Abstract
Obstructive sleep apnea (OSA) is often associated with hypertension and other cardiovascular diseases. Blood pressure (BP) variability is part of the assessment of cardiovascular risk. In OSA, BP variability has been studied mainly as very short-term (beat-by-beat) and short-term (24-hour BP profile) variability. BP measured on consecutive heartbeats has been demonstrated to be highly variable, due to repeated peaks during sleep, so that an accurate assessment of nocturnal BP levels in OSA may require peculiar methodologies. In 24-hour recordings, BP frequently features a “nondipping” profile, ie, <10% fall from day to night, which may increase cardiovascular risk and occurrence of major cardiovascular events in the nocturnal hours. Also, BP tends to show a large “morning BP surge”, a still controversial negative prognostic sign. Increased very short-term BP variability, high morning BP, and nondipping BP profile appear related to the severity of OSA. Treatment of OSA slightly reduces mean 24-hour BP levels and nocturnal beat-by-beat BP variability by abolishing nocturnal BP peaks. In some patients OSA treatment turns a nondipping into a dipping BP profile. Treatment of arterial hypertension in OSA usually requires both antihypertensive pharmacological therapy and treatment of apnea. Addressing BP variability could help improve the management of OSA and reduce cardiovascular risk. Possibly, drug administration at an appropriate time would ensure a dipping-BP profile.
Introduction
Obstructive sleep apnea (OSA) is a well-known cardiovascular risk factor. In patients with OSA, cardiovascular diseases have increased incidence and are associated with worse functional outcomes and increased mortality.Citation1 Systemic hypertension, which is often found in OSA, can importantly affect cardiovascular health. Also, altered blood pressure (BP) variability may carry some additional risk for higher incidence and faster progression of cardiovascular disease.Citation2,Citation3 Both the degree and pattern of BP variability have prognostic implications. Many methods and calculations have been proposed for assessment of BP variability, but physiological and clinical meanings of each of them are not always clear.Citation4,Citation5 In this article, after a brief review of assessment and implications of BP variability in the general population, we focus on BP variability in adult OSA, taking into account methodological, clinical, and therapeutic aspects.
Types of BP variability
BP variability can be defined as BP fluctuations occurring over time. It can be studied in the very short term (beat to beat), short term (24 hours), midterm (day to day), or long term (visit to visit).Citation2 Whatever the time scale, sympathetic activity is a determinant of BP variability in response to a variety of external and internal stimuli. In the very short term, respiratory influences, postural changes, emotional factors, or environmental conditions are a source of BP changes, while arterial and cardiopulmonary reflexes, arterial compliance, blood viscosity, and humoral factors modulate their amplitude.Citation2 Twenty-four-hour BP changes are affected by endocrine influences, genetic factors, degree of diurnal physical and mental activity, and more than anything else by the wake–sleep cycle.Citation6,Citation7 In normal subjects, BP decreases when falling asleep, reaches its lowest values and highest stability during slow-wave sleep (SWS), and shows the highest variability during rapid eye movement (REM) sleep.Citation8 Midterm and long-term BP variability are representative of BP burden and reflect the degree of control of BP over longer periods. Similarly to very short- and short-term variability, their increase is considered an unfavorable prognostic sign.Citation2
Measurement techniques should be appropriately chosen to study each kind of BP variability. Very short-term variability can be studied invasively by means of measurements performed with intra-arterial catheters, or noninvasively by means of a photoplethysmographic sensor applied at a finger using the volume-clamp methodology.Citation9,Citation10 In addition, a new noninvasive method to measure beat-to-beat BP at the wrist by applanation tonometry has been recently described.Citation11 Twenty-four-hour changes can be detected by BP measurements taken at different times of the day, eg, morning and evening, preferably with home measurements taken by patients themselves (home BP monitoring [HBPM]), or by instruments that automatically measure BP at fixed intervals during the 24 hours (ambulatory BPM [ABPM]). However, HBPM may provide information only on waking BP, whereas BP measurements during sleep can be obtained only with ABPM. For assessment of midterm BP variability, HBPM may be a better choice than ABPM, as ABPM may not be easily tolerated on consecutive days. Patients should preferably use validated automatic devices and receive appropriate training and instructions about the correct modalities to perform the measurements.Citation12 Similarly to midterm variability, long-term BP variability may be best monitored by HBPM. Whatever the type of BP assessment to perform, office BP measurements are far from ideal, mainly due to the common “white-coat effect”, which can lead to an overestimation of patients’ usual BP levels.Citation2 Evaluation of mid- and long-term variabilities is particularly useful in the management of hypertensive patients, but very little is known about their behavior in OSA. Therefore, we focus our attention on very short- and short-term variability.
Beat-to-beat BP measurements: very short-term BP variability
Beat-to-beat BP measurements are ideal to monitor fast changes in autonomic modulation, eg, in awake subjects performing the Valsalva maneuver, which is part of the assessment of patients with autonomic disorders, eg, orthostatic hypotension.Citation13 Among the most common applications of beat-to-beat BP recordings is the measurement of cardiac baroreflex sensitivity, which can be assessed both during wakefulness and during sleep. Its evaluation may be useful for prognostic purposes and to assess therapy effectiveness in several diseases, although present knowledge has not yet allowed us to adopt it in clinical routine.Citation14 During sleep, beat-to-beat measurements reveal sudden BP rises when events disturbing sleep occur, especially when associated with electroencephalography changes (cortical arousals).Citation15 BP surges can be a cause of increased nocturnal BP variability, as is usually observed in OSA. In this condition, beat-to-beat BPM may reveal important aspects of BP behavior that would be undetected by any other BP assessment method.
