?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
High light-to-energy conversion efficiency was achieved by applying novel TiO2 nanorod/nanoparticle (NR/NP) bilayer electrode in the N719 dye-sensitized solar cells. The short-circuit current density (JSC), the open-circuit voltage (VOC), the fill factor (FF), and the overall efficiency (η) were 14.45 mA/cm2, 0.756 V, 0.65, and 7.1%, respectively. The single-crystalline TiO2 NRs with length 200–500 nm and diameter 30–50 nm were prepared by simple hydrothermal methods. The dye-sensitized solar cells with pure TiO2 NR and pure TiO2 NP electrodes showed only a lower light-to-electricity conversion efficiency of 4.4% and 5.8%, respectively, compared with single-crystalline TiO2 NRs. This can be attributed to the new NR/NP bilayer design that can possess the advantages of both building blocks, ie, the high surface area of NP aggregates and rapid electron transport rate and the light scattering effect of single-crystalline NRs.
Introduction
Since the first report of a dye-sensitized solar cell (DSSC) in 1991 by O’Regan and Gratzel,Citation1 this system has aroused a lot of interest over the last decade due to its high efficiency, low cost, and simple preparation procedure.Citation2–Citation4 In general, a porous TiO2 nanoparticle (NP) film is used as an electron transport medium in DSSC.Citation5 Electron transport in such porous film is by trap-mediated diffusion, which is a slow mechanism.Citation6 A novel approach is explored to improve the photovoltaic performance of DSSC by using one-dimensional (1D) TiO2 nanomaterials,Citation7,Citation8 such as nanorods (NRs), nanotubes, and nanowires because 1D materials can improve electron transport properties and reduce light scattering.Citation9
In recent years, many 1D TiO2 materials, such as nanowires,Citation10 nanotubes,Citation11 and NRsCitation12 have been successively synthesized and applied on the DSSCs because they could provide direct pathways for electrons from the injection points to the FTO substrate and have the potential to increase the charge collection efficiency. However, most of these studies only applied pure 1D TiO2 nanomaterials on the DSSCs; few researches were reported for composite NR/NP electrode structured to complement the advantages of each other.Citation13,Citation14
In this article, we report a new promising bilayer design, a pure NP layer coated with pure single crystalline TiO2 NRs that have been synthesized by simple hydrothermal methods, and apply this new bilayer film electrode in DSSCs. Up to our knowledge, this is the first time the TiO2 NR/NP bilayer photoanode design has been applied in DSSCs. It is expected that the photovoltaic performance of DSSC can be improved by using this new TiO2 NR/NP bilayer design.
Experimental
Materials
Conducting glass plate (ITO glass, fluorine-doped SnO2 overlayer, sheet resistance 8 Ω/cm2, made by Beijing Building Material Factory, Beijing, China) was used as a substrate for precipitating TiO2 porous film and was cut into 0.25 cm2 sheets. Sensitizing dye cis-[(dcbH2)2Ru(SCN)2] was purchased from SOLARONIX SA (Aubonne, Switzerland). All other reagents (from Xilong Chemicals, Shantou, China) were used without further purification.
Preparation of TiO2 NRs
TiO2 NR was prepared according to the method reported in our previous work.Citation15 The preparation of the TiO2 NRs was described as follows: 1 g of TiO2 NPs prepared by sol-gel methodsCitation16,Citation17 was added into a 50 mL Teflon vessel containing an amount of hydroxides (NaOH/KOH = 1:1) as aqueous solution. The hydrothermal reaction was carried out at 200°C for 36 hours and then naturally cooled to room temperature, producing white Na2Ti3O7-xH2O and K2Ti3O7-xH2O precipitate. The white precipitate was isolated from the solution by centrifugating and washing with deionized water several times and dried at 70°C for 10 hours. For ion exchange, the sodium and potassium titanate NR was immersed into a 0.1M HNO3 solution for 6 hours, washed with deionized water for several times until the pH value of the solution was approximately 7, and then dried at 70°C for 10 hours. The obtained H-titanate NR was added into a 100 mL Teflon vessel, then filled with dilute HNO3 solution up to 80% of the total volume, and maintained at 180°C for 24 hours. The product was isolated from the solution by centrifugating and washing with deionized water for several times and dried at 70°C for 10 hours. The final step was to calcine the obtained sample at 450°C for 2 hours.
