Abstract
Insomnia is a common symptom, with chronic insomnia being diagnosed in 5–10% of adults. Although many insomnia patients use prescription therapy for insomnia, the health benefits remain uncertain and adverse risks remain a concern. While similar effectiveness and risk concerns exist for herbal remedies, many individuals turn to such alternatives to prescriptions for insomnia. Like prescription hypnotics, herbal remedies that have undergone clinical testing often show subjective sleep improvements that exceed objective measures, which may relate to interindividual heterogeneity and/or placebo effects. Response heterogeneity can undermine traditional randomized trial approaches, which in some fields has prompted a shift toward stratified trials based on genotype or phenotype, or the so-called n-of-1 method of testing placebo versus active drug in within-person alternating blocks. We reviewed six independent compendiums of herbal agents to assemble a group of over 70 reported to benefit sleep. To bridge the gap between the unfeasible expectation of formal evidence in this space and the reality of common self-medication by those with insomnia, we propose a method for guided self-testing that overcomes certain operational barriers related to inter- and intraindividual sources of phenotypic variability. Patient-chosen outcomes drive a general statistical model that allows personalized self-assessment that can augment the open-label nature of routine practice. The potential advantages of this method include flexibility to implement for other (nonherbal) insomnia interventions.
Introduction
Insomnia is among the most common clinical complaints, with a symptom prevalence in adults exceeding 30%, while more stringent diagnostic criteria place the prevalence of chronic insomnia in the 5–10% range.Citation1 There are many options to consider for treating insomnia. Cognitive behavioral therapy for insomnia (CBT-I) is the long-recognized first-line approach to chronic insomnia, as indicated by the American Academy of Sleep Medicine (AASM) practice parameters.Citation2 Although in-person CBT-I is not uniformly accessible, validated online optionsCitation3–Citation6 are increasingly available.
Although many chronic insomnia patients utilize prescription medications, adverse effects are well known and the risk–benefit balance remains a clinical challenge.Citation7–Citation9 In fact, there is extensive literature documenting the acute and chronic adverse effects associated with hypnotic drugs, including those approved by the Food and Drug Administration (FDA) and those used off-label.Citation7,Citation10–Citation13 Certain hypnotics may objectively worsen sleep: benzodiazepines might worsen breathing (although the evidence is inconsistentCitation14) and antidepressants might increase periodic limb movementsCitation15 or worsen breathing in some circumstances.Citation16 The objective improvements in sleep metrics by polysomnography (PSG) are modest; a meta-analysis that included “z-drug” hypnotics for primary insomnia indicated only 12.9 minutes faster average onset latency (less with benzodiazepines or antidepressants) and only 11.4 minutes of average additional total sleep time (TST).Citation17 Interestingly, subjective diary measures of latency and TST exceeded the objective metrics of benefit by 50–300%. It is perhaps not surprising that the same trends are observed for natural remedies under the broad category of complementary and alternative medicine (CAM). Clinical trial data are sparse aside from two remedies, melatonin and valerian, which have been tested in several studies each.Citation18–Citation20 The outcomes are similar to a pattern commonly observed in trials of prescription hypnotics, in that subjective sleep improvements often exceeded the objective improvements. For example, a recent meta-analysis of 18 randomized control trials (RCTs) of valerian for insomnia found little objective improvement, despite clear subjective benefit.Citation21
Recent reviews of the broader field of CAM therapy also report heterogeneous findings and emphasize methodological challenges.Citation22,Citation23 Despite these uncertainties, many adults turn to CAM remedies,Citation24,Citation25 with over one-third of adults reporting CAM use.Citation26 Epidemiological surveys indicate that the use of CAM is common among those with insomniaCitation27 (~5% reporting such use in 2002 National Health Interview Survey) as well as other systemic disorders that may be associated with disturbed sleep such as neurological conditionsCitation28 (44% reporting CAM use in the 2007 National Health Interview Survey) and chronic medical conditionsCitation29 (17% reporting mind–body therapies in the 2007 National Health Interview Survey). More recently, Bertisch et alCitation30 analyzed the subset of the 2007 National Health Interview Survey participants who reported insomnia symptoms and found that nearly half indicated using CAM therapy.