Twenty-four-hour BP: short-term BP variability
Many types of analyses may be done on 24-hour BP values, including separate measurements of waking and sleeping BP and the 24-hour BP profile. Today, it is commonly agreed that mean BP levels have a higher influence on cardiovascular risk than the 24-hour BP profile.Citation2,Citation16,Citation17 In particular, nocturnal BP levels are the most useful predictors of cardiovascular disease.Citation16,Citation18–Citation20 Thresholds for normal BP values, assessed by ABPM, have changed over time. Until recently, diurnal values ≥135/85 mmHg and nocturnal values ≥120/70 mmHg have been considered representative of diurnal and nocturnal hypertensive BP levels.Citation21 According to new guidelines, diurnal values ≥130/80 and nocturnal values ≥110/65 mmHg should be considered hypertensive.Citation12
However, some types of circadian BP changes also carry an independent risk, and the evaluation of the circadian BP profile helps to understand mechanisms governing BP changes better and establish more appropriate therapy in each hypertensive subject. In normal subjects, BP usually exhibits one peak early in the morning and one peak late in the afternoon or early evening.Citation6,Citation7 Either higher morning or evening BP have been reported, as some studies, mainly in Asian subjects, found higher values in the morning, while other studies, mainly in European subjects, in the evening.Citation22 It has been claimed that morning BP better predicts cardiovascular risk than evening BP,Citation23 particularly when the morning:evening BP ratio increases.Citation24
The 24-hour BP profile is evaluated mainly as change in mean BP from day to night. Since sleep is a major determinant of the diurnal change, usually causing a decrease in BP, BP measurements taken during waking and sleeping should be considered separately. For the sake of simplicity, fixed time ranges relevant to estimated waking and sleeping time are often used. They can be set as wide-fixed or narrow-fixed intervals. With the first method, all 24-hour measurements are taken into account and subdivided into waking and sleeping values. According to the other method, periods estimated as transition times between waking and sleeping are discarded.Citation25 Ideally, waking and sleeping measurements should be estimated individually based on sleep diaries or (better) on results of monitoring by instruments helping to define waking and sleeping times, like actigraphs.Citation26
Although definitions may vary,Citation19 the following BP 24-hour profiles are commonly considered: “dipping”, mean nocturnal reduction in BP by 10%–20%; “nondipping”, nocturnal reduction in BP between 0 and 10%; “reverse dipping” or “riser” pattern, increase in mean BP during the night; “extreme dipping”, nocturnal reduction in BP >20%. While the dipping pattern is considered physiological and associated with the lowest risk, nondipping and extreme-dipping patterns carry an intermediate risk and the reverse-dipping profile carries the highest risk of cardiovascular events.Citation27
Another way to look at the 24-hour BP variability is to compare nocturnal and morning BP to measure the “morning BP surge” (MBPS). BP normally increases after morning awakening.Citation6 An increase in cardiac output in young subjects and increased arterial stiffness in the elderly play a role in determining MBPS.Citation28 Elderly subjects usually have higher MBPS compared to young subjects. The definition of a normal MBPS rise is lacking, but a J- or U-shaped relationship between the degree of MBPS and cardiovascular risk has been proposed.Citation28
MBPS can be calculated following different criteria (). The most commonly used are: sleep-trough morning surge, defined as the difference between the average BP over 2 hours after waking and the average of three consecutive BP recordings, centered on the lowest reading during sleep; and the prewaking morning surge, defined as the difference between the average BP recordings 2 hours after waking and average BP recordings over 2 hours before waking up.Citation28,Citation29 The MBPS has been proposed as a potential contributor to the increased incidence of cardiovascular events in the morning hours. However, while some authors have confirmed the importance of the MBPS as a cardiovascular risk factor,Citation29–Citation31 others have reported opposite findings, with less risk in subjects with elevated MBPS.Citation32–Citation34
Figure 1 Measurements of morning blood pressure surge (MBPS).
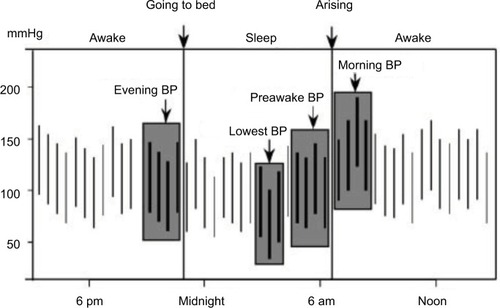
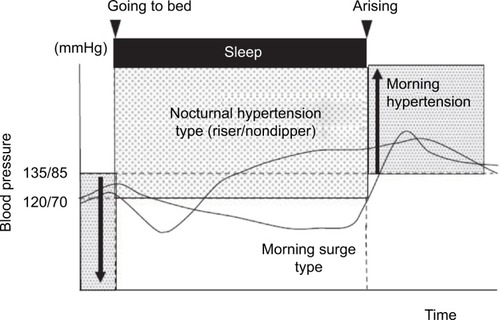
An apparently obvious objection raised to the MBPS concept is that subjects with high MBPS may actually show low nocturnal BP levels, which is difficult to reconcile with the well-known risk represented by nocturnal hypertension. Along the same lines, a high MBPS may be the effect of extreme dipping, such that it could be associated with increased cardiovascular risk due to very low nocturnal BP and not to high morning BP. Although an elevated MBPS is more common among extreme dippers, only a fraction of them have a high MBPS. According to one study, only 24% of extreme dippers have a high MBPS. Also, when extreme dipping and high MBPS coexist, increased cardiovascular risk appears to be associated more with the morning BP rise than with excessively low nocturnal BP.Citation29 Finally, a high MBPS, assessed by sleep-trough morning surge, may be associated with nocturnal hypertension in subjects who show a progressive increase in BP from the beginning to the end of the night ().Citation35
Figure 2 Different blood-pressure profiles in two subjects with high sleep-trough morning blood pressure surge (MBPS).
In clinical practice, ABPM is typically used in specialized hypertension clinics, especially for patients who have resistant or refractory hypertension. However, the importance and usefulness of ABPM measurements should lead to it being used clinically more often than it is at present.
BP variability in OSA
Patients with OSA are characterized by a high prevalence of arterial hypertension, which may exceed 50%,Citation36 and by high BP variability. In fact, OSA is frequently associated with factors, often interrelated, with important influences on the cardiocirculatory system, like oxidative stress, inflammation, endothelial dysfunction, and high sympathetic tone,Citation1 which increase during sleep as an immediate effect of respiratory disorders and remain high during wakefulness.Citation37 These factors may increase BP levels and variability chronically.
