Abstract
Background and objective
Abnormal resting-state functional connectivity (rs-FC) between the central executive network and the default mode network (DMN) in patients with obstructive sleep apnea (OSA) has been reported. However, the effect of OSA on rs-FC within the DMN subregions remains uncertain. This study was designed to investigate whether the rs-FC within the DMN subregions was disrupted and determine its relationship with clinical symptoms in patients with OSA.
Methods
Forty male patients newly diagnosed with severe OSA and 40 male education- and age-matched good sleepers (GSs) underwent functional magnetic resonance imaging (fMRI) examinations and clinical and neuropsychologic assessments. Seed-based region of interest rs-FC method was used to analyze the connectivity between each pair of subregions within the DMN, including the medial prefrontal cortex (MPFC), posterior cingulate cortex (PCC), hippocampus formation (HF), inferior parietal cortices (IPC), and medial temporal lobe (MTL). The abnormal rs-FC strength within the DMN subregions was correlated with clinical and neuropsychologic assessments using Pearson correlation analysis in patients with OSA.
Results
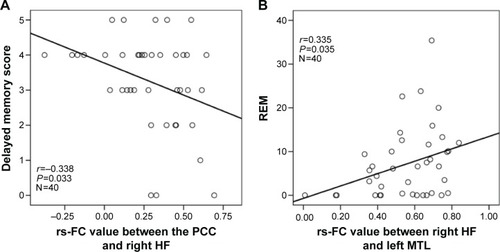
Compared with GSs, patients with OSA had significantly decreased rs-FC between the right HF and the PCC, MPFC, and left MTL. However, patients with OSA had significantly increased rs-FC between the MPFC and left and right IPC, and between the left IPC and right IPC. The rs-FC between the right HF and left MTL was positively correlated with rapid eye movement (r=0.335, P=0.035). The rs-FC between the PCC and right HF was negatively correlated with delayed memory (r=-0.338, P=0.033).
Conclusion
OSA selectively impairs the rs-FC between right HF and PCC, MPFC, and left MTL within the DMN subregions, and provides an imaging indicator for assessment of cognitive dysfunction in OSA patients.
Introduction
Obstructive sleep apnea (OSA) is a common sleep-related breathing disorder caused by repeated complete or partial collapse of the upper airway during sleep, resulting in intermittent hypoxia (IH), intermittent hypercapnia, and sleep fragmentation.Citation1 Based on the results of populations studies, it has been found that OSA affects approximately 5.7%–6.0% of middle-aged men and 2.4%–4.0% of middle-aged women.Citation2,Citation3 OSA has been shown to increase daytime sleepiness, road traffic accidents, stroke, hypertension, coronary artery disease, congestive heart failure, insulin resistance, and cardiovascular mortality.Citation4–Citation8 The common cognitive impairments, including impaired memory, learning, and attention have been commonly observed in patients with OSA, because of both sleep disturbances and hypoxemia.Citation9,Citation10 However, the underlying neural mechanisms remain unclear.
Structural and functional neuroimaging techniques have evolved and have been used to increase our understanding of neurocognitive processes and structural brain differences. Previous voxel-based morphometry (VBM) studies have reported conflicting results in patients with OSA,Citation11–Citation18 and failed to fully explain the differences in the pathophysiology or severity of disease. For example, one study failed to identify any regions of gray matter (GM) reduction.Citation14 Another study showed widespread loss of more than 20 foci of GM concentration.Citation17 Impaired hippocampus was a relatively consistent finding across different neuroimaging techniques in previous studies.Citation12,Citation13,Citation16,Citation17,Citation19 The hippocampus region is closely associated with neural processing of memory.Citation20 Prilipko et alCitation21 found that patients with OSA had a significant inactivation in the medial temporal regions within the default mode network (DMN) and a significant activation in the right ventral frontoparietal network during the tasks. In addition, Sweet et alCitation22 found a deactivation in the posterior cingulate and right postcentral gyrus within the DMN during continuous positive airway pressure (CPAP) withdrawal during working memory tasks using functional magnetic resonance imaging (fMRI) in patients with OSA. Magnetic resonance spectroscopy is a useful neuroimaging tool to measure changes in either the concentration or distribution of chemical substances. Bartlett et alCitation23 found an elevated N-acetylaspartate (NAA)-to-creatine (Cr) ratio (NAA/Cr) and lowered Cr levels in the left hippocampus area, which were associated with neurocognitive performance and OSA severity. O’Donoghue et alCitation24 found a decreased frontal NAA-to-choline (Cho) ratio (NAA/Cho) and hippocampus Cho/Cr ratio in patients with OSA, which persisted after CPAP treatment. However, in the recent years, resting-state fMRI (rs-fMRI)-based functional connectivity (FC) has been widely used for objective analysis of the brain’s functional connectome.Citation25,Citation26 Resting-state FC-based region of interest (ROI) has been regarded as a reliable technology.Citation27,Citation28 It has been increasingly used in studies of OSA and other sleep disorders.Citation29,Citation30 The DMN comprises multiple dissociated subregions, including medial prefrontal cortex (MPFC), posterior cingulate cortex (PCC), bilateral hippocampus formation (HF), bilateral inferior parietal cortices (IPC), and bilateral medial temporal lobe (MTL). Many studies have suggested that the DMN might be associated with the collection and evaluation of information,Citation31 self-referential mental activity,Citation32 extraction of episodic memory,Citation33 emotion and anxiety,Citation34,Citation35 and mind wandering or daydreaming.Citation36 Our previous study found that patients with OSA showed local abnormal spontaneous activity including part of the DMN using amplitude of low-frequency fluctuation (ALFF) and regional homogeneity (ReHo) method.Citation37,Citation38 These findings suggest that the DMN is associated with sleep disorder. Recent studies have confirmed abnormal rs-FC within the DMN subregions in various diseases, including multiple sclerosis, hepatic encephalopathy, and sleep deprivation.Citation39–Citation41 Although numerous neuroimaging studies have been conducted to identify structural and functional impairments in the brains of OSA patients, the effect of OSA on the intrinsic DMN node connectivity is still unknown. In this fMRI study, we hypothesized the rs-FC within the DMN subregions was disrupted in OSA patients.
