Abstract
The focus of this review was on the genioglossus (GG) muscle and its role in maintaining upper airway patency in both healthy individuals and obstructive sleep apnea (OSA) patients. This review provided an overview of GG anatomy and GG control and function during both wakefulness and sleep in healthy individuals and in those with OSA. We reviewed evidence for the role of the GG in OSA pathogenesis and also highlighted abnormalities in GG morphology, responsiveness, tissue movement patterns and neurogenic control that may contribute to or result from OSA. We summarized the different methods for improving GG function and/or activity in OSA and their efficacy. In addition, we discussed the possibility that assessing the synergistic activation of multiple upper airway dilator muscles may provide greater insight into upper airway function and OSA pathogenesis, rather than assessing the GG in isolation.
Introduction
The upper airway is an intricate structure that comprises nasal, pharyngeal and laryngeal regions. The upper airway has a key role in multiple functions, including breathing, vocalization and swallowing. To subserve these diverse functions, the upper airway is a highly malleable structure that lacks rigid bony support and therefore is susceptible to collapsing forces such as the negative intraluminal pressures that are generated during inspiration and positive extraluminal pressures exerted by the surrounding soft tissues. Muscles within the upper airway surround the collapsible segment. Many of these muscles are responsible for maintaining upper airway patency by opposing collapsing forces and thus are termed upper airway dilator muscles. These dilator muscles work synergistically to either stiffen the upper airway walls or enlarge the upper airway lumen so that optimal function can be achieved. During wakefulness, these muscles are effective in maintaining patency. However, with the onset of sleep, the activity of these muscles diminishes. In a susceptible individual, this loss of muscle activity during sleep may give rise to obstructive sleep apnea (OSA), a common sleep breathing disorder characterized by repeated partial or complete upper airway collapse during sleep.
There are at least 20 dilator muscles that surround the upper airway. These muscles can be broadly grouped according to their influence on key structures within the upper airway.Citation1,Citation2 The first group are muscles that control tongue protrusion, retraction, elevation and depression (genioglossus[GG], geniohyoid, styloglossus and hyoglossus). The second group are muscles that retract, stiffen, elevate and depress the soft palate (levator veli palatini, palatoglossus, palatopharyngeus, tensor veli palatini and musculus uvulae). The third group are muscles that connect to the hyoid bone to alter the position of both the tongue and the anterior pharyngeal wall (mylohyoid, geniohyoid, digastric, stylohyoid, omohyoid, sternohyoid and thyrohyoid). The final group of muscles close and stiffen the pharynx (superior, middle and inferior pharyngeal constrictor). There are also intrinsic muscles contained within the tongue that alter the shape of the tongue; however, whether these muscles are involved in the maintenance of upper airway patency is unclear and may depend on the level of activity in the extrinsic tongue muscles (GG, hyoglossus, styloglossus and palatoglossus). The focus of this review is the GG muscle as it is the most extensively studied dilator muscle and is believed to have a key role in the maintenance of upper airway patency during sleep and thus the pathogenesis of OSA.Citation3 This review provides an overview of the current perspectives on GG function during both wakefulness and sleep in healthy individuals and individuals with OSA.
Given the extensive literature on this topic, the current review primarily focuses on human studies. Further given that GG function during rapid eye movement (REM) sleep is not well studied and it is known to differ from non-rapid eye movement (NREM) sleep, this review primarily focuses on GG function during NREM sleep. This review is portioned into four distinct sections. The first section focuses on the GG in healthy individuals. The second section focuses on the GG in OSA. The third section focuses on methods to improve GG function and/or activity in OSA and their efficacy. The fourth and final section focuses on whether assessing the GG in isolation to understand upper airway patency is appropriate.
GG in healthy individuals
The GG is an extrinsic tongue muscle and the largest of the pharyngeal dilator muscles. The GG muscle originates from the mental spine of the mandible and fans out with a bulk of the fibers inserting into the body of the tongue.Citation4 The lowermost fibers extend backward and downward into the hyoid bone, while the uppermost fibers extend upward and anteriorly into the tip of the tongue. The GG muscle is innervated by the medial branch of the hypoglossal nerve (cranial nerve XII). Activation of the GG muscle results in the tongue moving anteriorly away from the posterior pharyngeal wallsCitation5 via contraction of the transverse GG fibers orientated horizontally and downward via the anterior GG fibers oriented vertically.Citation6 Therefore, the transverse fibers are likely more critical for the maintenance of upper airway patency. The activity of the GG muscle varies with the respiratory cycle, with augmented activity during inspiration and relatively reduced activity during expiration.
Determinants of GG activity
There are at least three key influences on GG activity; these include the central pattern generator, mechanical and chemical stimuli and changes in the state of sleep versus wakefulness (refer for a simplified schematic). The central pattern generator is located in the ventrolateral region of the medulla. This region comprises a small group of interneurons termed the pre-Bötzinger complex, which are thought to be critical for the generation of rhythmic respiration.Citation7 The activation of the GG and several other upper airway dilator muscles ~50–200 ms prior to the onset of diaphragmatic activity and beginning of inspirationCitation8,Citation9 is thought to reflect the activity of the central pattern generator. Tagged magnetic resonance imaging (MRI) of the upper airway shows that during normal breathing, the GG moves anteriorly by up to 1 mm prior to the start of inspiratory flow in healthy individuals.Citation5 This pre-activation is thought to prepare the airway for the onset of negative pressures generated during inspiration.
Figure 1 Schematic figure representing the major inputs (central pattern generator, chemoreceptor, mechanoreceptor and sleep–wake state) to the GG via the hypoglossal nerve (labeled XII).
Abbreviation: GG, genioglossus.