Measurement and characterization
The TiO2 NRs were observed with a JEM-2000EX transmission electron microscope (JEOL, Tokyo, Japan). The crystal structure of the titania was identified by X-ray diffraction (XRD) on a Bruker D8-ADVANCE X-ray diffractometer (Cairo Scientific Corp, Cairo, Egypt) at 40 kV and 40 mA for monochromatized Cu Kα radiation at 0.154 nm.
The photovoltaic test of DSSC was carried out by measuring the J–V character curves under simulated AM 1.5 solar illumination at 100 mW·cm−2 from a xenon arc lamp (XQ-500W; Shanghai Photoelectricity Device Company, Shangai, China) in ambient atmosphere; the fill factor (FF) and the overall light-to-electrical energy conversion efficiency (η) of DSSC were calculated according to the following equations:Citation18
where JSC is the short-circuit current density (mA·cm−2), VOC is the open-circuit voltage (V), Pin is the incident light power, and Jmax (mA·cm−2) and Vmax (V) are the current density and voltage at the point of maximum power output on the J–V curves, respectively.
All the measurements were performed in air. For each type of devices, a set of 5–8 devices was characterized to verify the reproducibility of the results.
The amount of chemisorbed dye was determined by a spectroscopic method by measuring the concentration of dye desorbed on the titania surface into a mixed solution of 0. 1M NaOH and ethanol (1:1 in volume fraction). The absorption spectrum was analyzed by UV-vis spectrophotometer (UV 2450; Shimadzu, Kyoto, Japan).
Preparation of electrode and assembly of cell
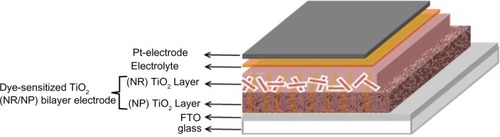
The TiO2 NR/NP electrode of DSSC was fabricated by layer assembly technique shown in . The first layer was TiO2 NP (approximately 9 μm in thickness) prepared by sol-gel method according to the method by Wang et al.Citation19 Then the second layer was TiO2 NR (approximately 3 μm in thickness), coated by using a doctor-blading technique. After air drying, the electrode was sintered at 450°C for 30 minutes and cooled down to 80°C. Then the calcined TiO2 electrode was immersed in ethanol solution of 2.5 × 10−4 M cis-[(dcbH2)2Ru(SCN)2] for 24 hours. After the substrate was adequately washed with anhydrous alcohol and dried in moisture-free air, the dye-sensitized TiO2 electrode was obtained. A DSSC was assembled by filling an electrolyte solution (0.6M tetrapropylammonium iodide, 0.1M iodine, 0. 1M lithium iodide, 0.5M 4-tertbutylpyridine in acetonitrile) between the dye-sensitized TiO2 electrode and a platinized conducting glass electrode. The two electrodes were clipped together, and a cyanoacrylate adhesive was used as sealant to prevent the electrolyte solution from leaking.
Results and discussion
Characterization of the TiO2 NRs
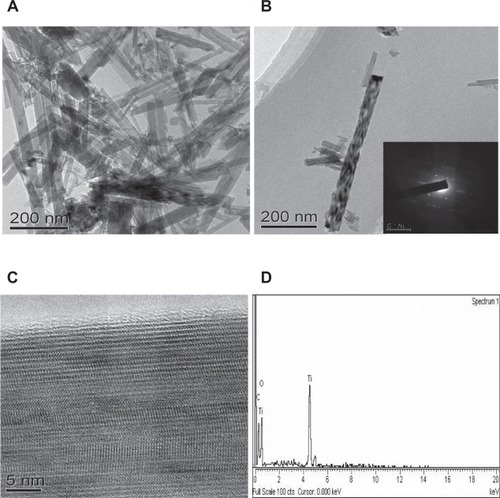
The detailed morphology of the TiO2 NRs was characterized with transmission electron microscopy (TEM) images, as shown in , . It can be seen that the synthesized titania has NR structure with length ranging from 200 to 500 nm and diameter 30–50 nm. The single crystallinity of the NRs was confirmed by the selected area electron diffraction pattern (inset in ) and the high-resolution TEM (). Energy dispersive X-ray analysis of the prepared TiO2 NRs () confirmed the purity of the prepared TiO2 NRs and the absence of residuals of Na or K ions.Citation20
Figure 2 A – B) TEM images of the TiO2 nanorod sample; inset in B) the corresponding SAED patterns of the nanorod. C) The HRTEM image of the nanorod. D) SEM-EDS element analysis of the nanorod.