To test the hypothesis, we first investigated the rs-FC patterns within DMN subregions using seed-based ROI correlation analyses methods in patients with OSA and in good sleepers (GSs). Next, we compared the intergroup differences in the rs-FC within the DMN subregion in patients with OSA and those with GSs. Finally, we evaluated the relationships between the altered rs-FC within the DMN subregions and disease severity and neuropsychologic performance in patients with OSA.
Materials and methods
Subjects
Forty male patients newly diagnosed with severe OSA and 40 male education- and age-matched GSs were included from the Sleep Monitoring Room of the Respiratory Department of The First Affiliated Hospital of Nanchang University. The inclusion and exclusion criteria for the patients with OSA and GSs were consistent with our previous studies.Citation37,Citation38 Inclusion criteria for the patients with OSA were male sex, age older than 22 years but younger than 60 years, and an apnea– hypopnea index (AHI) greater than 30. Inclusion criteria for GSs were male sex, age older than 22 years but younger than 60 years, and an AHI less than 5. The exclusion criteria for both patients with OSA and for GSs were 1) sleep disorders other than OSA, such as insomnia; 2) hypertension, diabetes, and respiratory and heart diseases; 3) neurodegenerative diseases, head injury, epilepsy, psychosis, depressive disorder, and hypothyroidism; 4) history of cerebrovascular disease; 5) alcohol or illicit drug abuse; 6) a structural lesion on brain MRI; and 7) MRI contraindications, such as claustrophobia, metallic implants, or devices in the body. Each candidate underwent a detailed clinical interview, sleep questionnaire, and overnight polysomnography (PSG). Informed written consent was obtained from all the subjects. The study was approved by the Medical Research Ethics Committee of The First Affiliated Hospital of Nanchang University.
Overnight PSG
The day before sleep studies, all GSs and patients with OSA were required to abstain from drinking caffeinated or alcoholic beverages. Overnight PSG monitoring was performed on all patients with OSA and GSs using the Respironics LE-Series physiological monitoring system (Alice 5 LE, Respironics, Orlando, FL, USA). PSG was recorded from approximately 10 pm to 6 am the next day. The electroencephalogram (EEG), electrooculogram (EOG), electrocardiogram, chin electromyogram (EMG), oral and nasal airflow, thoracic and abdominal movements, body position, oxygen saturation (SaO2), and snoring were recorded. According to the American Academy of Sleep Medicine (AASM) guidelines,Citation42 the EEG derivations (from frontal, central, occipital regions: F4/M1, C4/M1, O2/M1; and back up derivations: F3/M2, C3/M2, and O1/M2), EMG (located in the three chin electrodes and the middle of the right anterior tibialis), and EOG (located in the cornea and retina) were recorded. Sleep latency, total sleep time, sleep efficiency, sleep stages, arousal, and respiratory events were also recorded. According to the AASM manual, an obstructive apnea was defined as a reduction in airflow ≥90% lasting at least 10 seconds and associated with persistent respiratory effort; hypopnea was defined as a reduction in airflow ≥30% lasting at least 10 seconds and accompanied with a 4% or greater oxygen desaturation.Citation42 The AHI was calculated as the average of the total number of apnea and hypopnea events experienced per hour of sleep. The arousal index (AI) was computed as the mean number of EEG arousals per hour of sleep.