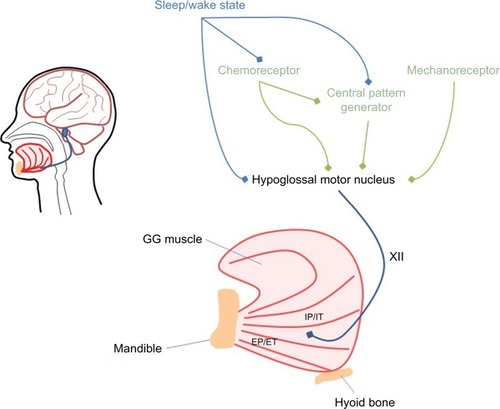
A major stimulus for GG muscle activity is negative pressure detected by the mechanoreceptors. The response of the GG to the sudden onset of negative pressure during wakefulness is brisk and occurs within ~35–55 ms.Citation10–Citation12 The magnitude of GG muscle activation is proportional to negative pressure.Citation10 A study that ventilated healthy individuals during wakefulness using a negative pressure ventilator demonstrated a high correlation between GG activation and negative pressure throughout the inspiratory cycle irrespective of the level of central respiratory drive, breathing rate and breathing volume.Citation13 Given that the application of topical anesthesia to the upper airway diminishes the negative pressure response,Citation14 it can be assumed that at least some of the mechanoreceptors are located locally close to or at the upper airway mucosal surface.
The GG also responds to chemical stimuli sensed by the central chemoreceptors located in the brainstem and the peripheral chemoreceptors located in the aorta and the carotid bodies. During wakefulness, GG muscle activity increases linearly in response to both hypoxia and hypercapnia.Citation15–Citation17 This response is not solely attributable to negative pressure generated by the diaphragm, as it is still present when negative pressure is minimized by the application of continuous positive airway pressure (CPAP).Citation17 Therefore, chemical stimuli likely increase GG activity by increasing central respiratory drive (via the central pattern generator) and via more marked negative pressure.
The other major determinant of GG activation is state. The transition from wakefulness to sleep is characterized by a fall in upper airway dilator muscle activity, a reduction in ventilation and an increase in upper airway resistance.Citation18–Citation20 The mechanisms responsible for this fall in activity are unclear. Likely contributors include decreased responsiveness to chemical stimuli during sleep and a loss of respiratory drive at sleep onset, which may reduce central pattern generator output and reduce negative pressure. However, there also appears to be a direct effect of wakefulness itself on upper airway dilator muscle activity. Studies that have held mechanical and chemical stimuli constant via application of CPAP or have eliminated respiratory drive with noninvasive ventilation have also demonstrated a decline in GG muscle activity from wakefulness to sleep,Citation21,Citation22 suggesting that wakefulness alone elicits GG activity. This is further supported by the observation that when an arousal from sleep or a return to wakefulness occurs, there is rapid restoration of upper airway dilator muscle activity.Citation18 This activation associated with the waking state has been termed the wakefulness stimulus.
Changes to GG control in sleep
In addition to the state-related changes in GG activity, there is also evidence that at least some aspects of GG control are altered with sleep. Following the initial fall at sleep onset, GG activity progressively increases during NREM sleepCitation18 such that activity reaches a level similar to or higher than wakefulness in established sleep.Citation23 The increase in GG activity after sleep onset is likely due to increased negative pressures and CO2 levels during NREM sleep, rather than a direct effect of sleep state. Some studies have shown that the GG response to negative pressure is slowed and diminished during NREM sleep relative to wakefulness.Citation11,Citation12 However, more recent work has demonstrated that when in the supine position, the speed and magnitude of the negative pressure response are equivalent to those observed during wakefulness.Citation24,Citation25 Similar findings have been demonstrated for the GG response to hypercapnia. In the lateral position, the hypercapnic response is minimal or absent during NREM sleep;Citation26,Citation27 however, in the supine position, the response is approximately half the magnitude of wakefulness.Citation17 The most likely explanation for these divergent findings between body positions is that GG activity does not increase significantly unless some degree of airway collapse is present as the GG appears to respond more effectively to combined respiratory stimuli rather than chemical or mechanical stimuli alone during sleep.Citation17,Citation27
The work presented so far has focused on multi-unit GG activity; however, a small body of work has assessed single motor units (SMUs), which represent the last common pathway of neural output and therefore provide valuable insight into GG motor control. There are six distinct SMU discharge patterns that have been identified within the GG.Citation28 There are units that fire only during inspiration (inspiratory phasic) or expiration (expiratory phasic). Then, there are units that fire throughout the respiratory cycle but have a higher discharge frequency during inspiration (inspiratory tonic) or expiration (expiratory tonic). Finally, there are units that fire throughout the respiratory cycle but have no respiratory modulation (tonic) or their modulation is unrelated to the respiratory cycle (tonic other). Recent data in healthy individuals have suggested that there is a geographical distribution of SMUs across the GG muscle with inspiratory units located more posteriorly and expiratory units located more anteriorly and close to the mandible ().Citation29 At the transition from wakefulness to sleep, ~70% of recorded motor units show minimal change, while the remaining 30% of units fall silent or reduce their respiratory modulation or their discharge frequency.Citation30 Inspiratory phasic and inspiratory tonic units are most affected by sleep onset, as ~50% of these unit types cease firing or reduce the proportion of the respiratory cycle over which they are active.Citation31 At arousal from sleep, previously silent inspiratory modulated units recommence firing.Citation32 As there is minimal change in discharge frequency at sleep onset and arousal, it is theorized that the changes in multi-unit GG activity typically observed at these transitions are predominately attributable to the recruitment/decruitment of SMUs.Citation33
Role of the GG in maintaining upper airway patency
Tagged MRI of the upper airway during wakefulness has demonstrated that the GG moves anteriorly during inspiration, enlarging the upper airway lumen during normal breathing.Citation5 During mild upper airway loading, anterior movement of the GG and enlargement of the upper airway are attenuated. This suggests that the increase in GG activity typically observed during loaded breathing acts to stiffen the upper airway. A recent study demonstrated that anterior movement of the GG during inspiration is greater in healthy individuals who have an anatomically narrow airway than in individuals who have a large upper airway.Citation34 Similarly, anterior movement is greater in individuals who have their tongue base angled toward the posterior airway wall compared to those who have their tongue base angled perpendicular to the posterior airway wall. Together these imaging studies suggest that in healthy individuals, the GG functions to both stiffen and dilate the upper airway. In individuals who have anatomical deficits, yet do not have OSA, this compensatory effect appears to be greater.