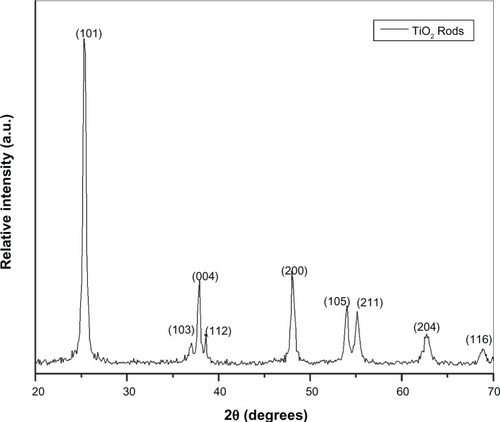
The XRD pattern of the TiO2 NRs is shown in . All diffraction peaks were assigned to pure anatase phase (JCPDS no. 21-1272)Citation21 without other crystalline byproducts. Moreover, the peaks are rather sharp, which indicates that the obtained TiO2 has relatively high crystallinity.
Photovoltaic characteristics and performance
shows the photocurrent density vs voltage (J–V) characteristics of a DSSC based on the hybrid NR/NP bilayer titania electrode (approximately 12 μm in total thickness). The performance of the DSSCs based on this bilayer film was measured under 1 Sun AM 1.5 simulated sunlight. The JSC, VOC, FF, and η of the bilayer electrode were found to be 14.45 mA/cm2, 0.756 V, 0.65, and 7.1%, respectively.
Figure 4 Photocurrent density–voltage (J–V) curves of DSSCs constructed with TiO2 pure (NP); pure (NR) and bilayer (NR/NP) photoanodes.
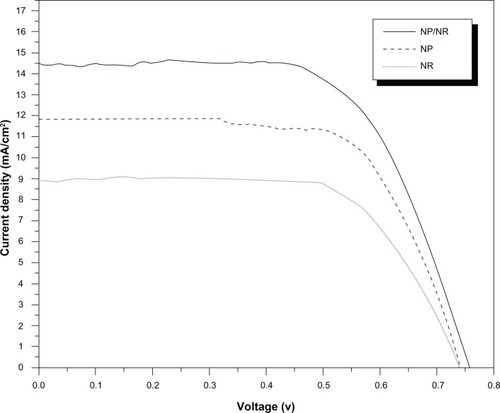
The comparison between photocurrent–voltage characteristics of the DSSC using the TiO2 NR/NP bilayer electrode with those of cells using pure TiO2 NR and NP electrodes (with the same film thickness) are shown in . The photoelectrical data of the DSSCs are summarized in . From the results in and the data in , it can be seen that high short photocurrent density, as well as high photovoltaic performance, has been obtained by applying the hybrid design TiO2 NR/NP bilayer electrode in the DSSC than that of pure NR and NP devices.