Neuropsychological assessments
All patients with OSA and GSs were evaluated with a self-reported sleep questionnaire for excessive daytime sleepiness using Epworth sleepiness scale (ESS), which asks the subject to rate his or her probability of falling asleep on a scale of increasing probability from 0 to 3 for eight different situations.Citation43 A score equal to or greater than 10 demonstrates excessive daytime sleepiness in OSA patients. Cognitive function was evaluated using the Montreal Cognitive Assessment (MoCA), including naming, executive function, calculation, attention, language, memory, abstraction, and orientation, with a total MoCA score lower than 26 indicating cognitive impairment.Citation44 If the length of subjects’ education was less than 12 years, one point was added to the total score, as education deviation adjustment.Citation44
MRI data acquisition
Imaging was performed on a 3.0 T MRI system with eight-channel head coil (Siemens, Munich, Germany). Earplugs were used to minimize scanner noise, and foam pads were used to reduce head movements. First, conventional T1-weighted imaging and T2-weighted imaging were performed. Then, the rs-fMRI images were collected using an echo planar imaging (EPI) sequence with the following parameters: repetition time (TR) =2,000 ms, echo time (TE) =30 ms, thickness =4.0 mm, gap =1.2 mm, field of view (FOV) =230 mm ×230 mm, matrix =64×64, and flip angle =90°. Each brain volume comprised 30 axial slices, and each functional run consisted of 240 volumes. During rs-fMRI scans, all subjects were instructed to keep their eyes closed, to be still, to think of nothing in particular, and not to fall asleep. Finally, high-resolution three-dimensional T1-weighted images were obtained by a brain volume sequence (TR =1,900 ms, TE =2.26 ms, thickness =1.0 mm, gap =0.5 mm, FOV =250 mm ×250 mm, matrix =256×256, flip angle =9°, 176 sagittal slices).
fMRI data preprocessing
Functional data were checked by MRIcro software (www.MRIcro.com) to exclude defective data. The rs-fMRI data were analyzed using the Data Processing Assistant for Resting-State fMRI Advanced Edition (DPARSFA; http://rfmri.org/DPARSFA) on the basis of MATLAB2010a (Mathworks, Natick, MA, USA). The first ten volumes of each subject were discarded due to the signal reaching equilibrium and the participants adapted to the scanning noise. The remaining 230 volumes were corrected for delay in acquisition time between different slices and corrected for geometrical displacements according to the estimated head movement. The Friston six-head motion parameters were computed by estimating translation in each direction and the angular rotation on each axis for each volume based on recent work showing that higher order models were more effective in removing head motion effects.Citation45,Citation46 Each subject showed a maximum displacement of less than 1.5 mm in any cardinal direction (x, y, z) and a maximum spin (x, y, z) of less than 1.5°. A multiple regression method was performed to remove possible sources of artifacts, including six estimated motion parameters, ventricular and white matter regions, and global signal.Citation47,Citation48 Subsequently, all functional data were spatially normalized to the Montreal Neurological Institute (MNI) EPI template, and resampled to 3×3×3 mm3 voxels and smoothed with a 6 mm full width at half maximum. Finally, a temporal filter (0.01–0.08 Hz) was applied to reduce the effect of low-frequency drift and high-frequency noise.
Definition of DMN subregions
In previous research,Citation38,Citation49,Citation50 we defined eight core subregions within the DMN: PCC (MNI coordinates: 0, −53, 26), MPFC (MNI coordinates: 0, 52, –6), left HF (MNI coordinates: −24, −22, −20), right HF (MNI coordinates: 24, −20, −22), left MTL (MNI coordinates: −43, −74, 28), right MTL (MNI coordinates: 47, −57, 20), left IPC (MNI coordinates: −29, 26, −28), and right IPC (MNI coordinates: 29, 26, −28). Next, the average time series of a 6 mm sphere centered at the peak coordinate of each subregion was extracted from each patient with OSA and GS, and the rs-FC map of each pairwise subregion was obtained.
rs-FC analysis of DMN subregions
For each patient with OSA and GS, Pearson correlation coefficients between the mean time series of each pairwise DMN subregion were computed and converted to z-values using Fisher’s r-to-z transformation to improve the normality.
Statistical analysis
The demographic and clinical data were compared between the two groups using independent sample t-test, which was conducted with Statistical Package for the Social Sciences version 19.0 (SPSS, Chicago, IL, USA). Differences were considered significant when P<0.05.
For the rs-FC within the DMN subregions, first, a single sample t-test was performed on individual z-value map of the 28 pairs of DMN subregion connectivities within each group. Then, we analyzed intergroup differences after adding the body mass index (BMI) and age as covariates of no interest using two independent sample t-tests. A corrected significance level of P<0.05, using the false-positive adjustment, was used to determine the statistical significance.
We investigated the relationship between clinical and neuropsychologic assessments and the altered rs-FC value of pairwise subregions within the DMN in patients with OSA. Each significant rs-FC value was extracted and used for ROI-based correlation analyses with clinical variables using linear correlation analysis. P<0.05 was deemed statistically significant.
Results
Demographic and clinical data
There were no significant differences between patients with OSA and GSs in age and years of education (P>0.05; ). The patients with OSA had significantly higher scores for BMI (t=7.43, P<0.001), AHI (t=17.20, P<0.001), SaO2 <90% (t=8.03, P<0.001), AI (t=8.09, P<0.001), and ESS (t=12.42, P<0.001), but a significantly lower score for rapid eye movement (REM) sleep (t=−8.03, P<0.001) and MoCA (t=−5.09, P=0.036) when compared with GSs.