The greatest peculiarities in BP behavior in OSA are seen during sleep. Pulsus paradoxus, ie, inspiratory reduction in BP ≥10 mmHg, occurs in obstructed breaths with strong respiratory efforts.Citation38,Citation39 More importantly, obstructive apnea termination is characterized by a BP peak lasting only a few seconds that is followed by a return of BP to or below baseline.Citation40 Therefore, the higher the number of apneas during sleep, the higher the number of BP oscillations. However, the amplitude of BP peaks is highly variable in relationship to sympathetic reactivity, which in turn is related to end-apneic hypoxemia, occurrence and strength of arousals at the end of each event, age of patients, and normotensive/hypertensive state.Citation41–Citation43 The rate and amplitude of BP peaks during sleep are fundamental determinants of the degree of variability of BP during the night and of the mean change in BP from day to night. Apneic events are less frequent in SWS,Citation44 and are usually longer and associated with worse hypoxemia in REM sleep.Citation45 Therefore, the respective duration of the various sleep stages may also influence nocturnal BP behavior in OSA through rate and severity of events in each of them. Due to the numerous, large, and rapid changes in BP during the night, information on BP in OSA is heavily affected by the method used for its assessment. Optimal detection of different parameters describing BP levels or variability may take advantage of different monitoring techniques.
Role of frequent diurnal and nocturnal BP measurements: very short-term variability
Continuous BP monitoring is not easily tolerated for prolonged periods of time, and may give rise to frequent artifacts, eg, due to limb movements. Therefore, beat-by-beat BP measurements are often performed during short representative time lapses. When recorded during wakefulness, their analysis is not more useful in OSA than in normal subjects, whereas they provide valuable and unique information on BP behavior in sleeping OSA subjects.
Intra-arterial measurements were already obtained in very early studies in OSA patients, and revealed the rapid oscillations associated with apneic eventsCitation40 that were later confirmed with the use of noninvasive techniques.Citation41,Citation43,Citation46–Citation50 Beat-by-beat evaluation of BP showed that during sleep, compared to the immediately preceding quiet wakefulness, mean BP falls if breathing is unobstructed, while it changes little during nonapneic snoring.Citation47 Conversely, in OSA, mean BP during sleep can be higher than during quiet wakefulness, with a trend to highest values during REM sleep.Citation46,Citation51
The advantage of beat-by-beat BP measurements in OSA is that they provide accurate information about very short-term variability in BP, although they are less accurate in the assessment of absolute BP values. During wakefulness, beat-by-beat measurements show increased variance in BP proportional to the severity of OSA.Citation52 Progression from quiet waking to non-REM to REM sleep is associated with an increasing coefficient of variation in BP values.Citation46 The SD of BP values during sleep is higher in OSA than in non-OSA subjects, although in both groups BP variability is similarly modulated by sleep stage, being lowest during SWS. Frequency of arousals and respiratory events are correlated with systolic and diastolic BP SD, although they do not fully explain them.Citation49
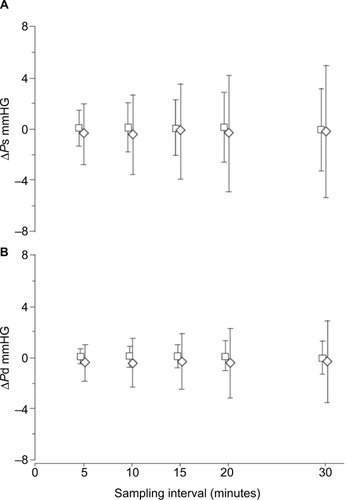
We used beat-by-beat BP measurements to compare accuracy of different sampling rates in the assessment of mean nocturnal BP in subjects with severe OSA and in non-OSA controls. In each subject, mean systolic and diastolic BP were calculated, averaging measurements taken on all heartbeats of one night. Then, mean BP values of the same night were measured, averaging values of heartbeats sampled each 5, 10, 15, 20 and 30 minutes. The mean values obtained from heartbeats sampled at each rate were compared to values calculated on all heartbeats. At all sampling intervals in both patients and controls, mean differences between mean systolic and diastolic nocturnal BP values calculated on sampled and all-night heartbeats were small, but the scatter of the differences was higher in the OSA group, especially when long sampling intervals were used (). In the OSA subjects, a sampling rate about three times that in the non-OSA subjects was necessary for a similarly accurate assessment of mean nocturnal BP.Citation53
Figure 3 Means ± SD of (A) ΔPs and (B) ΔPd values in control (□) and obstructive sleep apnea (◊) subjects for each sampling interval.
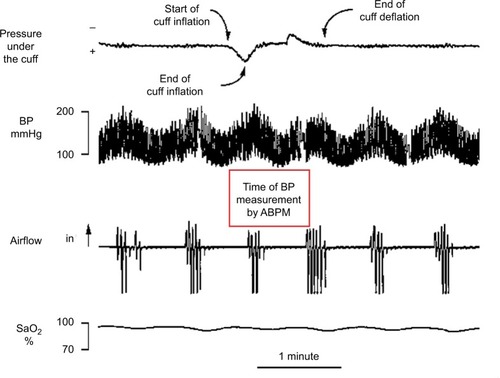
It has been claimed that cuff inflations for ABPM measurements can cause arousals, transiently increasing BP, so that BP levels detected by ABPM are higher than during undisturbed sleep.Citation54 In OSA subjects, this result proved true for systolic pressure, which is measured soon after brachial cuff inflation, but not for diastolic pressure, which is measured later so that it is less affected by the arousal.Citation55 Our experience with five sleepy patients with severe OSA was that the time taken by ABPM to perform each measurement was usually longer than an apneic cycle, ie, an apnea and the following ventilation interval, and rarely caused arousals prematurely interrupting the apnea occurring during the measurement ().Citation53 Therefore, frequent BP measurements by ABPM may not significantly disturb sleep and BP behavior in severe OSA patients.
Figure 4 Fragment of a polygraph recording in a representative sleepy patient with severe obstructive sleep apnea.