When considering CAM therapy for insomnia, the limited evidence basis must be balanced against the possibility that interindividual heterogeneity, which compromises trial power, could be consistent with subsets who respond positively. This is well known in other fields and has prompted a shift toward stratified trials based on genotype or phenotype. The single-patient extension of this idea is the so-called n-of-1 trial, which compares placebo against active drug in within-person alternating blocks.Citation31,Citation32 However, n-of-1 trials still require substantial resource investment and have not been consistently adopted.Citation33 The placebo component restricts implementation of this otherwise highly personalized approach to research protocols, that is, one cannot take a placebo-based n-of-1 approach in clinical practice. In addition, patient-defined goals may not be captured by the experimental question of whether the intervention is statistically superior to placebo. To bridge the gap between formal prospective trials (group-wise or n-of-1) and the common patient-care practice of “open label” interventions with qualitative outcome assessment, we propose a simple method that leverages principles of trial design while remaining personalized.
Methods
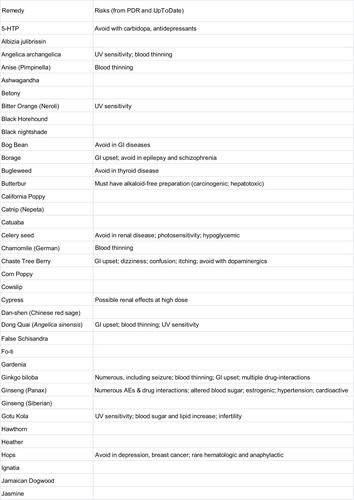
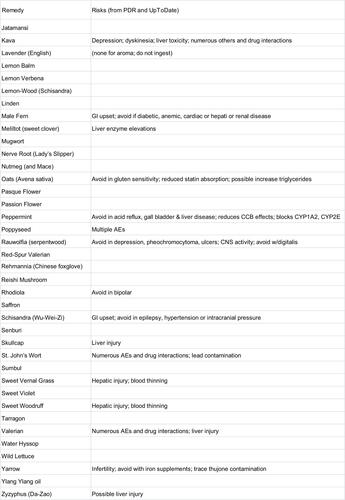
We performed a manual search of several print and online resources concerning herbal and supplement remedies of potential relevance for insomnia: The Physician’s Desk Reference for Herbal Medicines (PDR);Citation34 the Encyclopedia of Herbal Medicine,Citation35 The Drug and Natural Medicine Advisor (Time Life);Citation36 Medline Plus (https://medlineplus.gov/druginfo/herb_All.html), the National Center for Complementary and Integrative Health Database (https://nccih.nih.gov/health/herbsataglance.htm), and the Natural Medicines online database (http://naturaldatabase.therapeuticresearch.com/home.aspx?cs=&s=ND;paidaccess). In each case, we manually searched for language such as “insomnia”, “sleeplessness”, or “disturbed sleep” as an indication for the remedy. We also searched for related terms that might be indirectly related to sleep effects (“sedative”, “anxiety”, “tranquilizer”, “calming”, and “nervousness”). Agents were included in the figures if any of the above six sources indicated insomnia as an indication, or, if none specifically listed insomnia, we accepted the agent if two or more of the sources listed a related indirect term (above). Agents listed in a resource but only for indications other than sleep are labeled as “misc” to indicate other uses, while agents not listed at all are given a blank (“-”). Most of the remedies listed for insomnia were also listed for other indications; we did not systematically assess this aspect. We did not consider indications such as antidepressant effects or treatment of pain. We also reviewed adverse effect listings from the PDR of Herbal Medicine and from UpToDate.
