazole is a meaningful option, because it seems to have a therapeutic window at 150–210 ng/mL.Citation60 Levels beyond 210 ng/dL will lead to more side effects, and levels below 150 ng/dL will show subtherapeutic response and thus lead to more dropouts.
Conclusion
Clinically stable patients with schizophrenia on olanzapine who have evidence of metabolic syndrome can be successfully switched to aripiprazole, with improvement in various parameters of metabolic syndrome and without any significant change in efficacy measures. Switching is an option if careful cross-titration and close monitoring is possible. Careful clinical monitoring after a switch to aripiprazole might have been the reason that those who switched did not experience a higher rate of efficacy failures, compared with those who stayed on olanzapine.
Disclosure
There are no financial or other relationships that might lead to a conflict of interest. The authors report no other conflicts of interest in this work.
References
- ReavenGMSyndrome X: 6 years laterJ Int Med Suppl19947361322
- LakkaHMLaaksonenDELakkaTAThe metabolic syndrome and total and cardiovascular disease mortality in middle-aged menJAMA2002288212709271612460094
- PatelJKBuckleyPFWoolsonSCAFE InvestigatorsMetabolic profiles of second-generation antipsychotics in early psychosis: findings from the CAFE studySchizophr Res20091111–391619398192
- McEvoyJPMeyerJMGoffDCPrevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES IIISchizophr Res2005801193216137860
- MeyerJMNasrallahHAMcEvoyJPThe Clinical Antipsychotic Trials Of Intervention Effectiveness (CATIE) Schizophrenia Trial: clinical comparison of subgroups with and without the metabolic syndromeSchizophr Res200580191816125372
- AltamuraACBuoliMMauriMCHaloperidol versus second-generation antipsychotics in the long-term treatment of schizophrenia: a 4-year follow-up naturalistic studyJ Clin Psychopharmacol201131566166321881452
- JibsonMDTandonRNew atypical antipsychotic medicationsJ Psychiatr Res1998323–42152289793875
- DavisJMChenNClinical profile of an atypical antipsychotic: risperidoneSchizophr Bull2002281436112047021
- DavisJMChenNThe effects of olanzapine on the 5 dimensions of schizophrenia derived by factor analysis: combined results of the North American and international trialsJ Clin Psychiatry2001621075777111816864
- KarowANaberDSubjective well-being and quality of life under atypical antipsychotic treatmentPsychopharmacology (Berl)2002162131012107610
- ChongMYTanCHFujiiSAntipsychotic drug prescription for schizophrenia in East Asia: rationale for changePsychiatry Clin Neurosci2004581616714678459
- NewcomerJWSecond-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature reviewCNS Drugs200519Suppl 119315998156
- Pi-SunyerFXMedical hazards of obesityAnn Intern Med19931197 Pt 26556608363192
- NemeroffCBDosing the antipsychotic medication olanzapineJ Clin Psychiatry199758Suppl 1045499265916
- KinonBJThe routine use of atypical antipsychotic agents: maintenance treatmentJ Clin Psychiatry199859Suppl 1918229847048
- MarderSRMcQuadeRDStockEAripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trialsSchizophr Res2003612–312313612729864
- KasperSLermanMNMcQuadeRDEfficacy and safety of aripiprazole vs haloperidol for long-term maintenance treatment following acute relapse of schizophreniaInt J Neuropsychopharmacol20036432533714609439
- PigottTACarsonWHSahaARTorbeynsAFStockEGIngenitoGGAripiprazole Study GroupAripiprazole for the prevention of relapse in stabilized patients with chronic schizophrenia: a placebo-controlled 26-week studyJ Clin Psychiatry20036491048105614628980
- McQuadeRDJodyDKujawaMCarsonWHIwamotoTLong-term weight effects of aripiprazole versus olanzapineAmerican Psychiatric Association (APA) 156th Annual Meeting2003 May 17–22San Francisco, CA
- AllisonDBMentoreJLHeoMAntipsychotic-induced weight gain: a comprehensive research synthesisAm J Psychiatry1999156111686169610553730
- DanielDGZimbroffDLPotkinSGReevesKRHarriganEPLakshminarayananMZiprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Ziprasidone Study GroupNeuropsychopharmacology199920549150510192829
- HirschSRKisslingWBäumlJPowerAO’ConnorRA 28-week comparison of ziprasidone and haloperidol in outpatients with stable schizophreniaJ Clin Psychiatry200263651652312088164
- Swainston HarrisonTPerryCMAripiprazole: a review of its use in schizophrenia and schizoaffective disorderDrugs200464151715173615257633
- NewcomerJWMetabolic considerations in the use of antipsychotic medications: a review of recent evidenceJ Clin Psychiatry200768Suppl 1202717286524
- PotkinSGSahaARKujawaMJAripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorderArch Gen Psychiatry200360768169012860772
- KaneJMCarsonWHSahaAREfficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorderJ Clin Psychiatry200263976377112363115
- BurnsTChabannesJPDemyttenaereKSwitching antipsychotic medications: general recommendations and switching to amisulprideCurr Med Res Opin200218420120812201620
- WeidenPJMillerALLambertTJBuckleyPFThe art and science of switching antipsychotic medications, part 2J Clin Psychiatry2007681e0217284123
- WeidenPJBuckleyPFReducing the burden of side effects during long-term antipsychotic therapy: the role of “switching” medicationsJ Clin Psychiatry200768Suppl 61423