Whatever the time between measurements obtained at fixed intervals, ABPM may hardly be able to reveal extreme BP levels, which are often (but only transiently) reached in OSA at night. To overcome this problem, a nocturnal BPM method triggered by occurrence of oxygen desaturation has been developed.Citation56 This method shows a fair degree of reproducibility,Citation57 but the time required for a measurement and the short duration of the BP rise may reduce the accuracy of detection of BP peaks. However, it represents a clear advancement in the assessment of nocturnal BP in OSA with respect to ABPM.
Twenty-four-hour BP profile: short-term BP variability
Some information about the diurnal BP changes in OSA has been obtained with measurements taken in the evening and the next morning, after awakening. According to this methodology, the morning BP levels in OSA tend to exceed the evening ones. Most studies have reported that the evening-to-morning BP difference was correlated with OSA severity ().Citation48,Citation58–Citation64
Table 1 Studies based on morning and evening BP measurements
A large number of studies have been based on ABPM, with nocturnal BP measurements taken every 15, 20, or 30 minutes. Surprisingly, the first ABPM studies did not demonstrate increased nocturnal BP in OSA.Citation65,Citation66 Rather, one underscored the occurrence of large drops in BP in some subjects with mildly elevated apnea/hypopnea index (AHI) values, but large oxygen saturation falls. This observation remained isolated and unexplained. However, recruited patients were old, and increased arterial stiffness could be responsible for high diurnal BP values and blunted BP reactivity to sleep-respiratory disorders and arousals.Citation65 Instead, most of the following studies reported that patients with OSA showed a nondipping more commonly than a dipping BP pattern.Citation67–Citation72
In OSA, use of ABPM has also proved useful in highlighting high prevalence of masked hypertension, ie, normal office BP values with high mean values at the ABPM assessment. Masked hypertension in OSA could be a consequence of mean nocturnal BP exceeding normal levels.Citation73 However, two studies found that it could frequently also be due to high diurnal BP values detected only by ABPM.Citation74,Citation75
Other studies extended analysis of ABPM recordings beyond mean diurnal and nocturnal BP ().Citation76–Citation79 Altogether, OSA, and especially severe OSA, was associated not only with increased nocturnal but also with increased morning BP. Differences in diurnal and evening BP observed between subjects with and without OSA were milder, confirming the results obtained with waking evening and morning measurements described earlier.
Table 2 24-Hour BP characteristics in obstructive sleep apnea: beyond dipping and nondipping
Factors associated with high BP levels and nondipping profile observed at ABPM have been investigated in several cross-sectional studies. Significant relationships between increased AHI or oxygen desaturation index (number of oxygen saturation dips per hour of sleep) and reduced nocturnal BP dipping have been found in community and OSA populations.Citation59,Citation68,Citation69,Citation80 Other studies on OSA, while confirming a role of nocturnal hypoxemia, stressed the importance of arousals as possible determinants of nocturnal BP and 24-hour BP profile.Citation76,Citation81 By contrast, another study could not demonstrate differences between dippers and nondippers in the rate of arousals or SWS duration.Citation71 Differences between studies could be at least partially accounted for by variable relationships of the nondipping phenomenon in different subjects. In fact, according to some authors, in young OSA patients nondipping was correlated with the severity of respiratory disorders, and in elderly patients with reduced SWS and REM and increased stage 1 duration.Citation82 In a subsequent study, the same authors demonstrated that while a sustained high BP profile was associated with both OSA severity and altered sleep architecture, the MBPS was associated only with altered sleep architecture.Citation78 Morning BP was the object of another study that found that the severity of sleep-respiratory disorders in hypertensive subjects, though not directly affecting morning BP, was independently correlated with the MBPS.Citation83
Two longitudinal studies evaluated the incidence of a nondipping BP pattern in relationship to OSA in a general population cohort (the Wisconsin Sleep Cohort). In the first, an independent relationship between AHI values and incidence of systolic nondipping was demonstrated.Citation84 In the second, the risk of developing systolic and diastolic nondipping was significantly correlated with AHI values in REM, but not in non-REM sleep.Citation85
In summary, cross-sectional studies have shown that high-severity sleep-respiratory disorders and altered sleep architecture may be related to increased BP, especially at night, and to reduced nocturnal BP dipping, but data were inconsistent, possibly due to numerous confounding factors. Longitudinal studies, coming from only one cohort, support a role of sleep-respiratory disorders, especially if occurring during REM sleep, as determinants of nondipping BP.
Other measurements of BP variability
Besides the BP pattern over 24 hours, the SD of the measurements taken with ABPM has been commonly used as a parameter of variability.Citation68,Citation74,Citation86–Citation91 Other parameters have been coefficient of variation,Citation89 average real variability (ie, average of absolute differences between consecutive measurements),Citation89 time rate index (ie, rate of change of BP over time, defined as the first derivative values of BP by time).Citation87 Heterogeneous studies designs were evident. Two studies compared subjects with and without OSA,Citation87,Citation88 two compared hypertensive subjects with and without OSA,Citation89,Citation90 one compared subjects with mild–moderate and severe OSA,Citation91 one evaluated patients with moderate–severe OSA before and after continuous positive airway pressure (CPAP) treatment,Citation86 and one evaluated OSA subjects with normotension, established hypertension, or masked hypertension.Citation74 Therefore, results of these studies are hard to compare. Some relationship between OSA and BP variability was observed in most of them. However, depending on the study, an effect of OSA was variably apparent on systolic or diastolic BP or on 24-hour, diurnal, or nocturnal BP. We believe that ABPM may roughly catch BP variability in the nocturnal hours in OSA for the reasons explained, which could account for some disagreement among studies. Little information is available on mid- and long-term BP variability in OSA. No effect on morning-to-morning BP was reported during CPAP withdrawal for 2 weeks, which suggested that OSA does not significantly influence midterm BP variability.Citation92
Twenty-four-hour BP profile and cardiovascular risk
The characteristics of BP variability in OSA likely contribute to cardiovascular risk and to a peculiar temporal pattern of occurrence of cardiovascular events. Little direct evidence is available of a relationship between BP profile and cardiovascular risk in OSA. Among OSA patients studied with repeated morning and evening BP measurements, those with an MBPS showed higher high-sensitivity C-reactive protein levels.Citation61 Similarly, among OSA patients studied by ABPM, nondippers showed higher levels of IL2 and high-sensitivity C-reactive protein than dippers.Citation93,Citation94 Patients with both moderate–severe OSA and reverse-dipping BP profile showed worse brain white matter lesions than subjects with one condition alone.Citation95 In a retrospective study, it was observed that OSA patients who had shown a nondipping-BP profile had a higher incidence of stroke, coronary artery disease, and heart failure in a 43-month period.Citation96
A support to the role of the BP profile is also given by indirect evidence. While in the general population major cardiovascular events and sudden death occur preferentially in the morning hours,Citation6 in OSA their distribution in the 24-hour period seems to be different. Among subjects who had been studied by polysomnography and died from sudden cardiac death, the fatal events had a peak during the night if OSA had been diagnosed, and a nocturnal nadir in non-OSA subjects.Citation97 According to a recent larger investigation, sudden cardiac deaths were evenly distributed in the 24 hours in OSA patients, whereas non-OSA subjects died preferentially in the morning.Citation98 Two studies pointed out that in OSA patients, the onset of acute coronary syndrome was more frequent in the nocturnal hours, differently from subjects without OSA.Citation99,Citation100 In addition, a few studies showed that wakeup strokes were strongly associated with OSA.Citation101,Citation102 Multiple factors may be responsible for triggering cardiovascular events at night, but it is likely that the altered BP profile plays an important role. All these findings indicate that risk associated with OSA increases especially at night and not in the morning. Therefore, in OSA nocturnal hypertension and a nondipping or reverse-dipping pattern might influence cardiovascular risk more than MBPS.