Owing to technical constraints, tagged MRI of the GG cannot be performed during sleep. Endoscopy has been used to view the upper airway during sleep in OSA patients while they are under general anesthesiaCitation35 or during their normal sleep.Citation36 However, due to limitations regarding the precision of the measurement, it is best suited for identifying site of collapse and cannot provide detailed information regarding GG function. Further due to invasive instrumentation and the need for local and/or general anesthetics, flow patterns and muscle function under endoscopy may not replicate the normal conditions of the upper airway during sleep. Rather information regarding upper airway function during sleep is typically derived by assessing the relationship between peak airflow and pressures externally applied to the upper airway. A common measurement technique involves reducing pressure from an optimal level (at which the airway is completely patent) to a pressure at which airflow ceases, known as the pharyngeal critical closing pressure (Pcrit). Pcrit can be determined when the upper airway dilator muscles are passive by rapidly dropping pressure from optimal to suboptimal levels and measuring the consequential change in airflow within a few breaths.Citation37,Citation38 Alternatively, Pcrit can be determined when the upper airway dilator muscles are active by gradually reducing pressure and allowing an increase in muscle activity before the airflow measurements are made.Citation39 In healthy individuals, active Pcrit is strongly correlated (r = 0.83) to the responsiveness of the GG to negative pressure,Citation40 suggesting that the GG is key to the maintenance of upper airway patency during sleep.
Pharmacological studies have also provided insight into the role of the GG in maintaining upper airway patency. Animal studies have demonstrated that a loss of noradrenergic, histaminergic and serotonergic neuronal activity during sleep is likely responsible for the associated reduction in upper airway dilator muscle activity.Citation41–Citation45 The effects appear to be species specific. In cats, application of serotonin and histamine result in a significant increase in GG activity during wake and sleep, whereas noradrenaline has no excitatory effect.Citation41 The importance of serotonin to GG activity has also been demonstrated in dogs.Citation42 In contrast, for rats, both increases and decreases in serotonin have minimal effect on GG activity.Citation43,Citation44 However, when a noradrenergic antagonist is administered to awake rats, GG muscle activity is lowered to NREM levels.Citation45 Conversely, stimulation of alpha 1 adrenergic receptors in rats increases GG muscle activity during both wakefulness and NREM sleep. Based on these findings, a recent study administered 200 mg of desipramine, a tricyclic depressant that has strong noradrenergic effects, to healthy individuals prior to sleep.Citation46 Compared to placebo, desipramine did not alter phasic GG activity, GG responsiveness to negative pressure or sleep architecture. However, desipramine significantly increased tonic GG activity during NREM sleep (96% of the wakefulness levels) compared to placebo (75% of wakefulness levels) and also significantly reduced passive Pcrit by 0.6 cmH2O, indicating a less collapsible airway. Potassium channels are another mechanism by which GG activity has been shown to be reduced during sleep.Citation47 Recently, a potassium blocker was administered to humans prior to sleep. Significant but small increases in GG activity were observed for tonic activity during REM sleep.Citation48 It was theorized that a higher dosage may have resulted in larger effects, but such dosages are deemed unsafe for human use.
Therefore, studies of healthy individuals that have assessed the upper airway by use of imaging during wakefulness and collapsibility measures during sleep have demonstrated that the GG plays a key role in maintaining upper airway patency. However, much of what we know about the GG and its role in maintaining upper airway patency is derived primarily from the study of individuals with OSA. It is unclear whether GG function is abnormal in OSA and therefore gives rise to repetitive upper airway collapse or whether GG function is better or comparable to healthy individuals but not sufficient to overcome the other pathophysiological deficits that give rise to OSA, such as poor anatomy, oversensitive ventilatory control and low arousal thresholds.Citation49 In the following section, we have reviewed the role of the GG in OSA.
GG in OSA
Evidence for the role of the GG in the pathogenesis of OSA
The ability to maintain upper airway patency is thought to be critically dependent on a balance of forces, whereby poor upper airway anatomy is compensated for by the upper airway dilator muscles and that failure of the muscles to do so results in upper airway collapse.Citation3 As upper airway collapse does not occur during wakefulness, state-related changes in respiratory control and upper airway dilator muscle activity are deemed responsible for the inability to maintain a patent airway during sleep. The hypothesis that state related changes in respiratory/muscle control are critical in OSA is supported by the observation that during wakefulness, OSA patients have increased GG activation relative to healthy individuals.Citation20,Citation21,Citation50,Citation51 When CPAP is applied, the reduction in GG activation is greater in OSA patients than in healthy individuals,Citation50 implying that the enhanced activity is a compensatory response to negative pressure. The tonic component of GG activity is also greater in OSA patients than in healthy individuals. The mechanism responsible for this increase is unknown but theorized to be due to neural plasticity secondary to a chronic elevation in upper airway resistance.Citation50 Therefore, during wakefulness, the upper airway dilator muscles actively compensate for the anatomical deficits in OSA. At sleep onset, the OSA patients have a larger and more rapid fall in GG muscle activity than healthy individuals.Citation20,Citation21 Thus, this state-related loss of upper dilator muscle activation appears to unmask the anatomical deficits present in OSA patients.