Results and discussion
Natural remedies for sleep
An alphabetical list of herbal remedies is presented in and , with the relevant indications for each according to six sources (“Methods” section). The literature concerning herbal and natural supplement agents is heterogeneous in several important respects.
Figure 1 Natural remedies (alphabetical, A–J) listed by at least one of the six sources as possibly useful for sleep.
Abbreviations: Encyc, encyclopedia; Misc, miscellaneous; PDR, Physician’s Desk Reference for Herbal Medicines; NIH, National Institutes of Health; NCCIH, National Center for Complementary and Integrative Health Database.
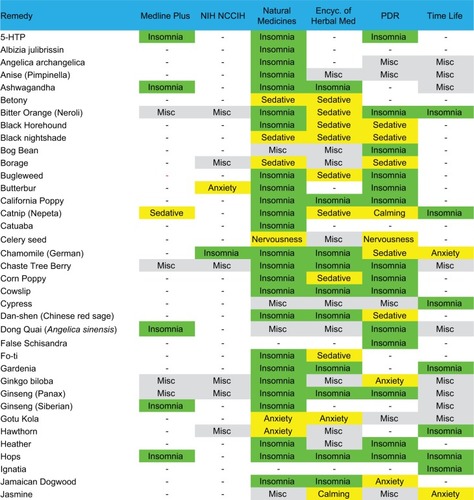
Figure 2 Natural remedies (alphabetical, J–Z) listed by at least one of the six sources as possibly useful for sleep.
Abbreviations: Encyc, encyclopedia; Misc, miscellaneous; PDR, Physician’s Desk Reference for Herbal Medicines; NIH, National Institutes of Health; NCCIH, National Center for Complementary and Integrative Health Database.
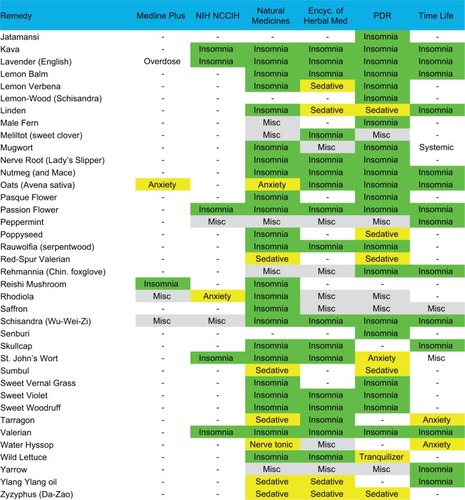
First, the substances often have several names apparently interchanged as equivalent in the literature. For example, according to the Natural Medicines Database, Bugleweed is also reportedly known as Ajuga, Archangle, Ashangee, Chanvre d’Eau, Green Wolf’s Foot, Gypsy Weed, Gypsywort, Hoarhound, Lycope, Lycope d’Amérique, Lycope d’Europe, Lycope de Virginie, Lycopi Herba, Lycopus Europea, Menta de Lobo, Patte-de-Loup, Paul’s Betony, Sweet Bugle, Virginia Water Horehound, Water Bugle, Water Horehound, and Wolf-strapp. By contrast, the Encyclopedia of Herbal Medicine lists only Gypsywort as a related species to Bugleweed. We did not conduct exhaustive cross-matching searches in this regard.
Second, different sources indicate different usages for the same agents. In some cases, agents were listed as helping insomnia in one source but as a stimulant in another source (peppermint and rhodiola). No single agent was listed as having a sleep or sleep-related usage in all six resources consulted. Most of the remedies were listed in 1–3 of the six sources, and a small subset appeared in 4–5 sources. A related issue is that the health benefits or indications may be reported differently across traditions, which were differentiated in some of the sources; we do not differentiate here.
Third, the terminology used for indications is nonstandardized and at times ill-defined from a medical standpoint, such as “nervous disorders”, which would be considered nonspecific. Heterogeneity of terminology is perhaps not surprising from the sleep medicine perspective, where terms like fatigue, sleepiness, hypersomnia, and fogginess may be used interchangeably by patients in clinical practice and may not be well distinguished even in research contexts.
