Abstract
Aims
Two specific objectives were established to quantify computer task performance among people with Duchenne muscular dystrophy (DMD). First, we compared simple computational task performance between subjects with DMD and age-matched typically developing (TD) subjects. Second, we examined correlations between the ability of subjects with DMD to learn the computational task and their motor functionality, age, and initial task performance.
Method
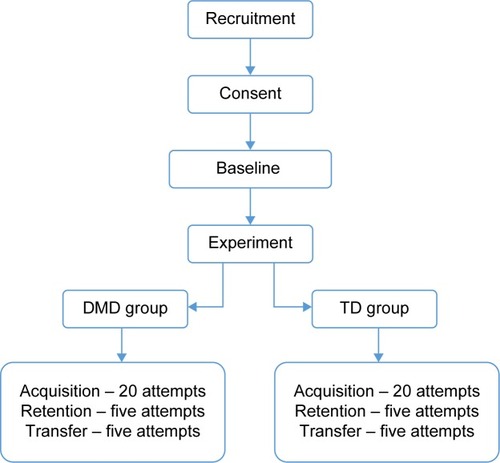
The study included 84 individuals (42 with DMD, mean age of 18±5.5 years, and 42 age-matched controls). They executed a computer maze task; all participants performed the acquisition (20 attempts) and retention (five attempts) phases, repeating the same maze. A different maze was used to verify transfer performance (five attempts). The Motor Function Measure Scale was applied, and the results were compared with maze task performance.
Results
In the acquisition phase, a significant decrease was found in movement time (MT) between the first and last acquisition block, but only for the DMD group. For the DMD group, MT during transfer was shorter than during the first acquisition block, indicating improvement from the first acquisition block to transfer. In addition, the TD group showed shorter MT than the DMD group across the study.
Conclusion
DMD participants improved their performance after practicing a computational task; however, the difference in MT was present in all attempts among DMD and control subjects. Computational task improvement was positively influenced by the initial performance of individuals with DMD. In turn, the initial performance was influenced by their distal functionality but not their age or overall functionality.
Introduction
Duchenne muscular dystrophy (DMD) is a severe, progressive disease that affects one in 3,600–6,000 live male births.Citation1 Affected individuals can have mildly delayed motor milestones and most are unable to run and jump due to proximal muscle weakness. Patients are generally diagnosed when toddlers and become wheelchair-dependent in their early teens.Citation2 While there is no cure, recent therapeutic advances have considerably extended the life span of individuals with DMD.Citation3,Citation4
Several studies have recently described DMD phenotype and genotype,Citation5,Citation6 heart problems,Citation7 postural adjustment,Citation8 physical training,Citation9 multidisciplinary clinical evaluations,Citation10,Citation11 therapeutic treatments,Citation12 and drug treatment.Citation13–Citation15 However, there is growing interest in the use of computers by subjects with DMD.Citation16,Citation17 Computers and assistive technology devices can maximize independence, productivity, and participation of persons with disabilities in academic programs, employment, recreation, and other activities. Importantly, new technologies enable people with severe limitations or even a total inability to control arm or hand movements to successfully perform everyday tasks, thus improving quality of life.Citation18
James and OrrCitation19 investigated progressive upper limb weakness in DMD and found that the shoulder and elbow were equally affected, with the hand being the least affected, suggesting that the degree of weakness is greatest proximally. This situation enables effective computer use with the hands but makes it more difficult to write given the need to move the elbow and arm.
Despite recognition of the importance of computer use for the functioning of people with disabilities, few studies have examined computer task performance by subjects with DMD. de Moura et alCitation20 used a computer task to evaluate automatic and voluntary visuospatial attention in subjects with DMD and healthy males. The results for participants with DMD were similar to that of younger children, suggesting that the disease is associated with delayed maturation of voluntary attention mechanisms. Stern et alCitation21 and Vilozni et alCitation22 used computer games to encourage respiratory efforts by patients with DMD and demonstrated that a properly utilized computer training program can improve ventilation performance and respiratory muscle endurance in DMD.
The present study was designed to characterize the performance of people with DMD in undertaking a basic computational task, and the results were interpreted from the theoretical framework of motor learning. Clinical studies into other diseases are already using the framework of motor learning in the rehabilitation of patients with cerebral palsy, stroke, and Down syndrome.Citation23–Citation29 However, there is limited research into motor learning in the habilitation and rehabilitation of people with DMD.Citation20,Citation30 Bendixen et alCitation31 reported that people with DMD exhibit very low levels of participation in skill-based activities, including those that require taking lessons to learn new skills.