How exactly the GG contributes to the occurrence or prevention of upper airway collapse in OSA is not yet entirely understood. Like healthy individuals, OSA patients modulate GG activity in response to changing upper airway conditions during sleep. When on fully therapeutic CPAP, there is no difference in the level of GG activation between OSA patients and healthy individuals.Citation39,Citation52 However, there are mixed findings as to whether GG activation is normal during obstruction as assessed via pressure drops during sleep. One study demonstrated that the slope of the relationship between peak GG activity and negative pressure does not differ between OSA patients and healthy individuals but that the latter individuals are able to achieve greater peak flows.Citation52 However, another study demonstrated that tonic (expiratory) GG activity increases at a slower rate in OSA patients.Citation53 Despite these contradictory findings, it is apparent that during sleep, OSA patients are able to increase their GG muscle activity to levels that substantially exceed wakefulness and yet the upper airway often remains occluded.Citation54,Citation55 A possible reason for this is that the level of GG activity present during wakefulness is not sufficient to overcome other physiological changes that occur and persist during sleep, such as low activity of the other dilator muscles,Citation18,Citation21 altered co-activation patterns with the other dilator musclesCitation54,Citation55 and low lung volumes.Citation56
OSA is also associated with altered GG tissue movement, morphology and neuropathy. Whether these abnormalities are a cause of OSA that is unmasked during sleep or a consequence of OSA is not yet clear and requires further examination. A description of each is given in the following section:
GG tissue movement
Imaging studies during wakefulness have demonstrated that healthy individuals typically show “en bloc” anterior movement of the whole posterior tongue.Citation57 For OSA patients, tongue movement is far more heterogeneous, with the exception of severe OSA patients (Apnea Hypopnea Index [AHI] > 30) who most commonly show minimal (<1 mm) posterior tongue movement (~70% of severe patients) or less commonly bidirectional movement (~25% of severe patients), where one part of the posterior tongue moves anteriorly while the other part moves posteriorly.Citation57 For individuals with mild-to-moderate OSA, the movement patterns are more variable with approximately one-third showing minimal movement, one-third showing oropharyngeal movement and one-third showing bidirectional movement. This bidirectional pattern appears to be unique to patients with OSA as it is rarely observed in healthy individuals.Citation57 Such abnormal tissue movement patterns may result from motor neuropathy, changes to GG structure, altered angles of insertion affecting mechanical action or reduced oral cavity space due to increased volume of the tongue and soft-tissue structures causing restricted movement.
GG morphology
Individuals with OSA also appear to have altered GG morphology. OSA patients have a higher proportion of type IIa fast twitch muscle fibers and a lower proportion of type I slow twitch fibers within the GG compared to healthy individuals.Citation58,Citation59 Type II muscle fibers are more susceptible to fatigue than type I muscle fibers. Indeed, when performing repeated intermittent tongue protrusions (5 seconds on at 70% of maximal force and 5 seconds off), OSA patients generate greater GG activity and exert more force but fatigue in half the time of healthy individuals.Citation60 Another study demonstrated that GG tongue protrusion force and endurance are not different between OSA patients and healthy individuals but that OSA patients take almost three times longer to recover their mean maximal force.Citation61 Alternatively, it has been demonstrated that obese OSA patients have similar tongue protrusion endurance to healthy individuals but that non-obese OSA patients fatigue more quickly than healthy indviduals.Citation62 Therefore, whether GG fatigue is greater in OSA patients and whether it contributes to OSA pathophysiology are unclear. In addition, OSA patients have also been documented to have reduced GG stiffness in the direction of the muscle fibers.Citation63 Lower stiffness is associated with a higher AHI and thus may contribute to OSA via an increase in compliance to collapsing pressures. Similarly, OSA patients have larger tongue volumes and increased tongue fat relative to healthy individuals, which is positively correlated to AHI.Citation64 Potential adverse effects of this include reduced upper airway aperture and GG contractile abnormalities.
GG neuropathy
There is also evidence to suggest that there are neurogenic changes in GG control of individuals with OSA. SMU studies have revealed that during wakefulness, the distribution of SMU types does not differ between OSA patients and healthy individuals but that OSA patients have larger and longer duration action potentials.Citation9 Furthermore, inspiratory phasic and inspiratory tonic units fire earlier in OSA patients relative to the onset of inspiration. These units also fire simultaneously in OSA patients, whereas for healthy individuals, inspiratory tonic units peak earlier than inspiratory phasic units. These abnormal firing patterns suggest that the output of the hypoglossal nucleus may be altered in OSA. More recent work has demonstrated that OSA patients show greater motor unit potential amplitude, duration and area in the posterior region of the GG than the anterior region, whereas healthy individuals show no difference in these regions.Citation65 Furthermore, when comparing motor unit parameters between healthy individuals and OSA patients, differences are present only for the posterior GG. This finding suggests that there may be muscular injury to this region in OSA patients.
Effectiveness of the GG in improving upper airway patency in OSA
There is some evidence to suggest that enhanced GG activity during sleep can improve upper airway patency at least in certain stages or body positions. A study that assessed OSA patients who were able to achieve stable breathing periods during sleep demonstrated that GG muscle activity was greater during these periods than during cyclical breathing.Citation66 It has also been observed that during slow-wave sleep, which is associated with a substantial reduction in obstructive events in OSA patients,Citation67 GG activation is greater relative to other sleep stages.Citation23,Citation66 It has been theorized that this enhanced activation is due to increased central drive during slow-wave sleep.Citation68 However, a recent study of healthy individuals demonstrated that the increased muscle activation is likely attributable to increased respiratory stimulation during slow-wave sleep.Citation69 Further evidence that enhanced GG activity may improve upper airway patency comes from a study by Sands et alCitation70 that compared GG activity in healthy individuals, overweight individuals with at least moderate OSA (AHI ≥15) and weight-matched individuals with no to minimal OSA (AHI <15). In the weight-matched individuals with no to minimal OSA, GG activity during sleep increased threefold from wakefulness relative to both the healthy individuals and the overweight individuals with at least moderate OSA. This finding suggests that in individuals who likely have anatomic predisposition to collapse, a substantial increase in GG activation during sleep is sufficient to keep the upper airway patent most of the time.