Table 1 Demographic and clinical data of patients with OSA and GSs
rs-FC of pairwise subregions within the DMN between patients with OSA and GSs
In the two groups, all pairwise DMN subregions showed strong correlation (). Compared with GSs, patients with OSA displayed significantly decreased rs-FC between the right HF and the PCC, MPFC, and left MTL. However, patients with OSA showed significantly increased rs-FC between left IPC and right IPC, and between the MPFC and left and right IPC (, ). Abnormal connection coefficients were extracted ().
Figure 1 Correlation matrix of the average time series of pairwise subregions within the DMN.
Abbreviations: FC, functional connectivity; DMN, default mode network; GSs, good sleepers; OSAs, patients with obstructive sleep apnea; MPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; HF, hippocampus formation; MTL, medial temporal lobe; IPC, inferior parietal cortices; R, right; L, left.
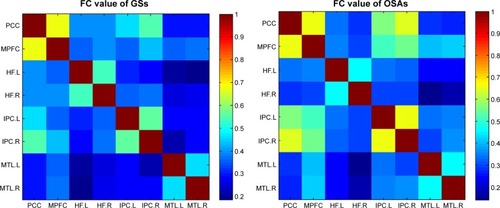
Figure 2 Compared with GSs, patients with OSA showed abnormal rs-FC of pairwise subregions within the DMN.
Abbreviations: GSs, good sleepers; OSA, obstructive sleep apnea; rs-FC, resting-state functional connectivity; DMN, default mode network; MPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; HF, hippocampus formation; MTL, medial temporal lobe; IPC, inferior parietal cortices; R, right; L, left.
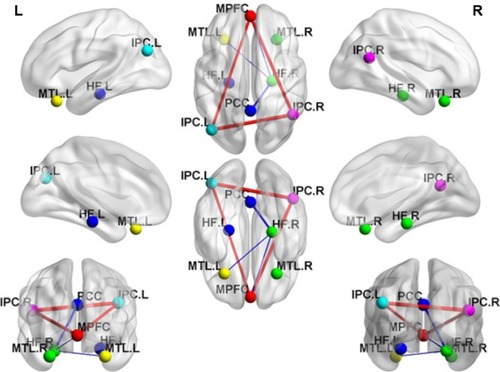
Figure 3 Mean correlation coefficient z-values of abnormal rs-FC of pairwise subregions within the DMN.
Abbreviations: GSs, good sleepers; OSAs, patients with obstructive sleep apnea; rs-FC, resting-state functional connectivity; MPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; HF, hippocampus formation; MTL, medial temporal lobe; IPC, inferior parietal cortices; R, right; L, left; DMN, default mode network.
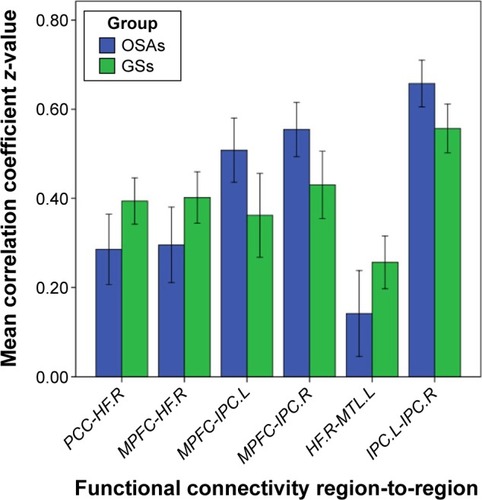
Table 2 Abnormal rs-FC of pairwise subregions within the DMN between patients with OSA and GSs
Correlations
In patients with OSA, the AHI score was significantly positively correlated with BMI (r=0.418, P=0.007), ESS (r=0.430, P=0.006), AI (r=0.732, P<0.001), and negatively correlated with nadir SaO2 (r=−0.499, P=0.001) and mean SaO2 (r=−0.467, P=0.002). BMI displayed significantly positive correlation with AI (r=0.546, P<0.001), and negative correlation with the nadir SaO2 (r=−0.613, P<0.001).
In patients with OSA, significant positive correlations were found between the rs-FC of the right HF with left MTL and the REM% (r=0.335, P=0.035). Significant negative correlations were found between the rs-FC of the PCC with right HF and delayed memory (r=−0.338, P=0.033; ).
Figure 4 Scatter plots demonstrate significant correlations between the rs-FC of pairwise subregions within the DMN and OSA severity and neuropsychologic scores in patients with OSA.
Abbreviations: OSA, obstructive sleep apnea; rs-FC, resting-state functional connectivity; REM, rapid eye movement; PCC, posterior cingulate cortex; HF, hippocampus formation; MTL, medial temporal lobe; DMN, default mode network.
Discussion
Abnormal rs-FC between the right anterior insula and the two anticorrelated cognitive-related networks (the central executive network and the DMN) has been reported in patients with OSA.Citation29 However, to the best of our knowledge, our study is the first to explore group differences between specific subregions within the DMN using seed-based ROI rs-FC method in patients with OSA. We found that patients with OSA displayed decreased rs-FC between the right HF and the PCC, MPFC, and left MTL, and concomitant increase in rs-FC between left IPC and right IPC and between the MPFC and left and right IPC, compared with GSs. Furthermore, we found that the rs-FC between the right HF and left MTL was positively correlated with the REM%, and the rs-FC between the PCC and right HF was negatively correlated with delayed memory. Our findings indicated aberrant rs-FC within the DMN subregions in patients with OSA during the resting state.