Table 1 The parameters of the dye-sensitized solar cells with the different TiO2 electrodes
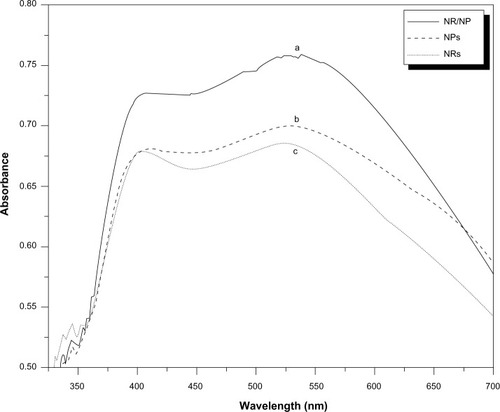
The adsorption of the dye molecules in the three TiO2 films is compared using UV–vis absorption spectra, which is shown in . The results clearly indicate that the absorption intensity of the adsorbed dye on the hybrid TiO2 NR/NP bilayer electrode is larger than the pure NP and pure NR electrodes with the same thickness. The amount of chemisorbed ruthenium dye onto the NR, NP, and NR/NP film electrodes are listed in and are determined as 2.1, 3.6, and 6.2 × 10−5 mol·cm−2, respectively. It means that hybrid NR/NP bilayer electrode can absorb more ruthenium dye than pure NR and pure NP do, which is ready for the enhancement of incident light harvest and improvement of light-to-electricity conversion efficiency of the DSSC.
Incident photon-to-current conversion efficiency
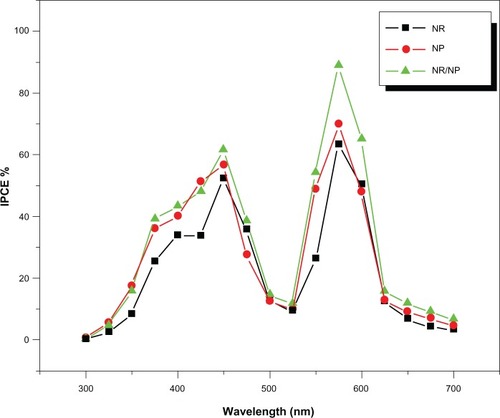
The incident photon-to-current conversion efficiency (IPCE) is defined as the ratio of the number of electrons in the external circuit produced by an incident photon at a given wavelength. Using EquationEquation (3)(3) , the IPCE values of the DSSCs with the NR/NP bilayer, NR and NP electrodes as a function of the illumination wavelength are shown in .
Figure 6 IPCE curves of the DSSCs based on pure NP, pure NR, and the NP/NR bilayer photoanodes.
where ISC is the short-circuit photocurrent (mA/cm2), Win is the incident light intensity (W/cm2), and λ is the wavelength (nm).
As expected, the DSSC with the NR/NP bilayer film showed higher conversion efficiency in the wavelength range 300–700 nm than that with pure NR and NP electrodes. The TiO2 NR/NP bilayer electrode shows a maximum IPCE of 88.9% at a wavelength of 575 nm, whereas the NR and NP electrodes show an IPCE of 63.5% and 70%, respectively. This resulted in a 22% improvement in the conversion efficiency and 27% in IPCE of the DSSC with NR/NP electrode than with NP electrode.
This large improvement in the photovoltaic performance of the new DSSC-based NR/NP bilayer design can be explained as, although the high surface area of TiO2 NP meets the requirement of adsorbing dye, it brings about, at the same time, many opportunities for the recombination of photoinjected electrons and the oxidized dye and/or the electron acceptors in the electrolyte.Citation21 The 1D TiO2 electrodes such as NRs can enhance the light harvesting,Citation22 straight pathway electron transport,Citation23 and also have no serious light loss due to back scattering. Therefore, the design of the TiO2 NR/NP bilayer film balances the surface area and the light scattering; thus, the performance of DSSC with TiO2 NR/NP bilayer electrode is higher than those with NP or NR electrode.
Conclusion
In summary, anatase TiO2 NR was successfully synthesized by hydrothermal method. High light-to-electricity conversion efficiency of 7.1% was achieved by applying high-efficient TiO2 NR/NP bilayer photoanode in DSSC compared with pure TiO2 NP as electrode, which shows the conversion efficiency of only 5.8%. This 22% enhancement is attributed to the balance between less light scattering for TiO2 NR and the more light harvesting for TiO2 NP.
Acknowledgments
The authors acknowledge the joint support of the National High Technology Research and Development Program of China (No. SQ2008AA03Z2470974), the National Natural Science Foundation of China (No. 50572030, 50842027), and Partnership and Ownership Initiative (ParOwn) of Egyptian Ministry of Higher Education and Scientific Research (Cycle 0709).