Does improving GG function and/or activity improve OSA?
GG stimulation
Several studies have directly stimulated the GG muscle in an attempt to improve upper airway patency. An early study of OSA patients demonstrated that electrical stimulation of the GG via intramuscular wire electrodes decreased Pcrit and shifted the pressure–flow relationship toward higher flow during sleep.Citation71 Another study of OSA patients under anesthesia also demonstrated reduced Pcrit and increased airflow with electrical stimulation of the GG,Citation72 with endoscopy revealing that the primary mechanism of action was enlargement of the pharyngeal region rather than an improvement in airway compliance. However, there were large interindividual differences in the relationship between the change in Pcrit and upper airway enlargement. Dotan et alCitation6 also assessed OSA patients under anesthesia and demonstrated that stimulation of the posterior segment of the GG reduced Pcrit to a greater extent than stimulation of the anterior segment. This may have occurred because anterior stimulation also contracts the vertical fibers involved in tongue depression, which may narrow rather than enlarge pharyngeal airway space. In addition, it was also observed by Dotan et alCitation6 that the larger the tongue and narrower the pharynx, the greater the reduction in Pcrit. Transcutaneous submental stimulation of the GG has also been assessed in OSA patients.Citation73 During wake, stimulation significantly increased tongue diameter. During sleep, stimulation reduced snoring, improved oxygenation and reduced the respiratory disturbance index by approximately two-thirds (from 28.1 to 10.2 events/h). Therefore, stimulation of the GG appears to be an effective means by which to reduce upper airway collapsibility in OSA.
Hypoglossal nerve stimulation
Other studies have attempted to increase upper airway patency via stimulating the hypoglossal nerve, which innervates all the intrinsic and extrinsic tongue muscles with the exception of the palatoglossus. While hypoglossal nerve stimulation has been feasible for several decades, there has been renewed interest for OSA patients who are noncompliant with CPAP. Hypoglossal nerve stimulation devices typically comprise a pulse generator that is implanted within the chest wall. An electrode cuff runs from the pulse generator to the hypoglossal nerve, and depending on where along the nerve the cuff is placed, different activation patterns can be achieved. Many of the recent hypoglossal nerve stimulation devices pace stimulation in phase with respiratory effort via a sensing lead.Citation74–Citation76 However, constant stimulation has also been trialed, where the stimulation is cycled through multiple nerve contact points to avoid nerve fatigue.Citation77 Hypoglossal nerve stimulation is preferred to direct stimulation of the GG muscle as the nerve branches that innervate the GG comprise mostly of efferent fibers and thus when stimulated are less likely to induce arousal than direct muscle stimulation.Citation78 Hypoglossal nerve stimulation has been shown to improve flow mechanics in individuals with OSA in a dose–response manner.Citation79 A recent meta-analysis has revealed a clinically significant reduction in AHI and oxygen desaturation index (ODI) by approximately half when using hypoglossal nerve stimulation compared to no treatment.Citation80 Interestingly, it has been shown that hypoglossal nerve stimulation only significantly improves AHI when the effect of stimulation causes protrusion of the tongue.Citation81 This implies that GG activation is critical to the effectiveness of hypoglossal nerve stimulation.
Non-myorelaxant sedative studies
Recent work has focused on non-myorelaxant sedatives as a potential method for increasing upper airway dilator muscle activity. The premise behind this is that by delaying arousal (with sedative use to raise the arousal threshold), respiratory stimuli can reach higher levels and therefore activate the dilator muscles to a greater extent.Citation82 In addition, arousals are also considered detrimental in OSA because they lead to hyperventilation and subsequent hypocapnia, which are theorized to reduce drive to the respiratory and upper airway dilator muscles on return to sleep.Citation82 To date, however, numerous studies have found that arousal does not result in GG muscle hypotoniaCitation83–Citation87 and non-myorelaxant sedatives do not have a consistent positive effect on the AHI.Citation82
Muscle training
Myofunctional therapy has also been proposed as a method for treating OSA. Myofunctional therapy typically comprises isometric and isotonic exercises directed at altering structures within both oral (lip and tongue) and oropharyngeal (soft palate and lateral pharyngeal wall) regions. A recent meta-analysis demonstrated a significant improvement in AHI (from a mean ± SD of 24.5 ± 14.3 events/h to 12.3 ± 11.8 events/h), minimum oxygen saturation, objective snoring and sleepiness symptoms in individuals with OSA following myofunctional therapy.Citation88 The mechanisms by which these exercises improve OSA are not yet understood but are theorized to be via increased muscle tone or decreased fat deposits within the upper airway.Citation88 Given these exercises are noninvasive, myofunctional therapy represents a promising future treatment direction for OSA. However, further work is required to determine the exercises that are most critical to improving upper airway patency.
Is assessing the GG in isolation appropriate?