OSA treatment and BP variability
In the management of OSA, the main focus is usually on the elimination of respiratory disorders during sleep. Treatment of OSA can reduce many symptoms and risk factors, usually to an extent that is proportional to the correction of the respiratory disorders.Citation103 It would also be important to reduce cardiovascular risk factors, including abnormal BP during sleep. For a long time, treatment of hypertensive patients has been aimed almost exclusively at maintaining normal BP levels, not rarely measured just as office diurnal BP values, which are often misleading. More recently, BP variability, including the 24-hour profile of BP, has been brought to the attention of clinicians as a possible target of treatment. A chronotherapy of hypertension, aimed at more carefully controlling nocturnal BP levels and converting a nondipping into a dipping BP profile, has been proposed as a convenient treatment strategy.Citation104
In OSA, antihypertensive drugs may reduce BP levels, but have at most a limited effect, if any, on sleep-respiratory disorders, such that they cannot substantially reduce nocturnal BP variability. Instead, elimination of respiratory disorders during sleep may be expected to prevent BP peaks and to reduce variability in BP during the night. Meta-analyses have consistently shown that on average, OSA treatment reduces BP by a small amount, with somewhat larger reduction in nocturnal levels.Citation105–Citation108 Effects are more evident in patients with severe OSA and with high compliance with treatment.Citation105,Citation107 CPAP treatment and pharmacological therapies have additive effects on BP,Citation109,Citation110 so the best strategy to treat hypertension and improve characteristics of BP variability in OSA usually includes both kinds of therapy.
A timely administration of antihypertensive drugs might reduce nocturnal BP and restore normal dipping, but few data are available on chronotherapy for altered BP in OSA. A crossover study demonstrated that when antihypertensive therapy was administered in the evening, nifedipine was more effective than carvedilol in decreasing mean and minimum nocturnal systolic BP, but only carvedilol decreased sleep BP peak.Citation111 A similar decrease in nocturnal BP peak was observed in a patient with evening administration of doxazosin.Citation112 A crossover study demonstrated that evening administration of valsartan, associated or not with amlodipine, was more effective than morning administration in reducing nocturnal BP and converting a nondipping into a dipping BP profile.Citation113 Instead, no advantage was found with evening compared to morning administration of perindopril in a randomized controlled trial conducted on hypertensive OSA patients.Citation110
Several studies, largely differing in design and objectives, have investigated effects of CPAP on BP variability (). One group of studies evaluated nocturnal BP by means of beat-by-beat measurements. They demonstrated that BP variability during sleep acutely decreased while CPAP was applied,Citation39,Citation46,Citation114 which is a consequence of a reduction in the number of BP peaks.Citation115 Furthermore, beat-by-beat BP variability before CPAP initiation was higher than immediately after CPAP withdrawal, following an approximately 5-month treatment period,Citation116 in agreement with the observation of lower postapneic BP peaks at CPAP withdrawal.Citation50 A second group of studies used ABPM and analyzed the change in 24-hour BP variability.Citation67,Citation70,Citation117–Citation123 The period of observation ranged between 1 day and 3 months, depending on the study. In all cases, some of the patients who were nondippers before CPAP initiation were found to be dippers after treatment, but the proportion of patients showing this change largely differed among studies. No effect of ≤1-week CPAP treatment on BP measurement SD during the day or night was reported.Citation86 Finally, a group of investigations evaluated BP variability with office or home BP measurements, and found some effect of CPAP treatment on waking systolic BP variability.Citation92,Citation124 In summary, CPAP treatment acutely decreases nocturnal very short-term BP variability. A few weeks of CPAP treatment may convert a nondipping into a dipping 24-hour BP profile in some patients and cause some decrease in diurnal BP variability, but more data are necessary to explore these aspects better. CPAP treatment may also decrease BP reactivity to sleep apneas,Citation50 such that acute CPAP withdrawal does not immediately revert BP variability to the level observed before treatment initiation.
Table 3 Changes in BP variability associated with CPAP treatment
Conclusion
Current knowledge suggests that BP behavior in OSA plays a role in the increased cardiovascular risk that is typical of this disorder, not only through its high diurnal and even more its nocturnal BP values but also through the characteristics of its variability. In fact, high very short-term variability, nondipping BP profile and high MBPS, which are all common in OSA, are known risk factors.