The current study aimed at verifying the quantitative performance of people with DMD in undertaking a computer task. Two specific objectives were established: 1) to compare the performances of typical developing (TD) age-matched controls and subjects with DMD on a simple computational task and 2) to establish correlations between DMD subject computational task performance and motor functionality, age, and initial maze task performance.
Methods
This study was approved by the Ethics Committee for review of research projects of the School of Medicine of ABC (Santo Andre, Brazil) (protocol number 12613013.4.0000.0082). Each participant’s legal guardian freely signed an informed consent form prior to study enrollment.
Participants
A total of 84 subjects participated, including 42 males with DMD and 42 age- and sex-matched TD individuals. The average age was 18.11±5.5 (min, 10; max, 32). The ages and scale scores of the two groups are shown in .
Table 1 Subject age and MFM scale scores
We evaluated all individuals with DMD at the Brazilian Association of Muscular Dystrophy (Associação Brasileira de Distrofia Muscular – ABDIM) who fit the inclusion criteria. The inclusion criteria for the DMD group were a diagnosis of DMD confirmed by a molecular method and/or protein expression and undergoing treatment at ABDIM. For the TD group, subjects had to be eligible males without any neuromuscular conditions. Exclusion criteria for participants in both groups were: not performing the task in a single trial-test (verbal and written instructions were provided before the experiment), presence of upper limb deformity or muscle weakness that prevented keyboard handling, or nonacceptance of participation in research by the participant and/or legal guardian by not signing the consent form and/or terms of agreement.
Instruments
Maze task
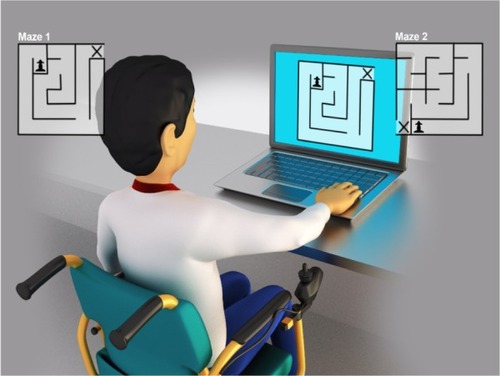
To verify computer task performance, we used a maze task proposed by Souza et alCitation32 and used by Possebom et al.Citation29 It consisted of traversing within a path on the computer screen in the shortest possible time. The maze had one entrance, one exit, and only one way to achieve the goal. Souza et alCitation32 stated that the maze task can be used in the diagnostic evaluation of individuals with changes in control and motor learning to identify aspects that are compromised during motor task execution. The maze task has the advantage that it can be adapted to many different subjects, including children, the elderly, and people with neurological disorders.
Therefore, two drawings of mazes with only one correct path were chosen. The labyrinth patterns in both mazes were distinct; however, the numbers of moves needed to accomplish the task were similar. illustrates the task variations for each stage of the experiment.
Motor Function Measure Scale
The Motor Function Measure (MFM) scale was used to characterize the sample and to compare maze task performances.Citation33,Citation34 The MFM scale consists of 32 task items in three dimensions (D1, D2, and D3) that provide a detailed profile of the physical impairment: D1, standing and transfers; D2, axial and proximal motor function; and D3, distal motor function. The scoring for each task uses a four-point scale based on the subject’s maximal abilities without assistance: 0, cannot initiate the task or maintain the starting position; 1, performs the task partially; 2, performs the task incompletely or imperfectly (with compensatory/uncontrolled movements or slowness); or 3, performs the task fully and “normally”. The 32 scores are summed to yield a total score expressed as the percentage of the maximum possible score (that obtained with no physical impairment); the lower the total score, the more severe the impairment.Citation35
Procedure
Participants or their legal guardians signed a consent form and underwent gross motor function evaluation as scored by two physiotherapists who discussed and arrived at a consensus regarding the MFM score.
After evaluating motor performance, participants completed the maze task individually in a room equipped with a computer, table, chair, and the evaluator responsible for providing instruction. The chair and footrest were adjusted according to the height and needs of the individual so that they would be properly positioned to view the beginning and end of the task. Before starting the task, the researcher demonstrated and described how to perform the task once; then, all the participants completed a single trial test to verify that they understood the instructions. They were asked to perform the task in the shortest time possible, taking the pawn to the “X” using the keyboard arrows (up, down, right, and left).