Obesity, age, and male sex were the main risk factors for OSA. Patients with OSA had significantly higher BMI compared with GSs in our study. Sforza et alCitation51 found that patients with OSA showed significant sex differences in clinical symptoms, and females with OSA exhibited a lower AHI, less severe hypoxia, and greater peripheral fat mass, and also frequently reported anxiety and depression. Previous studies had demonstrated that obesity and sex differences likely influence the resting-state brain activity.Citation52,Citation53 Controlling for the BMI and sex differences weakened the statistical differences in the rs-FC between patients with OSA and GSs. To confirm that our rs-FC findings were mainly due to OSA rather than obesity or sex differences, we compared the intergroup differences in rs-FC within the DMN subregions with BMI and age as covariates, and only adult male OSA patients were recruited. We still observed significant rs-FC between the right HF and PCC, MPFC, and left MTL with the same statistical threshold, suggesting that the reduced rs-FC was related to OSA rather than obesity.
Although the exact neural mechanisms underlying reduced rs-FC between the PCC and right HF were unknown, they may be related to structural or functional impairment in the PCC or right HF. Greicius et alCitation54 found that the PCC plays a pivotal role in the DMN, which has a strong anatomical relationship with the rest of the DMN. Moreover, the PCC displayed a strong reciprocal connection and other related memory structures, such as the HF, and played a critical role in episodic memory retrieval.Citation55 Previous studies found that hippocampal structural or functional impairments were relatively consistent across different neuroimaging techniques in OSA.Citation12,Citation13,Citation16,Citation17,Citation19 A few studies have showed abnormal deactivation and metabolism in the PCC in patients with OSA.Citation22,Citation56 Our previous study found that patients with OSA showed significantly lower ALFF value in the PCC, and displayed a significant positive correlations with the nadir SaO2, suggesting that IH may be an important factor underlying PCC dysfunction in OSA.Citation38 Prilipko et alCitation57 found that patients with OSA displayed abnormal inactivation in the DMN during working memory tasks and significant positive correlation with behavioral performance, suggesting that inhibition of the resting activity in the DMN plays an important role in cognitive impairment. A previous study has shown greater ipsilateral HF atrophy and reduced FC between the ipsilateral HF and the PCC in patients with temporal lobe epilepsy.Citation58 Consistent with these results, our study found that patients with OSA displayed significantly decreased rs-FC between the PCC and right HF. Furthermore, the rs-FC between the PCC and right HF displayed significant negative correlation with delayed memory score, suggesting that reduced rs-FC between the PCC and right HF might be an imaging indicator of cognitive impairment in OSA.
Previous studies have shown that the MTL subsystem, composed of the MPFC, posterior IPC, retrosplenial cortex, parahippocampal cortex, and HF, showed increased activity in participants during episodic decisions and that the MPFC was the most important nodal hub.Citation59 Zhang et alCitation60 found that patients with OSA demonstrated decreased rs-FC and reduced GMV in the MPFC using independent component analysis and VBM methods, respectively, suggesting that the structural and functional impairments in the MPFC might be a candidate mechanism for the cognitive and emotional deficits. A previous study showed reduced FC between the MPFC and the HF in patients with depression and schizophrenia.Citation61 The prefrontal cortex and HF were vulnerable to impairment by sleep disruption and IH,Citation62,Citation63 and decreased task-related activation in the MPFC and hippocampal atrophy were frequently reported in patients with OSA.Citation64,Citation65 The hippocampus and MPFC play distinct and important roles in emotional processing, spatial memory, and navigation.Citation66,Citation67 Our study found that OSA was associated with significantly decreased rs-FC between the right HF and the MPFC, probably related to IH and sleep fragmentation, respectively, resulting in neuronal deficits in the HF and the MPFC.Citation68,Citation69
A previous study has found that cerebrovascular reactivity of the MTL was negatively correlated with duration of nocturnal hypoxemia, suggesting a possible pathophysiological mechanism for hippocampal injury.Citation70 The volume of the HF and the MTL atrophy were also reported, which is associated with memory impairment.Citation71 Consistent with the previous study, our study found significantly decreased rs-FC between right HF and left MTL in patients with OSA. We also found that the rs-FC between the right HF and left MTL was positively correlated with REM sleep. REM sleep has been shown to be necessary for cortical synaptic plasticity and for the acquisition of spatial and nonspatial memory.Citation72 Fragmented REM sleep resulting from REM-sleep-specific respiratory events and related arousal might have resulted in low REM sleep amount in patients with OSA. Low REM sleep may play an important role in the HF and the MTL for memory impairment in patients with OSA.