Fourth, the sources differed in their listing of adverse effects and drug interactions.
Safety considerations for natural remedies
Natural remedies may be perceived as safer than prescriptions, although risk concerns have been raised for many supplements.Citation37,Citation38 The six sources we assessed often included general suggestions and cautions regarding uncertainties surrounding the preparations, such as purity and standardization. Strikingly, a recent report indicated that the majority of herbal remedies sampled had contamination, substitution, or use of fillers not indicated in the labeling.Citation39
and contain adverse event listings from the PDR for Herbal Medicines and UpToDate. Because the safety of these agents has not been studied with rigor, the absence of reported risks should not be interpreted as a demonstration of safety. Many cautioned against use in pregnancy. Several remedies in the UpToDate listing for hepatic injury (often transient, but can be severe) are found in : valerian, mistletoe, skullcap, and kava. Some common remedies had extensive detail of reported adverse risks, such as valerian, ginseng, ginko, and kava; readers are directed to the PDR for additional details. Other resources are also available in this regard. The NIH maintains a public database for searching for liver toxicity for drugs and supplements (http://www.livertox.nih.gov/). In addition, UpToDate recommends www.consumerlab.com, for checking which brands have undergone independent testing, and the FDA has a web resource for safety and recalls that includes foods and herbals (http://www.fda.gov/Safety/Recalls/).
Pragmatic challenges of requiring conventional evidence standards for herbals
The paucity of data for herbal therapy for insomnia is in principle addressable by randomized placebo controlled trials. However, the time horizon and resources required for such testing across even a subset of the remedies listed herein is daunting and seems unlikely to occur even with optimistic funding projections. The n-of-1 trial approach, despite being an important innovation, is unlikely to solve this evidence problem, as it is still resource intensive and requires research protocols, in part due to the placebo component of the design. Yet, many patients continue to seek advice about or actively self-medicate with alternatives to prescription agents, indicating a need to bridge this evidence gap.
Providers may vary in their comfort level and risk tolerance in these situations.Citation37,Citation38 Moreover, individuals arguably consider their personal perspective over what is reported in RCTs. The disconnect between patients’ views and those of some providers is illustrated by data suggesting patients were not talking to their providers about CAM, believed physicians did not know enough about CAM, and would continue to use CAM even if clinical trials showed lack of efficacy.Citation40 Even for FDA-approved hypnotics, it seems unlikely that patients are anchoring their usage decisions upon the absolute number of extra minutes of sleep versus placebo from an RCT. Thus, guidance for how to think about their goals and leverage their diaries via a semiquantitative approach can be empowering, especially for patients who may be overwhelmed at their situation, finding it challenging to identify patterns or assess anything beyond a gestalt feeling in response to a new intervention.
A practical clinical method for guided self-testing
There are several motivating factors driving our approach, which are in line with common clinical practice when pharmacological or behavioral therapy trials are pursued. Self-tracking with diaries is often standard in clinical practice, but how the entries drive decisions and actions remains a qualitative exercise. Any method should ideally promote certain patient care goals. Borrowing from the concept of statistical power from traditional group-wise clinical trials, the idea is to apply quantitative guidance to a single person measuring their sleep over multiple nights. Providing guidance even for simple questions like “how long should I try this?” can be structured, drawing from diary entries in a more quantitative manner, and incorporating the reality of night-to-night variability that is common in chronic insomnia.Citation41–Citation43 What specific aspects of sleep should be tracked is also highly personal, though diaries commonly inquire about sleep latency, number of awakenings, TST, etc.