- FleischhackerWWMcQuadeRDMarcusRNArchibaldDSwaninkRCarsonWHA double-blind, randomized comparative study of aripiprazole and olanzapine in patients with schizophreniaBiol Psychiatry200965651051718986646
- PaeCUSerrettiAChiesaAImmediate versus gradual suspension of previous treatments during switch to aripiprazole: results of a randomized, open label studyEur Neuropsychopharmacol200919856257019442491
- StroupTSMcEvoyJPRingKDSchizophrenia Trials NetworkA randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP)Am J Psychiatry2011168994795621768610
- NewcomerJWCamposJAMarcusRNA multicenter, randomized, double-blind study of the effects of aripiprazole in overweight subjects with schizophrenia or schizoaffective disorder switched from olanzapineJ Clin Psychiatry20086971046105618605811
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in AdultsExecutive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III)JAMA2001285192486249711368702
- HengDMaSLeeJJModification of the NCEP ATP III definitions of the metabolic syndrome for use in Asians identifies individuals at risk of ischemic heart diseaseAtherosclerosis2006186236737316112123
- American Psychiatric AssociationDiagnostic and Statistical Manual and Mental Disorders4th ed text revWashington DCAmerican psychiatric association2000
- KaySRFiszbeinAOplerLAThe Positive and Negative Syndrome Scale (PANSS) for schizophreniaSchizophr Bull19871322612763616518
- GuyWECDEU Assessment Manual for Psychopharmacology – Revised (DHEW Publ No ADM 76–338)Rockville, MDUS Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs1976218222
- ArmitagePBerryGMatthewsJNSAnalysing means and proportionsArmitagePBerryGMatthewsJNSStatistical Methods in Medical Research4th edMalden, MABlackwell Science Ltd200283146
- LiebermanJAStroupTSMcEvoyJPClinical Antipsychotic Trials of Intervention Effectiveness (CATIE) InvestigatorsEffectiveness of antipsychotic drugs in patients with chronic schizophreniaN Engl J Med2005353121209122316172203
- MeyerJMDavisVGGoffDCChange in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: prospective data from phase 1Schizophr Res20081011–327328618258416
- NasrallahHAMetabolic findings from the CATIE trial and their relation to tolerabilityCNS Spectr2006117 Suppl 7323916816798
- StroupTSLiebermanJAMcEvoyJPCATIE InvestigatorsEffectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychoticAm J Psychiatry2006163461162216585435
- De HertMKalnickaDvan WinkelRWampersMHanssensLVan EyckDTreatment with rosuvastatin for severe dyslipidemia in patients with schizophrenia and schizoaffective disorderJ Clin Psychiatry200667121889189617194266
- WuRRZhaoJPJinHLifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trialJAMA2008299218519318182600
- WuRRZhaoJPGuoXFMetformin addition attenuates olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients: a double-blind, placebo-controlled studyAm J Psychiatry2008165335235818245179
- McQuadeRDStockEMarcusRA comparison of weight change during treatment with olanzapine or aripiprazole: results from a randomized, double-blind studyJ Clin Psychiatry200465Suppl 18475615600384
- ChrzanowskiWKMarcusRNTorbeynsANyilasMMcQuadeRDEffectiveness of long-term aripiprazole therapy in patients with acutely relapsing or chronic, stable schizophrenia: a 52-week, open-label comparison with olanzapinePsychopharmacology (Berl)2006189225926617058105
- HasnainMViewegWVFredricksonSKBeatty-BrooksMFernandezAPandurangiAKClinical monitoring and management of the metabolic syndrome in patients receiving atypical antipsychotic medicationsPrim Care Diabetes20093151519083283
- McCueREWaheedRUrcuyoLComparative effectiveness of second-generation antipsychotics and haloperidol in acute schizophreniaBr J Psychiatry200618943344017077434
- TakeuchiHRemingtonGA systematic review of reported cases involving psychotic symptoms worsened by aripiprazole in schizophrenia or schizoaffective disorderPsychopharmacology (Berl)2013228217518523736279
- American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of ObesityConsensus development conference on antipsychotic drugs and obesity and diabetes; ppDiabetes Care200427259660114747245
- CitromeLOlanzapine: interpreting the label changeInt J Clin Pract200761121960196217983436
- NasrallahHAAtypical antipsychotic-induced metabolic side effects: insights from receptor-binding profilesMol Psychiatry2008131273517848919
- ReynoldsGPHillMJKirkSLThe 5-HT2C receptor and antipsychoticinduced weight gain – mechanisms and geneticsJ Psychopharmacol2006204 Suppl151816785265
- Matsui-SakataAOhtaniHSawadaYReceptor occupancy-based analysis of the contributions of various receptors to antipsychotics-induced weight gain and diabetes mellitusDrug Metab Pharmacokinet200520536837816272755
- NewcomerJWHauptDWThe metabolic effects of antipsychotic medicationsCan J Psychiatry200651848049116933585
- HendersonDCCaglieroECopelandPMGlucose metabolism in patients with schizophrenia treated with atypical antipsychotic agents: a frequently sampled intravenous glucose tolerance test and minimal model analysisArch Gen Psychiatry2005621192815630069
- HahnMKWoleverTMArenovichTAcute effects of single-dose olanzapine on metabolic, endocrine, and inflammatory markers in healthy controlsJ Clin Psychopharmacol201333674074624100786
- SparshattATaylorDPatelMXKapurSA systematic review of aripiprazole – dose, plasma concentration, receptor occupancy, and response: implications for therapeutic drug monitoringJ Clin Psychiatry201071111447145620584524