The IPC is implicated in attention processing,Citation73 and is unintentionally or obligatorily occupied in the recall, consolidation, and retrieval of episodic memory information.Citation74,Citation75 Previous studies described overactivation of the right IPC during a two-back verbal working memory after acute withdrawal from positive airway pressure treatment in OSA.Citation76 Similarly, Ayalon et alCitation77 found that patients with OSA had increased brain activation in the left IPC, and was negatively correlated with recall during verbal learning task, suggesting that patients with OSA displayed an adaptive compensatory recruitment response. Consistent with the findings, our study found that patients with OSA had significantly increased rs-FC between left and right IPC, and between the MPFC and left and right IPC. These brain regions may be involved in an adaptive compensatory response for cognitive impairment in patients with OSA.
Limitations
Several limitations should be considered when interpreting our results. First, considering the convention of sample collection and homogeneity, we only recruited adult males with severe OSA. Females, children, and mild-to-moderate OSA cases need to be investigated in future studies. Previous observational studies demonstrated that children with OSA had increased executive dysfunction and depression symptoms compared with the normal children.Citation78,Citation79 However, the underlying neural mechanism is not clear. Second, we only observed the rs-FC within the DMN subregions. Diffusion tensor imaging technology enables elucidation of the anatomical basis of abnormal rs-FC within the DMN subregions. Finally, we only studied the abnormal rs-FC within the DMN subregions before treatment in OSA. Additional investigations are needed to explore whether the abnormal rs-FC within the DMN subregions can be improved after treatment.
Conclusion
In this study, we investigated alterations in connectivity of pairwise subregions within the DMN in patients with OSA using seed-based ROI rs-FC methods. We found that OSA selectively impairs the rs-FC between right HF and PCC, MPFC, and left MTL within the DMN subregions, thereby providing an imaging indicator for the assessment of cognitive dysfunction in patients with OSA.
Acknowledgments
This work was supported by the Natural Science Foundation of China (grant number 81560285), Jiangxi Provincial Department of Natural Science Foundation Project (grant number 20132BAB205100), Jiangxi Provincial Department of Science and Technology Support Program (grant numbers 20132BBG70061 and 20141BBG70026), and Jiangxi Provincial Department of Graduate Innovation Foundation (grant number YC2015-S082).
Disclosure
None of the authors have received payment or services from a third party (government, commercial, private foundation, etc) for any aspect of the submitted work at any time. The authors report no conflicts of interest in this work.
References
- JordanASMcSharryDGMalhotraAAdult obstructive sleep apnoeaLancet2014383991873674723910433
- LiuJHWeiCZHuangLYPrevalence of signs and symptoms suggestive of obstructive sleep apnea syndrome in Guangxi, ChinaSleep Breath201418237538224072550
- FranklinKALindbergEObstructive sleep apnea is a common disorder in the population – a review on the epidemiology of sleep apneaJ Thorac Dis2015781311132226380759
- Teran-SantosJJimenez-GomezACordero-GuevaraJThe association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-SantanderN Engl J Med19993401184785110080847
- OldenburgOLampBFaberLSleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patientsEur J Heart Fail20079325125717027333
- StrohlKPBrownDBCollopNATS Ad Hoc Committee on Sleep Apnea, Sleepiness, and Driving Risk in Noncommercial Drivers. An official American Thoracic Society clinical practice guideline: sleep apnea, sleepiness, and driving risk in noncommercial driversAm J Respir Crit Care Med2013187111259126623725615
- FosterGDSandersMHMillmanRObstructive sleep apnea among obese patients with type 2 diabetesDiabetes Care20093261017101919279303
- SaunamakiTJehkonenMDepression and anxiety in obstructive sleep apnea syndrome: a reviewActa Neurol Scand2007116527728817854419
- EnglemanHMDouglasNJSleep 4: sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndromeThorax200459761862215223874
- XuMYangYZhangJJLevels of neuroglobin in serum and neurocognitive impairments in Chinese patients with obstructive sleep apneaSleep Breath201317257358222674396
- KambaMInoueYHigamiSCerebral metabolic impairment in patients with obstructive sleep apnea: an independent association of obstructive sleep apnea with white matter changeJ Neurol Neurosurg Psychiatry200171333433911511706
- MaceyPMHendersonLAMaceyKEBrain morphology associated with obstructive sleep apneaAm J Respir Crit Care Med2002166101382138712421746
- MorrellMJMcRobbieDWQuestRAChanges in brain morphology associated with obstructive sleep apneaSleep Med20034545145414592287
- O’DonoghueFJBriellmannRSRochfordPDCerebral structural changes in severe obstructive sleep apneaAm J Respir Crit Care Med2005171101185119015699018