We use patient-specific simple inputs to create personalized sleep strategies that can be applied to any chosen remedy to improve sleep. These personalized strategies use patient-specific goals as a form of power calculation and goal checking for self-testing. illustrates a visual algorithm for the process. The individual is first guided through the process of simplifying the many facets of sleep that could be tracked in principle into a binary label of “good” or “bad” sleep. This seemingly simple step overcomes two key challenges. First, having multiple sleep features involved in a treatment goal complicates self-testing by reducing power (i.e., prolonging the time needed to evaluate effects). Second, different patients attribute different importance to sleep features, which requires customization of diaries and of power calculations. Collapsing patient-determined factors into a binary outcome allows the approach to generalize across any patient-derived definition of “good” sleep and allows any patient to participate in this guided process using a common end point: what is your goal percentage of good nights? This is akin to how composite end points are used in large RCTs or how “effect size” seeks to render different outcomes on a common scale.
Figure 3 Flow chart describing the steps of guided self-testing.
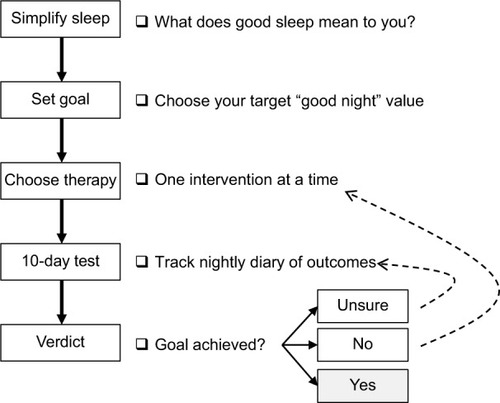
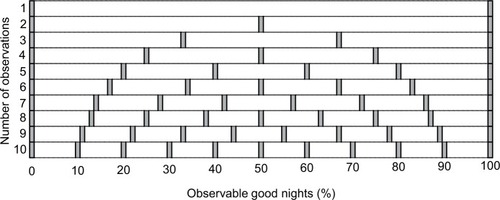
Once the sleep goal and the remedy are chosen, an initial 10-night assessment is undertaken. The precise number of nights is less important than the implications of what value is chosen, as a trade-off between duration of the test, and resolution of probability of a good night. illustrates the challenges with assessing sleep with small numbers of observations. For a binary outcome (good vs bad night), if only one observation is made, the only observable proportion of good nights is 0 or 100% – neither is likely to be the true probability. For two nights, the only observable proportions are 0, 50, or 100% – also a poor resolution of probability. Brief trials, such as one to two nights, create two important inferential risks: 1) overconfidence (if the result happens to be 100% good) or 2) overpessimism (if the result happens to be 0% good). Consider a fair coin flipped twice, where heads indicates a good night, and tails indicates a bad night: there is a 50% chance that the observed two-flip outcome will be extreme (HH and TT are each 25% chance) rather than the correct estimate of the true probability of 50% (HT and TH are each 25% chance). With increasing number of observations, one increases the resolution of possible observed proportions of good nights. For 10 nights, the observable proportions have a more clinically interpretable coverage of intervals (0, 10, 20%, etc). Those wishing higher resolution would track themselves for additional nights (e.g., 20 nights for 5% intervals).
Figure 4 Observable proportions of good nights, assuming binary outcome (good vs bad) across a range of 1–10 nights.
After the 10-night trial, the resulting observed percentage of good nights is compared to the binomial distribution of 10 “trials” under the assumption that the user-chosen goal probability of good nights has been achieved. If the observed percentage of good nights is within the acceptable “buffer” range, the remedy could be continued, assuming no adverse effects have occurred. For example, a patient might choose a goal of 70% good nights, while recognizing that any given 10-night period might have slightly more or slightly less observed good nights, even if the goal is met, that is, if 0.7 is the “true” probability. If the proportion is too far below the acceptable buffer, a new remedy can be tried (, dotted arrow back to “choose therapy” step). If the value is close but not within the buffer range of the goal, then the individual has the option to pursue an additional 10 nights (, dotted arrow back to “10-day test” step).