While the focus of the current review is on the GG, assessing this muscle in isolation may be problematic. It is highly likely that upper airway patency is dependent on the co-activation of multiple dilator muscles working synergistically to stiffen and dilate the airway. There is some recent evidence that suggests that failure to maintain patency is due to asynchrony with other upper airway dilator muscles.Citation54,Citation55 A study assessed patterns of activation of the GG and retractor muscles (styloglossus and hyoglossus) at equivalent levels of esophageal pressure during both wakefulness and sleep in OSA patients. To achieve this, patients breathed through inspiratory resistors during wakefulness to simulate the range of pressures expected to occur during obstructive events. During sleep, nasal pressure was dropped to induce obstructive events associated with increasing negative pressures and GG activation. During the obstructive events induced during sleep, GG activation prior to arousal was twice as high as the level attained at equivalent esophageal pressures obtained during wakefulness. In contrast, retractor muscle activity was only half the level of wakefulness values.Citation54 A similar study assessed GG and peri-pharyngeal muscle (styloglossus, geniohyoid, sternohyoid and sternocleidomastoid) activation patterns in both healthy individuals and OSA patients.Citation55 During the obstructive events induced during sleep, GG activity was approximately two-fold the level of wakefulness, whereas for the peri-pharyngeal muscles, activity increased to only two-thirds of the wakefulness level, with no difference in patterns between healthy individuals and OSA patients. This suggests that during sleep, the GG is able to substantially exceed the levels required to keep the pharyngeal airway patent during wakefulness, whereas other peri-pharyngeal muscles do not show the same level of activation. This asynchrony between the GG muscle and the peri-pharyngeal muscles during sleep may explain the inability to maintain airway patency, despite enhanced activation by the GG. Anticipated adverse mechanical effects of this altered activation pattern during sleep include reduced upper airway stiffness due to hypotonia of the peri-pharyngeal muscles and abnormal contraction of the GG due to a shortening of its length because of hypotonia of the muscles that insert into the hyoid bone.Citation55 Together these studies suggest that it is necessary to study the synergistic activation of multiple upper airway dilator muscles to gain a better understanding of the role of the GG in OSA pathophysiology.
Summary
The GG appears to be critical to the maintenance of upper airway patency during both wakefulness and sleep. The role of the GG in OSA pathogenesis is not completely understood. While there are reported abnormalities in responsiveness, morphology, tissue movement and neurogenic control of the GG in OSA patients, how each of these contribute to upper airway collapse and/or impaired flow-mechanics is unclear and requires further exploration via imaging or assessment of mechanical parameters. Furthermore, given the evidence that improved GG activity has a positive effect on upper airway collapsibility and OSA, future work should focus on developing feasible techniques to increase GG activity that are acceptable to OSA patients. Additionally, given the findings that other upper airway muscles may lag in respect to activation during sleep relative to the GG, future work should examine how asynchrony between the upper airway muscles contributes to OSA pathogenesis.
Disclosure
The authors report no conflicts of interest in this work.
References
- AyappaIRapoportDMThe upper airway in sleep: physiology of the pharynxSleep Med Rev20037193312586528
- WhiteDPYounesMKObstructive sleep apneaCompr Physiol201222541259423720258
- RemmersJEDeGrootWJSauerlandEKAnchAMPathogenesis of upper airway occlusion during sleepJ Appl Physiol Respir Env Exerc Physiol1978446931938670014
- BerkovitzBKBHollandGRMoxhamBChapter 3: regional topography of the mouth and related areasOral Anatomy, Histology and Embryology5th edEdinburghElsevier20187091
- ChengSButlerJEGandeviaSCBilstonLEMovement of the human upper airway during inspiration with and without inspiratory resistive loadingJ Appl Physiol20111101697520966195
- DotanYGolibrodaTOlivenRParameters affecting pharyngeal response to genioglossus stimulation in sleep apnoeaEur Respir J201138233834721177842
- EckertDJButlerJEChapter 16: respiratory Physiology: understanding the control of ventilationKrygerMRothTWCDPrinciples and Practice of Sleep Medicine6th edPhiladelphia, PAElsevier2015167173
- StrohlKPHensleyMJHallettMSaundersNAIngramRHActivation of upper airway muscles before onset of inspiration in normal humansJ Appl Physiol Respir Env Exerc Physiol19804946386426777347
- SaboiskyJPButlerJEMcKenzieDKNeural drive to human genioglossus in obstructive sleep apnoeaJ Physiol2007585113514617916615
- HornerRLInnesJAMurphyKGuzAEvidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in manJ Physiol199143615292061830
- WheatleyJRMezzanotteWSTangelDJWhiteDPInfluence of sleep on genioglossus muscle activation by negative pressure in normal menAm Rev Respir Dis199314835976058368629
- HornerRLInnesJAMorrellMJSheaSAGuzAThe effect of sleep on reflex genioglossus muscle activation by stimuli of negative airway pressure in humansJ Physiol199447611411518046629
- AkahoshiTWhiteDPEdwardsJKBeauregardJSheaSAPhasic mechanoreceptor stimuli can induce phasic activation of upper airway muscles in humansJ Physiol2001531pt 367769111251050
- HornerRLInnesJAHoldenHBGuzAAfferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesiaJ Physiol199143631442061834
- OnalELopataMO’ConnorTDDiaphragmatic and genioglossal electromyogram responses to isocapnic hypoxia in humansAm Rev Respir Dis198112432152176792954