Among the characteristics of BP variability in OSA, the most peculiar is the periodic recurrence of short BP peaks. However, this is not exclusive to OSA, as nocturnal BP peaks can be observed in other conditions with periodicity, in particular in patients with periodic leg movements.Citation125 Sometimes, BP peaks reach very high levels. It is conceivable that a precise assessment of nocturnal BP that accurately takes into account values and duration of peaks carries prognostic significance. In fact, it has been found that maximum BP values measured over several days may be better related to several cardiovascular outcomes than mean values.Citation126 Today, a precise detection of hypertensive peaks is not easy in common clinical practice. Research of new monitoring systems is in progress. The new techniques appear promising,Citation56,Citation57 although they still need to be better validated and probably refined.
Once BP variability is evaluated, effective strategies should be applied to improve it. BP variability may be reduced with treatment of disordered breathing events or with a pharmacological treatment directly addressing BP. As described earlier, many studies have observed a reduction in BP variability and an improvement of BP profile with CPAP treatment, but not all findings have been in agreement. It is unknown whether differing interindividual effects of CPAP on BP variability contribute to the variable efficacy of CPAP. Knowledge on pharmacological treatment of hypertension to be preferentially used in OSA is still limited,Citation127,Citation128 and the few available data on chronotherapy for hypertension in OSA are inconsistent. Besides, more experience is required with regard to combined effects of treatment of OSA by CPAP, weight loss, or other treatments and antihypertensive pharmacological therapy.
Today, although OSA is believed to increase cardiovascular risk, cardiovascular benefits of OSA treatment are questioned, based on the results of randomized controlled trials.Citation129 Research is on the way for a personalized approach to the treatment of several diseases, including OSA.Citation130 Individual characteristics of BP variability could be one factor to take into consideration in the management of OSA patients and in the choice of a specific therapeutic approach to reduce the cardiovascular burden of the disease.
Disclosure
The authors report no conflicts of interest in this work.
References
- JavaheriSBarbeFCampos-RodriguezFSleep apnea: types, mechanisms, and clinical cardiovascular consequencesJ Am Coll Cardiol201769784185828209226
- ParatiGOchoaJELombardiCBiloGAssessment and management of blood-pressure variabilityNat Rev Cardiol201310314315523399972
- StevensSLWoodSKoshiarisCBlood pressure variability and cardiovascular disease: systematic review and meta-analysisBMJ2016354i409827511067
- StergiouGSParatiGHow to best assess blood pressure? The ongoing debate on the clinical value of blood pressure average and variabilityHypertension20115761041104221536987
- TaylorKSHeneghanCJStevensRJAdamsECNunanDWardAHeterogeneity of prognostic studies of 24-hour blood pressure variability: systematic review and meta-analysisPLoS One2015105e012637525984791
- PortaluppiFTiseoRSmolenskyMHHermidaRCAyalaDEFabbianFCircadian rhythms and cardiovascular healthSleep Med Rev201216215116621641838
- SmolenskyMHHermidaRCPortaluppiFCircadian mechanisms of 24-hour blood pressure regulation and patterningSleep Med Rev20173341627076261
- MuraliNSSvatikovaASomersVKCardiovascular physiology and sleepFront Biosci20038s636s65212700080
- ImholzBPWielingWvan MontfransGAWesselingKHFifteen years experience with finger arterial pressure monitoring: assessment of the technologyCardiovasc Res19983836056169747429
- MaggiRViscardiVFurukawaTBrignoleMNon-invasive continuous blood pressure monitoring of tachycardic episodes during interventional electrophysiologyEuropace201012111616162220837572
- DueckRGoedjeOCloptonPNoninvasive continuous beat-to-beat radial artery pressure via TL-200 applanation tonometryJ Clin Monit Comput2012262758322258303
- WheltonPKCareyRMAronowWS2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelinesHypertension20187161269132429133354
- GoldsteinDSCheshireWPJrBeat-to-beat blood pressure and heart rate responses to the Valsalva maneuverClin Auton Res201727636136729052077
- PinnaGDMaestriRla RovereMTAssessment of baroreflex sensitivity from spontaneous oscillations of blood pressure and heart rate: proven clinical value?Physiol Meas201536474175325798657
- MorganBJCrabtreeDCPuleoDSBadrMSToiberFSkatrudJBNeurocirculatory consequences of abrupt change in sleep state in humansJ Appl Physiol (1985)1996805162716368727549
- HansenTWLiYBoggiaJThijsLRichartTStaessenJAPredictive role of the nighttime blood pressureHypertension201157131021079049
- AsayamaKSchutteRLiYHansenTWStaessenJABlood pressure variability in risk stratification: what does it add?Clin Exp Pharmacol Physiol2014411823573998
- FagardRHCelisHThijsLDaytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertensionHypertension2008511556118039980
- YanoYKarioKNocturnal blood pressure and cardiovascular disease: a review of recent advancesHypertens Res201235769570122378470
- CuspidiCSalaCValerioCNegriFManciaGNocturnal hypertension and organ damage in dippers and nondippersAm J Hypertens201225886987522573011
- ManciaGFagardRNarkiewiczK2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC)J Hypertens20133171281135723817082
- AparicioLSBarochinerJCuffaroPEDeterminants of the morning-evening home blood pressure difference in treated hypertensives: the HIBA-Home StudyInt J Hypertens2014201456925925580283
- KarioKSaitoIKushiroTMorning home blood pressure is a strong predictor of coronary artery disease: the HONEST studyJ Am Coll Cardiol201667131519152727150682
- MatsuiYEguchiKShibasakiSAssociation between the morning-evening difference in home blood pressure and cardiac damage in untreated hypertensive patientsJ Hypertens200927471272019516171