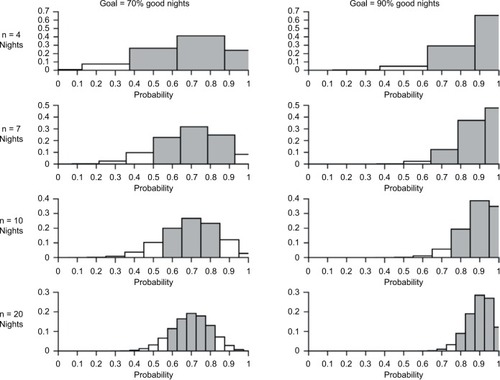
It is up to the individual to decide how much certainty they wish to achieve in estimating their probability of a good night. For any sequence of nights, the maximum likelihood estimate of the true probability of a good night is equal to the observed probability of a good night. The number of nights assessed to derive the observed probability will shape how confident one is with that estimate. illustrates the binomial distribution for n = 4 nights to n = 20 nights (rows), for goal percentage of good nights of 70 or 90% (left and right columns). The gray shading indicates a range of “acceptable” buffer around the goal. This can be adjusted and personalized but is set here for illustration with a bound of ±10% of the goal proportion of good nights. To summarize, our proposed approach has key advantages that draw directly from patient care and translate directly into clinical guidance.
Figure 5 Histograms of binomial distributions across different true probabilities of a good night and different number of nights tested.
Simplifying diary tracking to a common terminology (good vs bad) allows adaptation to any personal definition of what “good” sleep may be. This streamlines diary interpretation and allows the method to generalize. It does not preclude individuals tracking other details, akin to secondary or exploratory aims in a clinical trial.
The diary tracking feeds directly into the process. By linking diary tracking results directly with patient-driven decisions using basic statistical methods, the effort of self-tracking feeds directly into guided but flexible decision making.
There is no requirement for baseline diary pattern tracking, because the outcome is purely “goal” focused, rather than the typical clinical trial approach of showing a significant difference from pretreatment baseline values.
The method mitigates the risk of reacting to a single night, intuitively paralleling how we could not judge the fairness of a coin based on one toss. Instead of responding to “noise” of individual nights, it provides a context for patients to embrace their own nightly variability so it no longer represents a fear of uncertainty.
The results are by definition assured to be relevant to the individual. In this way, we can reconcile the realities of response heterogeneity in a chronic disease with highly individualized causes, contributors, and treatment goals.
Disadvantages of the method are also recognized. That the outcomes of sleep are forced into a simplified binary answer may strike some individuals as oversimplified in that they cannot comfortably render their complex issue into such a categorization. Certainly, multifactor outcomes can be modeled but require larger self-testing durations to analyze in this manner (akin to the standard practice of defining one outcome, often a composite outcome, in clinical trials, to establish trial power). Further, we recognize that the process does not engage any placebo role. However, the costs and infrastructure required to conduct placebo-controlled testing, which necessarily involves research protocols rather than routine clinical practice, are circumvented. Additionally, the individual’s goals of therapy may not involve statistical proof of difference from placebo or statistical proof of difference from baseline – the two major goals of prospective randomized trials. Finally, the results of any individual’s testing may not generalize beyond that individual. However, combining individualized results can inform future population tudies, and a meta-analysis of n-of-1 trials, for example, has been reported.Citation33
Conclusion
The adaptive method naturally integrates with what already happens in a qualitative manner for patient self-assessments to provide a structure upon which already accepted clinical goals can be actively supported. The goal-oriented strategy of self-testing is distinct from the traditional statistical comparison of superiority of postintervention sleep versus baseline sleep, which may resonate less with patients who may have goals not well captured by traditional methods. Instead, the approach augments usual care (i.e., open label) of prescribing while remaining personalized. For patients and providers who wish to consider these agents after careful discussion and consideration of the uncertainties regarding safety and efficacy, such a system could provide a useful and practical clinical framework that serves multiple clinical goals.