Disclosure
The authors report no conflicts of interest in this work.
References
- O’ReganBGrätzelMA low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 filmsNature1991353737739
- GrätzelMPhotoelectrochemical cellsNature200141433834411713540
- WuJHLanZLinJMA novel thermosetting gel electrolyte for stable quasi-solid-state dye-sensitized solar cellsAdv Mater20071940064011
- WuJHaoSLanZAn all-solid-state dye-sensitized solar cell-based poly(N-alkyl-4-vinyl-pyridine iodide) electrolyte with efficiency of 5.64%J Am Chem Soc2008130115681156918693733
- ItoSMurakamiTNComtePFabrication of thin film dye sensitized solar cells with solar to electric power conversion efficiency over 10%Thin Solid Films200851646134619
- KopidakisNBenksteinKDVan de LagemaatJFrankAJTransport-limited recombination of photocarriers in dye-sensitized nanocrystalline TiO2 solar cellsJ Phys Chem B19991071130711315
- SongMYAhnYRJoSMKimDYTiO2 single-crystalline nanorod electrode for quasi-solid-state dye-sensitized solar cellsAppl Phys Lett200587113113
- AdachiMMurataYOkadaIYoshikawaSFormation of titania nanotubes and applications for dye-sensitized solar cellsJ Electrochem Soc2003150G488G493
- YipCMakCDjurisicAHsuYChanWDye-sensitized solar cells based on TiO2 nanotube/porous layer mixed morphologyAppl Phys A200892589593
- AdachiMMurataYTakaoJJiuJSakamotoMWangFHighly efficient dye-sensitized solar cells with a titania thin-film electrode composed of a network structure of single-crystal-like TiO2 nanowires made by the “oriented attachment” mechanismJ Am Chem Soc2004126149431494915535722
- UchidaSChibaRTomihaMMasakiNShiraiMApplication of titania nanotubes to a dye-sensitized solar cellElectrochemistry200270418420
- JiuJTIsodaSWangFMAdachiMDye-sensitized solar cells based on a single-crystalline TiO2 nanorod filmJ Phys Chem B20061102087209216471788
- RoyPKimDParamasivamISchmukiPImproved efficiency of TiO2 nanotubes in dye sensitized solar cells by decoration with TiO2 nanoparticlesElectrochem Commun20091110011004
- TanBWuYDye-sensitized solar cells based on anatase TiO2 nanoparticle/nanowire compositesJ Phys Chem B2006110159321593816898747
- HafezHSSynthesis of highly-active single-crystalline TiO2 nanorods and its application in environmental photocatalysisMater Lett20096314711474
- KasugaTHiramatsuMHosonASekinoTNiiharaKTitania nanotubes prepared by chemical processingAdv Mater19991113071311
- NogueiraAFFloresICde FreitasJNLongoCde PaoliMWinnischoferHDye-sensitized solar cells based on TiO2 nanotubes and a solid-state electrolyteJ Photochem Photobiol A2007189153160
- GrätzelMPerspectives for dye-sensitized nanocrystalline solar cellsProg Photovoltaic Res Applic20008171185
- WangPZakeeruddinSMComtePCharvetRBakerRHGrätzelMEnhance the performance of dye-sensitized solar cells by co-grafting amphiphilic sensitizer and hexadecylmalonic acid on TiO2 nanocrystalsJ Phys Chem B20031071433614341
- YoshidaRSuzukiYYoshikawaSSyntheses of TiO2(B) nanowires and TiO2 anatase nanowires by hydrothermal and post-heat treatmentsJ Solid State Chem200517821792185
- Joint Committee on Powder Diffraction, International Centre for Diffraction DataPDF-2 release2001Newtown Square, PAICDD2001
- WangYYangHXuHDNA-like dye-sensitized solar cells based on TiO2 nanowire-covered nanotube bilayer film electrodesMater Lett201064164166
- WeiQHirotaKTajimaKHashimotoKDesign and synthesis of TiO2 nanorod assemblies and their application for photovoltaic devicesChem Mater20061850805087