- MaceyPMKumarRWooMABrain structural changes in obstructive sleep apneaSleep200831796797718652092
- CanessaNCastronovoVCappaSFObstructive sleep apnea: brain structural changes and neurocognitive function before and after treatmentAm J Respir Crit Care Med2011183101419142621037021
- JooEYTaeWSLeeMJReduced brain gray matter concentration in patients with obstructive sleep apnea syndromeSleep201033223524120175407
- MaceyPMKumarRYan-GoFLSex differences in white matter alterations accompanying obstructive sleep apneaSleep201235121603161323204603
- TorelliFMoscufoNGarreffaGCognitive profile and brain morphological changes in obstructive sleep apneaNeuroimage201154278779320888921
- BurgessNMaguireEAO’KeefeJThe human hippocampus and spatial and episodic memoryNeuron200235462564112194864
- PrilipkoOHuynhNSchwartzSThe effects of CPAP treatment on task positive and default mode networks in obstructive sleep apnea patients: an fMRI studyPLoS One2012712e4743323227139
- SweetLHJerskeyBAAloiaMSDefault network response to a working memory challenge after withdrawal of continuous positive airway pressure treatment for obstructive sleep apneaBrain Imaging Behav20104215516320502992
- BartlettDJRaeCThompsonCHHippocampal area metabolites relate to severity and cognitive function in obstructive sleep apneaSleep Med20045659359615511707
- O’DonoghueFJWellardRMRochfordPDMagnetic resonance spectroscopy and neurocognitive dysfunction in obstructive sleep apnea before and after CPAP treatmentSleep2012351414822215917
- FristonKJFunctional and effective connectivity: a reviewBrain Connect201111133622432952
- Van DijkKRHeddenTVenkataramanAIntrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimizationJ Neurophysiol2010103129732119889849
- FoxMDSnyderAZVincentJLThe human brain is intrinsically organized into dynamic, anticorrelated functional networksProc Natl Acad Sci U S A2005102279673967815976020
- FranssonPSpontaneous low-frequency BOLD signal fluctuations:an fMRI investigation of resting-state default mode of brain function hypothesisHum Brain Mapp2005261152915852468
- ZhangQQinWHeXXFunctional disconnection of the right anterior insula in obstructive sleep apneaSleep Med20151691062107026298780
- YeoBTTandiJCheeMWFunctional connectivity during rested wakefulness predicts vulnerability to sleep deprivationNeuroimage20151711114715825700949
- GusnardDARaichleMERaichleMESearching for a baseline: functional imaging and the resting human brainNat Rev Neurosci200121068569411584306
- GusnardDAAkbudakEShulmanGLMedial prefrontal cortex and self-referential mental activity: relation to a default mode of brain functionProc Natl Acad Sci U S A20019874259426411259662
- CabezaRDolcosFGrahamRSimilarities and differences in the neural correlates of episodic memory retrieval and working memoryNeuroimage200216231733012030819
- SimpsonJRJrDrevetsWCSnyderAZEmotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxietyProc Natl Acad Sci U S A200198268869311209066
- SimpsonJRJrSnyderAZGusnardDAEmotion-induced changes in human medial prefrontal cortex: I. During cognitive task performanceProc Natl Acad Sci U S A200198268368711209065
- MasonMFNortonMIVan HornJDWandering minl brain activity in male patients with severe obstructive sleep apnea: a resting-state functional magnetic resonance imaging studyNeuropsychiatr Dis Treat2014101819182625278755
- PengDCDaiXJGongHHAltered intrinsic regional brain activity in male patients with severe obstructive sleep apnea: a resting-state functional magnetic resonance imaging studyNeuropsychiatr Dis Treat2014101819182625278755
- LiHJDaiXJGongHHAberrant spontaneous low-frequency brain activity in male patients with severe obstructive sleep apnea revealed by resting-state functional MRINeuropsychiatr Dis Treat20151120721425653530
- ZhouFZhuangYGongHAltered inter-subregion connectivity of the default mode network in relapsing remitting multiple sclerosis: a functional and structural connectivity studyPLoS One201497e10119824999807
- QiRFZhangLJXuQAbnormal functional connectivity within the default mode network in patients with HBV-related cirrhosis without hepatic encephalopathy revealed by resting-state functional MRIBrain Res20141576738024907446
- DaiXJLiuCLGongHHLong-term sleep deprivation decreases the default spontaneous activity and connectivity pattern in healthy male subjects: a resting-state fMRI studyNeuropsychiatr Dis Treat20151176177225834451
- IberCAncoli-IsraelSChessonAfor the American Academy of Sleep MedicineThe AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications1st edWestchester, ILAmerican Academy of Sleep Medicine2007
- BillingsMERosenCLAuckleyDPsychometric performance and responsiveness of the functional outcomes of sleep questionnaire and sleep apnea quality of life instrument in a randomized trial: the HomePAP studySleep201437122017202425325491
- NasreddineZSPhillipsNABédirianVThe Montreal Cognitive Assessment, MoCA: a brief screening tool for mild Cognitive impairmentJ Am Geriatr Soc20055369569915817019
- SatterthwaiteTDElliottMAGerratyRTAn improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity dataNeuroimage20136424025622926292