Acknowledgments
We thank Jessica Kelly for assistance with early aspects of the literature review. Kate Romero and Balaji Goparaju are cofirst authors. This was not an industry-supported study. We thank Dr Suzanne Bertisch for useful discussions.
Supplementary materials
Disclosure
Dr Bianchi has received funding from the Department of Neurology, Massachusetts General Hospital, the Center for Integration of Medicine and Innovative Technology, the Milton Family Foundation, the MGH-MIT Grand Challenge, and the American Sleep Medicine Foundation. Dr Bianchi has a patent pending on a home sleep monitoring device. Dr Bianchi has research contracts with MC10 and Insomnisolv, a consulting agreement with McKesson and International Flavors and Fragrances, received payment for educational material from Oakstone Publishing, and has provided expert testimony in sleep medicine. Dr Bianchi serves as a medical monitor for Pfizer. Dr Westover has received funding from the NIH (NIH-NINDS 1K23NS090900). The other authors report no conflicts of interest in this work.
References
- OhayonMMEpidemiology of insomnia: what we know and what we still need to learnSleep Med Rev2002629711112531146
- Schutte-RodinSBrochLBuysseDDorseyCSateiaMClinical guideline for the evaluation and management of chronic insomnia in adultsJ Clin Sleep Med20084548750418853708
- EspieCALuikAICapeJDigital cognitive behavioural therapy for insomnia versus sleep hygiene education: the impact of improved sleep on functional health, quality of life and psychological well-being. Study protocol for a randomised controlled trialTrials201617125727216112
- van der ZweerdeTLanceeJSlottjePCost-effectiveness of i-Sleep, a guided online CBT intervention, for patients with insomnia in general practice: protocol of a pragmatic randomized controlled trialBMC Psychiatry2016168527038786
- LanceeJvan StratenAMorinaNKaldoVKamphuisJHGuided online or face-to-face cognitive behavioral treatment for insomnia: a randomized wait-list controlled trialSleep201639118319126414893
- ChengSKDizonJComputerised cognitive behavioural therapy for insomnia: a systematic review and meta-analysisPsychother Psychosom201281420621622585048
- LaderMBenzodiazepines revisited – will we ever learn?Addiction2011106122086210921714826
- KripkeDFLangerRDKlineLEHypnotics’ association with mortality or cancer: a matched cohort studyBMJ Open201221e000850
- BianchiMTThomasRJEllenbogenJMHypnotics and mortality riskJ Clin Sleep Med20128435135222893762
- KripkeDFHypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefitF1000Res2016591827303633
- Antai-OtongDThe art of prescribing. Risks and benefits of non-benzodiazepine receptor agonists in the treatment of acute primary insomnia in older adultsPerspect Psychiatr Care200642319620016916422
- GlassJLanctotKLHerrmannNSprouleBABustoUESedative hypnotics in older people with insomnia: meta-analysis of risks and benefitsBMJ20053317526116916284208
- AllainHBentue-FerrerDPolardEAkwaYPatatAPostural instability and consequent falls and hip fractures associated with use of hypnotics in the elderly: a comparative reviewDrugs Aging200522974976516156679
- MasonMCatesCJSmithIEffects of opioid, hypnotic and sedating medications on sleep-disordered breathing in adults with obstructive sleep apnoeaCochrane Database Syst Rev20157CD01109026171909
- YangCWhiteDPWinkelmanJWAntidepressants and periodic leg movements of sleepBiol Psychiatry200558651051416005440
- EckertDJMalhotraAWellmanAWhiteDPTrazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal thresholdSleep201437481181924899767
- BuscemiNVandermeerBFriesenCThe efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTsJ Gen Intern Med20072291335135017619935