During the acquisition phase, all groups attempted the maze 20 times, and then after 5 minutes, they performed five more repetitions of Maze 1 for the retention phase. Finally, for the transfer phase, they performed five repetitions in Maze 2. The study design task is shown in .
Data analysis
The results were obtained using blocks (five attempts each) for all study phases (acquisition, retention, and transfer). This approach was adopted to minimize the impact of variable individual values.Citation36,Citation37 Analyses of variance were used to make comparisons between groups (DMD and TD groups) and block attempts (acquisition and practice effects: first acquisition block versus last acquisition block; retention: last acquisition block versus retention block; transfer: retention block versus transfer block) with repeated measures for the factor block. Tukey honest significant difference post hoc testing was also performed. We used Pearson correlation coefficients to assess the degree of association between variables. Regression analysis considering improvement in movement time (MT) in the first and final practice blocks was performed to determine which factors (age, MFM-tot, MFM-D3) influenced the degree of learning during practice for the DMD group. We considered findings to be significant at P<0.05.
Results
Practice effects
Significant differences were found between the DMD and TD groups, F(1, 82) =268.3, P<0.001, ηCitation2=0.77; the first and last acquisition blocks, F(1, 82) =69.58, P<0.01, ηCitation2=0.46; and interaction for block by group, F(1, 82) =14.44, P<0.01, ηCitation2=0.15. Post hoc comparisons indicated a significant decrease in MT between the first and last acquisition blocks, but only for the DMD group (8.4 and 5.7 seconds, respectively) and not for the TD group (4.4 and 3.4 seconds, respectively; ). In addition, the TD group showed a shorter MT than the DMD group with practice. The results are detailed in .
Figure 3 Representation of the trial blocks for both groups.
Abbreviations: A1, first acquisition block; A2, second acquisition block; A3, third acquisition block; A4, fourth acquisition block; CI, confidence interval; DMD, Duchenne muscular dystrophy; R, retention test block; T, transfer test block; TD, typical development.
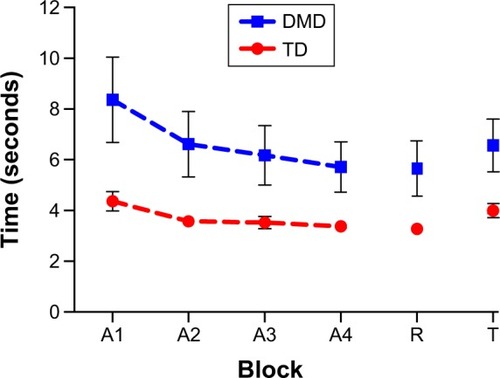
Table 2 Mean and standard deviations of the blocks in each study phase of the study. In addition the mean difference and 95% CI are also shown
Retention
The comparison of the final practice and retention blocks did not reveal any significant main or interaction effects by block. That is, the patterns of MT in the final practice and retention blocks were similar for both groups, indicating consolidating learning in the DMD group. However, the main effect for group persisted, F(1, 82) =20.91, P<0.001, ηCitation2=0.20.
Transfer
There were significant effects of block, F(1, 82) =63.84, P<0.001, ηCitation2=0.44, indicating that MT increased from retention to transfer (DMD group 5.7 and 6.6 seconds; TD 3.3 and 4.0 seconds, respectively). The significant main effect of group also remained, F(1, 82) =256.5, P<0.001, ηCitation2=0.21. There was no interaction between the two factors. Finally, proactive interference was examined by comparing MT during the transfer and first acquisition blocks. This revealed significant effects for block, F(1, 82) =22.21, P<0.01, ηCitation2=0.21 and block by group, F(1, 82) =9.72, P<0.01, ηCitation2=0.11. Post hoc comparisons indicated that MT during transfer was shorter than during the first acquisition block. However, this was only the case for the DMD group (8.4 and 6.6 seconds, respectively) and not for the TD group (4.4 and 4.0 seconds, respectively). We also observed a significant main effect for group, F(1, 82) =23.68, P<0.001, ηCitation2=0.22.
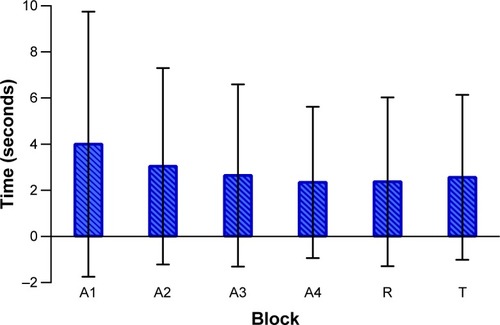
The differences between groups for each trial are shown in .