- O’BrienEParatiGStergiouGAmbulatory blood pressure measurement: what is the international consensus?Hypertension201362698899424060895
- CrespoCFernándezJRAboyMMojónAClinical application of a novel automatic algorithm for actigraphy-based activity and rest period identification to accurately determine awake and asleep ambulatory blood pressure parameters and cardiovascular riskChronobiol Int2013301–2435423130607
- SallesGFReboldiGFagardRHPrognostic effect of the nocturnal blood pressure fall in hypertensive patients: the Ambulatory Blood Pressure Collaboration in Patients with Hypertension (ABC-H) meta-analysisHypertension201667469370026902495
- KarioKPrognosis in relation to blood pressure variability: pro side of the argumentHypertension20156561163116925916727
- KarioKPickeringTGUmedaYMorning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective studyCirculation2003107101401140612642361
- LiYThijsLHansenTWPrognostic value of the morning blood pressure surge in 5645 subjects from 8 populationsHypertension20105541040104820212273
- PierdomenicoSDPierdomenicoAMCoccinaFLapennaDPorrecaEPrognostic value of nondipping and morning surge in elderly treated hypertensive patients with controlled ambulatory blood pressureAm J Hypertens201730215916527838624
- IsraelSIsraelABen-DovIZBursztynMThe morning blood pressure surge and all-cause mortality in patients referred for ambulatory blood pressure monitoringAm J Hypertens201124779680121490694
- VerdecchiaPAngeliFMazzottaGDay-night dip and early-morning surge in blood pressure in hypertension: prognostic implicationsHypertension2012601344222585951
- BombelliMFodriDTosoERelationship among morning blood pressure surge, 24-hour blood pressure variability, and cardiovascular outcomes in a white populationHypertension201464594395025156170
- KarioKTime for focus on morning hypertension: pitfall of current antihypertensive medicationAm J Hypertens2005182 Pt 114915115752939
- SawatariHChishakiAAndoSIThe epidemiology of sleep disordered breathing and hypertension in various populationsCurr Hypertens Rev2016121121726778203
- MarroneORiccobonoLSalvaggioAMirabellaABonannoABonsignoreMRCatecholamines and blood pressure in obstructive sleep apnea syndromeChest199310337227778449058
- ShiomiTGuilleminaultCStoohsRSchnittgerILeftward shift of the interventricular septum and pulsus paradoxus in obstructive sleep apnea syndromeChest199110048949021914603
- AliNJDaviesRJFleethamJAStradlingJRThe acute effects of continuous positive airway pressure and oxygen administration on blood pressure during obstructive sleep apneaChest19921016152615321600769
- CoccagnaGMantovaniMBrignaniFParchiCLugaresiEContinuous recording of the pulmonary and systemic arterial pressure during sleep in syndromes of hypersomnia with periodic breathingBull Physiopathol Respir (Nancy)197285115911724348639
- TunYOkabeSHidaWNocturnal blood pressure during apnoeic and ventilatory periods in patients with obstructive sleep apnoeaEur Respir J19991461271127710624754
- LofasoFGoldenbergFd’OrthoMPCosteAHarfAArterial blood pressure response to transient arousals from NREM sleep in nonapneic snorers with sleep fragmentationChest199811349859919554636
- PlanèsCLeroyMFayetGAegerterPFoucherARaffestinBExacerbation of sleep-apnoea related nocturnal blood-pressure fluctuations in hypertensive subjectsEur Respir J200220115115712166563
- RatnavadivelRChauNStadlerDYeoAMcEvoyRDCatchesidePGMarked reduction in obstructive sleep apnea severity in slow wave sleepJ Clin Sleep Med20095651952420465017
- FindleyLJWilhoitSCSurattPMApnea duration and hypoxemia during REM sleep in patients with obstructive sleep apneaChest19858744324363979129
- SforzaECapecchiVLugaresiEHaemodynamic effects of short-term nasal continuous positive airway pressure therapy in sleep apnoea syndrome: monitoring by a finger arterial pressure deviceEur Respir J1992578588631499711
- DaviesRJCrosbyJVardi-VisyKClarkeMStradlingJRNoninvasive beat to beat arterial blood pressure during non-REM sleep in obstructive sleep apnoea and snoringThorax19944943353398202903
- SforzaELugaresiEDeterminants of the awakening rise in systemic blood pressure in obstructive sleep apnea syndromeBlood Press1995442182257496560
- LeroyMvan SurellCPilliereRShort-term variability of blood pressure during sleep in snorers with or without apneaHypertension19962869379438952580
- MarroneOSalvaggioABonsignoreMRInsalacoGBonsignoreGBlood pressure responsiveness to obstructive events during sleep after chronic CPAPEur Respir J200321350951412662010
- GroteLHeitmannJKöhlerUPenzelTPeterJHWichertPAssessment of the nocturnal blood pressure relative to sleep stages in patients with obstructive sleep apneaZ Kardiol199685Suppl 3112114
- NarkiewiczKMontanoNCogliatiCvan de BornePJDykenMESomersVKAltered cardiovascular variability in obstructive sleep apneaCirculation19989811107110779736593
- MarroneORomanoSInsalacoGBonsignoreMRSalvaggioABonsignoreGInfluence of sampling interval on the evaluation of nocturnal blood pressure in subjects with and without obstructive sleep apnoeaEur Respir J200016465365811106208
- DaviesRJJenkinsNEStradlingJREffect of measuring ambulatory blood pressure on sleep and on blood pressure during sleepBMJ199430869328208238167489
- HeudeEBourginPFeigelPEscourrouPAmbulatory monitoring of blood pressure disturbs sleep and raises systolic pressure at night in patients suspected of suffering from sleep-disordered breathingClin Sci (Lond)199691145508774259
- ShirasakiOKuwabaraMSaitoMTagamiKWashiyaSKarioKDevelopment and clinical application of a new technique for detecting ‘sleep blood pressure surges’ in sleep apnea patients based on a variable desaturation thresholdHypertens Res201134892292821614003
- KuwabaraMHamasakiHTomitaniNShigaTKarioKNovel triggered nocturnal blood pressure monitoring for sleep apnea syndrome: distribution and reproducibility of hypoxia-triggered nocturnal blood pressure measurementsJ Clin Hypertens (Greenwich)2017191303727411291