- BrzezinskiAVangelMGWurtmanRJEffects of exogenous melatonin on sleep: a meta-analysisSleep Med Rev200591415015649737
- BuscemiNVandermeerBHootonNThe efficacy and safety of exogenous melatonin for primary sleep disorders. A meta-analysisJ Gen Intern Med200520121151115816423108
- BentSPadulaAMooreDPattersonMMehlingWValerian for sleep: a systematic review and meta-analysisAm J Med2006119121005101217145239
- Fernandez-San-MartinMIMasa-FontRPalacios-SolerLSancho-GomezPCalbo-CaldenteyCFlores-MateoGEffectiveness of Valerian on insomnia: a meta-analysis of randomized placebo-controlled trialsSleep Med201011650551120347389
- LeachMJPageATHerbal medicine for insomnia: a systematic review and meta-analysisSleep Med Rev20152411225644982
- SarrisJByrneGJA systematic review of insomnia and complementary medicineSleep Med Rev20111529910620965131
- KaufmanDWKellyJPRosenbergLAndersonTEMitchellAARecent patterns of medication use in the ambulatory adult population of the United States: the Slone surveyJAMA2002287333734411790213
- ClarkeTCBlackLIStussmanBJBarnesPMNahinRLTrends in the use of complementary health approaches among adults: United States, 2002–2012Natl Health Stat Report201579116
- TindleHADavisRBPhillipsRSEisenbergDMTrends in use of complementary and alternative medicine by US adults: 1997–2002Altern Ther Health Med20051114249
- PearsonNJJohnsonLLNahinRLInsomnia, trouble sleeping, and complementary and alternative medicine: analysis of the 2002 national health interview survey dataArch Intern Med2006166161775178216983058
- WellsREPhillipsRSSchachterSCMcCarthyEPComplementary and alternative medicine use among US adults with common neurological conditionsJ Neurol2010257111822183120535493
- BertischSMWeeCCPhillipsRSMcCarthyEPAlternative mind-body therapies used by adults with medical conditionsJ Psychosom Res200966651151919446710
- BertischSMWellsRESmithMTMcCarthyEPUse of relaxation techniques and complementary and alternative medicine by American adults with insomnia symptoms: results from a national surveyJ Clin Sleep Med20128668169123243402
- DuanNKravitzRLSchmidCHSingle-patient (n-of-1) trials: a pragmatic clinical decision methodology for patient-centered comparative effectiveness researchJ Clin Epidemiol2013668 SupplS21S2823849149
- LillieEOPatayBDiamantJIssellBTopolEJSchorkNJThe n-of-1 clinical trial: the ultimate strategy for individualizing medicine?Per Med20118216117321695041
- PunjaSBukutuCShamseerLN-of-1 trials are a tapestry of heterogeneityJ Clin Epidemiol201676475627079847
- GruenwaldJBrendlerTJaenickeCPDR for Herbal Medicines4thMontvale, NJThomson Healthcare, Inc.2007
- ChevallierAEncyclopedia of Herbal Medicine2ndNew York, NYDK2016
- The Drug & Natural Medicine AdvisorRichmond, VATime-Life Books2002
- AsharBHRowland-SeymourAAdvising patients who use dietary supplementsAm J Med20081212919718261493
- GlissonJKWalkerLAHow physicians should evaluate dietary supplementsAm J Med2010123757758220493463
- NewmasterSGGrguricMShanmughanandhanDRamalingamSRagupathySDNA barcoding detects contamination and substitution in North American herbal productsBMC Med20131122224120035
- BlendonRJDesRochesCMBensonJMBrodieMAltmanDEAmericans’ views on the use and regulation of dietary supplementsArch Intern Med2001161680581011268222
- BaronKGReidKJMalkaniRGKangJZeePCSleep variability among older adults with insomnia: associations with sleep quality and cardiometabolic disease riskBehav Sleep Med201715214415726745754
- BuysseDJChengYGermainANight-to-night sleep variability in older adults with and without chronic insomniaSleep Med2010111566419962939
- SuhSNowakowskiSBernertRAClinical significance of night-to-night sleep variability in insomniaSleep Med201213546947522357064