Figure 4 Difference between the groups in each trial block (mean ± standard deviation).
Abbreviations: A1, first acquisition block; A2, second acquisition block; A3, third acquisition block; A4, fourth acquisition block; R, retention test block; T, transfer test block; DMD, Duchenne muscular dystrophy; TD, typical development.
We evaluated the linear correlation between block attempts and the functional scale scores. The results show significant moderate negative correlations between the MFM-D3 and trial blocks (), indicating that subjects who performed relatively well on the MFM-D3 required less time to complete the maze.
Table 3 Correlation coefficient considering trial blocks with age and functional scale results
The regression analysis revealed a significant finding F(4, 37) =42.61, P<0.001, r2=0.82, resulting in the following equation: improvement =0.462× first practice block. In other words, only the MT in the first practice block predicted the degree of learning; motor tests performance did not contribute. To understand which factors influenced performance in the first practice block, another regression analysis was performed between the first block of the acquisition phase with age, MFM-tot, and MFM-D3. There was a significant finding for the regression model, F(3.38) =6.31, P=0.001, r2=0.33, resulting in the following equation: performance in the first practice block =−0.219× MFM-D3. In other words, only MFM D3 could predict performance in the first practice block.
Discussion
People suffering from severe disabilities can benefit from the use of assistive technologies that facilitate communication, house-environment management, and mobility according to the user’s residual motor abilities.Citation38 Determining the computing task performance of individuals with disabilities can help develop important strategies to maintain or improve their functionality.
Considering the maze task, the results show that only participants in the DMD group had significant performance differences between the beginning and end of the acquisition phase, with reduced time to perform the task. Subjects with DMD were able to maintain their performance, and although there was a significant difference between retention and transfer, the MT values of transfer were below that at baseline (Block A1). These results suggest that individuals with DMD were able to adapt to the task and improve their performance with training. Similarly, Savion-Lemieux and PenhuneCitation39 observed a reduction in time to execute a given task after training, verifying that learning occurred.
Conversely, we found that participants in the TD group did not improve their performance during training. The MT verified in the first acquisition block was already close to their fastest performance, suggesting that the task was easier for the TD group.
Regarding the difference between groups, the performance of individuals with DMD was inferior to the TD group for all phases of the task. This demonstrates that despite their ability to learn a motor task on the computer, their disease hindered their functional performance. DMD is characterized by progressive muscle weaknessCitation40 due to myofibril deterioration, which results in slower movement.Citation41,Citation42 Other musculoskeletal factors may have contributed to difficulties in task execution, such as increased connective tissue (fibrosis) which accompanies the muscular changesCitation43 and fatigue in DMD.Citation44
Studies comparing DMD and TD individuals also found performance differences. Mattar and SobreiraCitation45 studied hand weakness, and Nakafuji and TsujiCitation30 evaluated perceptual motor processes and bilateral transfer in individuals with DMD and age-matched controls; both studies found significant differences between the two groups. Their results suggest that protocols used for TD individuals might yield different results than for subjects with DMD.
Linear regression revealed that the age of participants with DMD in our study did not influence maze performance. Given the progressive nature of DMD and that the age of our sample (mean ± standard deviation, 18±5.5; range: 10–32 years) was greater than the average age reported in several other studies,Citation30,Citation46–Citation48 it is not surprising that older subjects had worse task performance.
We observed significant moderate negative correlations between the MFM-D3 and trial blocks (), indicating that persons who perform relatively well on the MFM-D3 require less time to complete the maze and suggesting that performance was related to arm motor skill. However, the linear correlation demonstrated that the execution time in block A1 was the only variable capable of predicting participant performance in the acquisition phase of the computer task. Again, the level of MFM-D3 (ie, upper limb motor function) could predict performance in the initial block of attempts (block A1). Previous studies that assessed motor learning and bilateral transfer of learning in individuals with DMD also reported that motor dysfunction affected the evaluated performances.Citation30,Citation46
The current study has several limitations that should be mentioned. First, we used a convenience sample, and most evaluated individuals were older than 10 years, meaning that we were unable to obtain data from individuals with DMD with good hand function (<10 years old). The second concern is that although the maze used in the computational task was simple, with low cognitive demand, we did not perform formal cognitive assessments. Future studies should employ more complicated maze designs that require more cognitive participation to solve the task, allowing the evaluation of diverse neuropsychologic aspects of planning, execution, spatial organization, and implicit memory. Such work will facilitate the generalization of the results for more complex computing activities such as those carried out in activities of daily living.