- OnalELopataMO’ConnorTDDiaphragmatic and genioglossus electromyogram responses to CO2 rebreathing in humansJ Appl Phsyiol198150510521055
- LoY-LJordanASMalhotraAGenioglossal muscle response to CO2 stimulation during NREM sleepSleep200629447047716676780
- WorsnopCJKayAPierceRJKimYTrinderJActivity of respiratory pump and upper airway muscles during sleep onsetJ Appl Physiol19988539089209729564
- FogelRBTrinderJMalhotraAWithin-breath control of genioglossal muscle activation in humans: Effect of sleep-wake stateJ Physiol2003550389991012807995
- MezzanotteWSTangelDJWhiteDPInfluence of sleep onset on upper-airway muscle activity in apnea patients versus normal controlsAm J Respir Crit Care Med1996153188018878665050
- FogelRBTrinderJWhiteDPThe effect of sleep onset on upper airway muscle activity in patients with sleep apnoea versus controlsJ Physiol2005564254956215695240
- LoY-LJordanASMalhotraAInfluence of wakefulness on pharyngeal airway muscle activityThorax200762979980517389755
- BasnerRCRinglerJSchwartzsteinRMWeinbergerSEWeissJWPhasic electromyographic activity of the genioglossus increases in normals during slow-wave sleepRespir Physiol19918321892002068416
- EckertDJMcEvoyRDGeorgeKEThomsonKJCatchesidePGGenioglossus reflex inhibition to upper-airway negative-pressure stimuli during wakefulness and sleep in healthy malesJ Physiol2007581pt 31193120517395627
- MalhotraATrinderJFogelRBPostural effects on pharyngeal protective reflex mechanismsSleep20042761105111215532204
- PillarGMalhotraAFogelRBUpper airway muscle responsiveness to rising PCO2 during NREM sleepJ Appl Phsyiol20008912751282
- StanchinaMLMalhotraAFogelRBGenioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleepAm J Respir Crit Care Med200216594594911934719
- SaboiskyJPButlerJEFogelRTonic and phasic respiratory drives to human genioglossus motoneurons during breathingJ Neurophysiol20069542213222116306175
- LuuBMuceliSSaboiskyJMotor unit territories in human genioglossus estimated with multi-channel intramuscular electrodesJ Appl Physiol (1985)2018124366467129357517
- BaileyEFFridelKWRiceADSleep/wake firing patterns of human genioglossus motor unitsJ Neurophysiol20079863284329117928550
- WilkinsonVMalhotraANicholasCLDischarge patterns of human genioglossus motor units during sleep onsetSleep200831452553318457240
- WilkinsonVMalhotraANicholasCLDischarge patterns of human genioglossus motor units during arousal from sleepSleep201033337938720337197
- TrinderJJordanASNicholasCLDischarge properties of upper airway motor units during wakefulness and sleepProg Brain Res2014212597525194193
- ChengSBrownECHattAButlerJEGandeviaSCBilstonLEHealthy humans with a narrow upper airway maintain patency during quiet breathing by dilating the airway during inspirationJ Physiol2014592214763477425217376
- VroegopAVVandervekenOMBoudewynsANDrug-induced sleep endoscopy in sleep-disordered breathing: Report on 1,249 casesLaryngoscope2014124379780224155050
- MarquesMGentaPRSandsSAEffect of sleeping position on upper airway patency in obstructive sleep apnea is determined by the pharyngeal structure causing collapseSleep2017403
- SchwartzARO’DonnelCPBaronJThe hypotonic upper airway in obstructive sleep apnea. Role of structures and neuromuscular activityAm J Respir Crit Care Med1998157105110579563718
- GoldARSchwartzARThe pharyngeal critical pressure: the whys and hows of using nasal continuous positive airway pressure diagnosticallyChest19961104107710888874271
- PatilSPSchneiderHMarxJJGladmonESchwartzARSmithPLNeuromechanical control of upper airway patency during sleepJ Appl Physiol2007102254755617008440
- Philip-JoëtFMarcISérièsFEffects of genioglossal response to negative airway pressure on upper airway collapsibility during sleepJ Appl Physiol1996805146614748727528
- NeuzeretPCSakaiKGormandFApplication of histamine or serotonin to the hypoglossal nucleus increases genioglossus muscle activity across the wake-sleep cycleJ Sleep Res200918111312119250178
- VeaseySPanckeriKHoffmanEPackAHendricksJThe effects of serotonin antagonists in an animal model of sleep-disordered breathingAm J Respir Crit Care Med199615327767868564132
- SoodSMorrisonJLLiuHHornerRLRole of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping ratsAm J Respir Crit Care Med2005172101338134716020803
- SoodSRaddatzELiuXLiuHHornerRLInhibition of serotonergic medullary raphe obscurus neurons suppresses genioglossus and diaphragm activities in anesthetized but not conscious ratsJ Appl Physiol200610061807182116484356
- ChanESteenlandHWLiuHHornerRLEndogenous excitatory drive modulating respiratory muscle activity across sleep-wake statesAm J Respir Crit Care Med2006174111264127316931636
- Taranto-MontemurroLEdwardsBASandsSADesipramine increases genioglossus activity and reduces upper airway collapsibility during non-REM sleep in healthy subjectsAm J Respir Crit Care Med2016194787888526967681
- GraceKPHughesSWShahabiSHornerRLK+ Channel modulation causes genioglossus inhibition in REM sleep and is a strategy for reactivationRespir Physiol Neurobiol2013188327728823872455
- Taranto-MontemurroLSandsSAAzarbarzinAEffect of 4-aminopyridine on genioglossus muscle activity during sleep in healthy adultsAnn Am Thorac Soc20171471177118328387543
- EckertDJWhiteDPJordanASMalhotraAWellmanADefining phenotypic causes of obstructive sleep apnea: Identification of novel therapeutic targetsAm J Respir Crit Care Med20131888996100423721582
- FogelRBMalhotraAPillarGGenioglossal activation in patients with obstructive sleep apnea versus control subjectsAm J Respir Crit Care Med2001164112025203011739130
- MezzanotteWSTangelDJWhiteDPWaking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism)J Clin Invest199289157115791569196
- JordanASWellmanAHeinzerRCMechanisms used to restore ventilation after partial upper airway collapse during sleep in humansThorax2007621086186717412778