- YanCGCheungBKellyCA comprehensive assessment of regional variation in the impact of head micromovements on functional connectomicsNeuroimage20137618320123499792
- GuoWJiangJXiaoCDecreased resting-state interhemispheric functional connectivity in unaffected siblings of schizophrenia patientsSchizophr Res2014152117017524325975
- SaadZSGottsSJMurphyKTrouble at rest: how correlation patterns and group differences become distorted after global signal regressionBrain Connect201221253222432927
- HeddenTVan DijkKRBeckerJADisruption of functional connectivity in clinically normal older adults harboring amyloid burdenJ Neurosci20092940126861269419812343
- ZhangDRaichleMEDisease and the brain’s dark energyNat Rev Neurol201061152820057496
- SforzaEChouchouFColletPSex differences in obstructive sleep apnoea in an elderly French populationEur Respir J20113751137114320817711
- DaiXJGongHHWangYXGender differences in brain regional homogeneity of healthy subjects after normal sleep and after sleep deprivation: a resting-state fMRI studySleep Med201213672072722503940
- BlackWRLeppingRJBruceASTonic hyper-connectivity of reward neurocircuitry in obese childrenObesity (Silver Spring)20142271590159324634397
- GreiciusMDSupekarKMenonVResting-state functional connectivity reflects structural connectivity in the default mode networkCereb Cortex2009191727818403396
- TortaDMCaudaFDifferent functions in the cingulate cortex, a metaanalytic connectivity modeling studyNeuroimage20115642157217221459151
- YaouhiKBertranFClochonPA combined neuropsychological and brain imaging study of obstructive sleep apneaJ Sleep Res200918364819250174
- PrilipkoOHuynhNSchwartzSTask positive and default mode networks during a parametric working memory task in obstructive sleep apnea patients and healthy controlsSleep201134329330121358846
- KemmotsuNKucukboyaciNEChengCEAlterations in functional connectivity between hippocampus and prefrontal cortex as a correlate of depressive symptoms in temporal lobe epilepsyEpilepsy Behav201329355255924176688
- Andrews-HannaJRReidlerJSSepulcreJFunctional-anatomic fractionation of the brain’s default networkNeuron201065455056220188659
- ZhangQWangDQinWAltered resting-state brain activity in obstructive sleep apneaSleep201336565165923633747
- GenzelLDreslerMCornuMMedial prefrontal-hippocampal connectivity and motor memory consolidation in depression and schizophreniaBiol Psychiatry201577217718625037555
- BeebeDWGozalDObstructive sleep apnea and the prefrontal cortex: towards a comprehensive model; linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficitsJ Sleep Res200211111611869421
- GozalDDanielJMDohanichGPBehavioral and anatomical correlates of chronic episodic hypoxia during sleep in the ratJ Neurosci20012172442245011264318
- ZhangXMaLLiSA functional MRI evaluation of frontal dysfunction in patients with severe obstructive sleep apneaSleep Med201112433534021398177
- MorrellMJJacksonMLTwiggGLChanges in brain morphology in patients with obstructive sleep apnoeaThorax2010651090891420861295
- CrossRLKumarRMaceyPMNeural alterations and depressive symptoms in obstructive sleep apnea patientsSleep20083181103110918714782
- MaguireEAGadianDGJohnsrudeISNavigation-related structural change in the hippocampi of taxi driversProc Natl Acad Sci U S A20009784398440310716738
- MorrellMJTwiggGNeural consequences of sleep disordered breathing: the role of intermittent hypoxiaAdv Exp Med Biol2006588758817089881
- RowBWLiuRXuWIntermittent hypoxia is associated with oxidative stress and spatial learning deficits in the ratAm J Respir Crit Care Med20031671548155312615622
- PrilipkoOHuynhNThomasonMEAn fMRI study of cerebrovascular reactivity and perfusion in obstructive sleep apnea patients before and after CPAP treatmentSleep Med201415889289824916094
- DaulatzaiMAEvidence of neurodegeneration in obstructive sleep apnea: Relationship between obstructive sleep apnea and cognitive dysfunction in the elderlyJ Neurosci Res201524117
- Romcy-PereiraRPavlidesCDistinct modulatory effects of sleep on the maintenance of hippocampal and medial prefrontal cortex LTPEur J Neurosci200420123453346215610178
- SupekarKUddinLQPraterKDevelopment of functional and structural connectivity within the default mode network in young childrenNeuroimage201052129030120385244
- FosterDJWilsonMAReverse replay of behavioural sequences in hippocampal place cells during the awake stateNature2006440708468068316474382
- WigGSGraftonSTDemosKEMedial temporal lobe BOLD activity at rest predicts individual differences in memory ability in healthy young adultsProc Natl Acad Sci U S A200810547185551856019001272
- AloiaMSSweetLHJerskeyBATreatment effects on brain activity during a working memory task in obstructive sleep apneaJ Sleep Res200918440441019765205
- AyalonLAncoli-IsraelSKlemfussZIncreased brain activation during verbal learning in obstructive sleep apneaNeuroimage20063141817182516626972
- EspositoMAntinolfiLGallaiBExecutive dysfunction in children affected by obstructive sleep apnea syndrome: an observational studyNeuropsychiatr Dis Treat201391087109423976855
- CarotenutoMEspositoMParisiLDepressive symptoms and childhood sleep apnea syndromeNeuropsychiatr Dis Treat2012836937322977304