Conclusion
We observed improved computational task performance among participants with DMD following practice. However, a difference in MT was observed in all attempts among individuals from both groups.
The improvement on the computational task was positively influenced by the baseline performance level of individuals with DMD, which was itself affected by distal functionality, suggesting that better functionality leads to better performance. It was not associated with age or the overall functionality of individuals with DMD.
Author contributions
All authors participated in study design, data acquisition and interpretation, and manuscript drafting and revision. All authors read and gave final approval to the version submitted for publication.
Acknowledgments
This study received financial support from the FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo, process number 2012/16970-6).
Disclosure
The authors report no conflicts of interest in this work.
References
- BushbyKFinkelRBirnkrantDJDiagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial managementLancet Neurol201091779319945913
- KinaliMArechavala-GomezaVFengLLocal restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept studyLancet Neurol200981091892819713152
- EagleMBaudouinSVChandlerCGiddingsDRBullockRBushbyKSurvival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilationNeuromuscul Disord2002121092692912467747
- SnowWMAndersonJEJakobsonLSNeuropsychological and neurobehavioral functioning in Duchenne muscular dystrophy: a reviewNeurosci Biobehav Rev201337574375223545331
- PeddareddygariLRPillaiBHNochlinDSharerLRGrewalRPPhenotype-genotype analysis of dystrophinopathy caused by duplication mutation in Dystrophin gene in an African patientAfr Health Sci201111460760922649443
- FosterHPopplewellLDicksonGGenetic therapeutic approaches for Duchenne muscular dystrophyHum Gene Ther201223767668722647146
- BelloLMelaciniPPezzaniRCardiomyopathy in patients with POMT1-related congenital and limb-girdle muscular dystrophyEur J Hum Genet201220121234123922549409
- JoverMSchmitzCBosdureEChabrolBAssaianteCAnticipatory postural adjustments in a bimanual load-lifting task in children with Duchenne muscular dystrophyNeurosci Lett2006403327127516750880
- JansenMde GrootIJvan AlfenNGeurtsAChPhysical training in boys with Duchenne muscular dystrophy: the protocol of the No Use is Disuse studyBMC Pediatr2010105520691042
- BushbyKConnorEClinical outcome measures for trials in Duchenne muscular dystrophy: report from International Working Group meetingsClin Investig (Lond)20111912171235
- BushbyKFinkelRBirnkrantDJDiagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary careLancet Neurol20109217718919945914
- JureticNJorqueraGCaviedesPJaimovichERiverosNElectrical stimulation induces calcium-dependent up-regulation of neuregulin-1beta in dystrophic skeletal muscle cell linesCell Physiol Biochem2012295−691993022613991
- MalikVRodino-KlapacLRMendellJREmerging drugs for Duchenne muscular dystrophyExpert Opin Emerg Drugs201217226127722632414
- RamanVYacobDTobiasJDDexmedetomidine-ketamine sedation during upper gastrointestinal endoscopy and biopsy in a patient with Duchenne muscular dystrophy and egg allergyInt J Crit Illn Inj Sci201221404322624101
- MerliniLGennariMMalaspinaEEarly corticosteroid treatment in 4 Duchenne muscular dystrophy patients: 14-year follow-upMuscle Nerve201245679680222581531
- LeebRLeeFKeinrathCSchererRBischofHPfurtschellerGBrain-computer communication: motivation, aim, and impact of exploring a virtual apartmentIEEE Trans Neural Syst Rehabil Eng200715447348218198704
- Ron-AngevinRDiaz-EstrellaABrain-computer interface: changes in performance using virtual reality techniquesNeurosci Lett2009449212312719000739
- BurgstahlerSComdenDLeeSMArnoldABrownKComputer and cell phone access for individuals with mobility impairments: an overview and case studiesNeuroRehabilitation201128318319721558625
- JamesWVOrrJFUpper limb weakness in children with Duchenne muscular dystrophy – a neglected problemProsthet Orthot Int1984821111136483591
- de MouraMCDo ValleLEResendeMBPintoKOVisuospatial attention disturbance in Duchenne muscular dystrophyDev Med Child Neurol2010522e10e1520002115
- SternLMSeegerBRLittleJManagement and prognosis of Duchenne muscular dystrophyAust Paediatr J19831921351366626058