- McGinleyBMSchwartzARSchneiderHKirknessJPSmithPLPatilSPUpper airway neuromuscular compensation during sleep is defective in obstructive sleep apneaJ Appl Physiol2008105119720518403451
- DotanYPillarGSchwartzAROlivenAAsynchrony of lingual muscle recruitment during sleep in obstructive sleep apneaJ Appl Physiol2015118121516152425814639
- OlivenRCohenGDotanYSomriMSchwartzAROlivenAAlteration in upper airway dilator muscle co-activation during sleep: comparison of patients with OSA and healthy subjectsJ Appl Physiol2018124242142929191983
- BallardRDIrvinCGMartinRJPakJPandeyRWhiteDPInfluence of sleep on lung volume in asthmatic patients and normal subjectsJ Appl Physiol1990685203420412361905
- BrownECChengSMcKenzieDKButlerJEGandeviaSCBilstonLERespiratory movement of upper airway tissue in obstructive sleep apneaSleep20133671069107623814344
- SérièsFSimoneauJAPierreSStMarcICharacteristics of the genioglossus and musculus uvulae in sleep apnea hypopnea syndrome and in snorersAm J Respir Crit Care Med19961536187018748665048
- CarreraMBarbeFSauledaJTomasMGomezCAgustiAGPatients with obstructive sleep apnea exhibit genioglossus dysfunction that is normalized after treatment with continuous positive airway pressureAm J Respir Crit Care Med19991591960196610351945
- EckertDLoYSaboiskyJJordanAWhiteDMalhotraASensorimotor function of the upper-airway muscles and respiratory sensory processing in untreated obstructive sleep apneaJ Appl Physiol201111161644165321885797
- BlumenMBPerez De La SotaAQuera-SalvaMAFrachetBChabolleFLofasoFTongue mechanical characteristics and genioglossus muscle EMG in obstructive sleep apnoea patientsRespir Physiol Neurobiol2004140215516415134663
- CarreraMBarbeFSauledaJEffects of obesity upon genioglossus structure and function in obstructive sleep apnoeaEur Respir J200423342542915065833
- BrownECChengSMckenzieDKButlerJEGandeviaSCBilstonLETongue stiffness is lower in patients with obstructive sleep apnea during wakefulness compared with matched control subjectsSleep201538453754425409103
- KimAMKeenanBTJacksonNTongue fat and its relationship to obstructive sleep apneaSleep201437101639164825197815
- ZhangHYeJYHuaLInhomogeneous neuromuscular injury of the genioglossus muscle in subjects with obstructive sleep apneaSleep Breath201519253954525107373
- JordanASWhiteDPLoY-LAirway dilator muscle activity and lung volume during stable breathing in obstructive sleep apneaSleep200932336136819294956
- RatnavadivelRChauNStadlerDYeoAMcEvoyRDCatchesidePGMarked reduction in obstructive sleep apnea severity in slow wave sleepJ Clin Sleep Med20095651952420465017
- McSharryDGSaboiskyJPDeyoungPA mechanism for upper airway stability during slow wave sleepSleep201336455556323565001
- HicksACoriJMJordanASMechanisms of the deep, slow-wave, sleep-related increase of upper airway muscle tone in healthy humansJ Appl Physiol201712251304131228255086
- SandsSAEckertDJJordanASEnhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apneaAm J Respir Crit Care Med2014190893093725191791
- OlivenAO’HearnDJBoudewynsAUpper airway response to electrical stimulation of the genioglossus in obstructive sleep apneaJ Appl Physiol20039552023202914555669
- OlivenRTovNOdehMInteracting effects of genioglossus stimulation and mandibular advancement in sleep apneaJ Appl Physiol200910651668167319228985
- SteierJSeymourJRaffertyGFContinuous transcutaneous submental electrical stimulation in obstructive sleep apnea: a feasibility studyChest20111404998100721454399
- StrolloPJSooseRJMaurerJTUpper-Airway Stimulation for Obstructive Sleep ApneaN Engl J Med2014370213914924401051
- EastwoodPRBarnesMWalshJHTreating obstructive sleep apnea with hypoglossal nerve stimulationSleep201134111479148622043118
- Van De HeyningPHBadrMSBaskinJZImplanted upper airway stimulation device for obstructive sleep apneaLaryngoscope201212271626163322549513
- MwengeGBRombauxPDuryMLengeleBRodensteinDTargeted hypoglossal neurostimulation for obstructive sleep apnoea: A 1-year pilot studyEur Respir J201341236036722599356
- KezirianEJBoudewynsAEiseleDWElectrical stimulation of the hypoglossal nerve in the treatment of obstructive sleep apneaSleep Med Rev201014529930520116305
- SchwartzARBarnesMHillmanDAcute upper airway responses to hypoglossal nerve stimulation during sleep in obstructive sleep apneaAm J Respir Crit Care Med2012185442042622135343
- CertalVFZaghiSRiazMHypoglossal nerve stimulation in the treatment of obstructive sleep apnea: A systematic review and meta-analysisLaryngoscope201512551254126425389029
- HeiserCMaurerJTSteffenAFunctional outcome of tongue motions with selective hypoglossal nerve stimulation in patients with obstructive sleep apneaSleep Breath20152055356026315466
- JordanASO’DonoghueFJCoriJMTrinderJPhysiology of arousal in obstructive sleep apnea and potential impacts for sedative treatmentAm J Respir Crit Care Med2017196781482128399379
- CoriJMThorntonTO’DonoghueFJArousal-induced hypocapnia does not reduce genioglossus activity in obstructive sleep apneaSleep2017406
- JordanASCoriJMDawsonAArousal from sleep does not lead to reduced dilator muscle activity or elevated upper airway resistance on return to sleep in healthy individualsSleep2015381535925325511
- CoriJMNicholasCLBaptistaSInspiratory-resistive loading increases the ventilatory response to arousal but does not reduce genioglossus muscle activity on the return to sleepJ Appl Physiol2012113690991622815388
- JordanASEckertDJWellmanATrinderJMalhotraAWhiteDPTermination of respiratory events with and without cortical arousal in obstructive sleep apneaAm J Respir Crit Care Med2011184101183119121836132
- CoriJMRochfordPDDonoghueFJOTrinderJJordanASThe influence of CO2 on genioglossus muscle after-discharge following arousal from sleepSleep20174011
- CamachoMCertalVAbdullatifJMyofunctional therapy to treat obstructive sleep apnea: a systematic review and meta-analysisSleep201538566967525348130