- VilozniDBar-YishayEGurIShapiraYMeyerSGodfreySComputerized respiratory muscle training in children with Duchenne muscular dystrophyNeuromuscul Disord1994432492557919973
- MirelmanAMaidanIHermanTDeutschJEGiladiNHausdorffJMVirtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson’s disease?J Gerontol A Biol Sci Med Sci201166223424021106702
- BurdeaGCCioiDKaleAJanesWERossSAEngsbergJRRobotics and gaming to improve ankle strength, motor control, and function in children with cerebral palsy – a case study seriesIEEE Trans Neural Syst Rehabil Eng201321216517322773059
- ThorpeDEValvanoJThe effects of knowledge of performance and cognitive strategies on motor skill learning in children with cerebral palsyPediatr Phys Ther200214121517053676
- WinsteinCJMeriansASSullivanKJMotor learning after unilateral brain damageNeuropsychologia199937897598710426521
- LevinMFKnautLAMagdalonECSubramanianSVirtual reality environments to enhance upper limb functional recovery in patients with hemiparesisStud Health Technol Inform20091459410819592789
- Torriani-PasinCBonuzziGMSoaresMAPerformance of Down syndrome subjects during a coincident timing taskInt Arch Med2013611523618314
- PossebomWFSilvaTDdRéAHNAprendizagem motora em pessoas com síndrome de Down: tarefa de labirinto no computadorTemas sobre Desenvolvimento20131045460
- NakafujiATsujiKLearning and transfer in two perceptual-motor skills in Duchenne muscular dystrophyPercept Mot Skills200193233935211769887
- BendixenRMSenesacCLottDJVandenborneKParticipation and quality of life in children with Duchenne muscular dystrophy using the International Classification of Functioning, Disability, and HealthHealth Qual Life Outcomes2012104322545870
- SouzaDEFrançaFRCamposTFTeste de labirinto: instrumento de análise na aquisição de uma habilidade motoraRev Bras Fisioter2006103355360
- BerardCPayanCHodgkinsonIFermanianJA motor function measure for neuromuscular diseases. Construction and validation studyNeuromuscul Disord200515746347016106528
- IwabeCPfeilstikerBNucciAMedida da função motora: versão da escala para o português e estudo de confiabilidadeRev Bras Fisioter200812417424
- VuillerotCPayanCGirardotFResponsiveness of the motor function measure in neuromuscular diseasesArch Phys Med Rehabil2012931222512256.e122705238
- SchmidtRABjorkRANew conceptualizations of practice: common principles in three paradigms suggest new concepts for trainingPsychol Sci199234207217
- de Mello MonteiroCBMassettiTda SilvaTDTransfer of motor learning from virtual to natural environments in individuals with cerebral palsyRes Dev Disabil201435102430243724981192
- CincottiFMattiaDAloiseFNon-invasive brain-computer interface system: towards its application as assistive technologyBrain Res Bull200875679680318394526
- Savion-LemieuxTPenhuneVBThe effects of practice and delay on motor skill learning and retentionExp Brain Res2005161442343115551084
- DeconinckNDanBPathophysiology of Duchenne muscular dystrophy: current hypothesesPediatr Neurol20073611717162189
- CaromanoFANiitsumaLYVainzofMZatzMCorrelação entre o tempo de realização de diferentes atividades físicas por portadores de distrofia muscular de DuchenneRevista de Terapia Ocupacional da Universidade de São Paulo2003143133140
- Sienko ThomasSBuckonCENicoriciABagleyAMcDonaldCMSussmanMDClassification of the gait patterns of boys with Duchenne muscular dystrophy and their relationship to functionJ Child Neurol20102591103110920587736
- KlinglerWJurkat-RottKLehmann-HornFSchleipRThe role of fibrosis in Duchenne muscular dystrophyActa Myol201231318419523620650
- CorradoATascaEFatigue in muscular dystrophiesNeuromuscul Disord201222Suppl 3S214S22023182642
- MattarFLSobreiraCHand weakness in Duchenne muscular dystrophy and its relation to physical disabilityNeuromuscul Disord200818319319818207403
- NairKPVasanthAGourie-DeviMDisabilities in children with Duchenne muscular dystrophy: a profileJ Rehabil Med200133414714911506211
- CottonSMVoudourisNJGreenwoodKMAssociation between intellectual functioning and age in children and young adults with Duchenne muscular dystrophy: further results from a meta-analysisDev Med Child Neurol200547425726515832549
- MentoGTarantinoVBisiacchiPSThe neuropsychological profile of infantile Duchenne muscular dystrophyClin Neuropsychol20112581359137721999586