- HoffsteinVMateikaJEvening-to-morning blood pressure variations in snoring patients with and without obstructive sleep apneaChest199210123793841735259
- StradlingJRBarbourCGlennonJLangfordBACrosbyJHWhich aspects of breathing during sleep influence the overnight fall of blood pressure in a community population?Thorax200055539339810770821
- WangYYangQFengJCaoJChenBThe prevalence and clinical features of hypertension in patients with obstructive sleep apnea hypopnea syndrome and related nursing strategiesJ Nurs Res2016241414726859736
- Lavie-NevoKPillarGEvening-morning differences in blood pressure in sleep apnea syndrome: effect of genderAm J Hypertens200619101064106917027829
- MokrosŁKuczyńskiWFranczakŁBiałasiewiczPMorning diastolic blood pressure may be independently associated with severity of obstructive sleep apnea in non-hypertensive patients: a cross-sectional studyJ Clin Sleep Med201713790591028502282
- TingHLoHSChangSYPost- to pre-overnight sleep systolic blood pressures are associated with sleep respiratory disturbance, pro-inflammatory state and metabolic situation in patients with sleep-disordered breathingSleep Med200910772072518952496
- HuangYCLinCYLanCCComparison of cardiovascular co-morbidities and CPAP use in patients with positional and non-positional mild obstructive sleep apneaBMC Pulm Med20141415325257571
- McGintyDBeahmESternNLittnerMSowersJReigeWNocturnal hypotension in older men with sleep-related breathing disordersChest19889423053113396408
- WilcoxIGrunsteinRRCollinsFLDoyleJMKellyDTSullivanCECircadian rhythm of blood pressure in patients with obstructive sleep apneaBlood Press1992142192221345219
- PankowWNabeBLiesAKohlFVLohmannFWInfluence of obstructive sleep apnoea on circadian blood pressure profileJ Sleep Res19954Suppl 110210610607184
- NabeBLiesAPankowWKohlFVLohmannFWDeterminants of circadian blood pressure rhythm and blood pressure variability in obstructive sleep apnoeaJ Sleep Res19954Suppl 19710110607183
- SuzukiMGuilleminaultCOtsukaKShiomiTBlood pressure “dipping” and “non-dipping” in obstructive sleep apnea syndrome patientsSleep19961953823878843529
- AkashibaTMinemuraHYamamotoHKosakaNSaitoOHorieTNasal continuous positive airway pressure changes blood pressure “non-dippers” to “dippers” in patients with obstructive sleep apneaSleep199922784985310566903
- LoredoJSAncoli-IsraelSDimsdaleJESleep quality and blood pressure dipping in obstructive sleep apneaAm J Hypertens2001149 Pt 188789211587154
- MaYSunSPengCKFangYThomasRJAmbulatory blood pressure monitoring in Chinese patients with obstructive sleep apneaJ Clin Sleep Med201713343343927855748
- BaguetJPBoutinIBarone-RochetteGHypertension diagnosis in obstructive sleep apnea: self or 24-hour ambulatory blood pressure monitoring?Int J Cardiol201316752346234723176771
- BaguetJPLévyPBarone-RochetteGMasked hypertension in obstructive sleep apnea syndromeJ Hypertens200826588589218398330
- DragerLFDiegues-SilvaLDinizPMObstructive sleep apnea, masked hypertension, and arterial stiffness in menAm J Hypertens201023324925420019671
- NodaAOkadaTHayashiHYasumaFYokotaM24-Hour ambulatory blood pressure variability in obstructive sleep apnea syndromeChest19931035134313478486008
- NagataKOsadaNShimazakiMDiurnal blood pressure variation in patients with sleep apnea syndromeHypertens Res200831218519118360036
- SasakiNOzonoRYamauchiRThe relationship between morning hypertension and sleep quality in patients with obstructive sleep apnea syndromeClin Exp Hypertens201335425025623530964
- ChoJSIhmSHKimCJObstructive sleep apnea using Watch-Pat 200 is independently associated with an increase in morning blood pressure surge in never-treated hypertensive patientsJ Clin Hypertens (Greenwich)201517967568126033308
- SeifFPatelSRWaliaHKObstructive sleep apnea and diurnal nondipping hemodynamic indices in patients at increased cardiovascular riskJ Hypertens201432226727524351803
- NodaAYasumaFOkadaTYokotaMInfluence of movement arousal on circadian rhythm of blood pressure in obstructive sleep apnea syndromeJ Hypertens200018553954410826555
- SasakiNOzonoRYamauchiRAge-related differences in the mechanism of nondipping among patients with obstructive sleep apnea syndromeClin Exp Hypertens201234427027722559060
- LiXLiJLiuKAssociation between sleep disorders and morning blood pressure in hypertensive patientsClin Exp Hypertens201840433734328956652
- HlaKMYoungTFinnLPeppardPESzklo-CoxeMStubbsMLongitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort studySleep200831679580018548823
- MokhlesiBHagenEWFinnLAHlaKMCarterJRPeppardPEObstructive sleep apnoea during REM sleep and incident non-dipping of nocturnal blood pressure: a longitudinal analysis of the Wisconsin Sleep CohortThorax201570111062106926307037
- BaoXNelesenRALoredoJSDimsdaleJEZieglerMGBlood pressure variability in obstructive sleep apnea: role of sympathetic nervous activity and effect of continuous positive airway pressureBlood Press Monit20027630130712488649
- SteinhorstAPGonçalvesSCOliveiraATInfluence of sleep apnea severity on blood pressure variability of patients with hypertensionSleep Breath201418239740124092449
- KeXSunYYangRAssociation of 24 h-systolic blood pressure variability and cardiovascular disease in patients with obstructive sleep apneaBMC Cardiovasc Disord20171728729212465
- ShiJPiaoJLiuBObstructive sleep apnea increases systolic and diastolic blood pressure variability in hypertensive patientsBlood Press Monit201722420821228394772
- ShaoLHeizhatiMYaoXInfluences of obstructive sleep apnea on blood pressure variability might not be limited only nocturnally in middle-aged hypertensive malesSleep Breath201822237738429150775
- MartynowiczHPorębskaIPorębaRMazurGBrzeckaANocturnal blood pressure variability in patients with obstructive sleep apnea syndromeAdv Exp Med Biol201695291527562150
- LettauFSchwarzEIStradlingJRKohlerMBlood pressure variability in obstructive sleep apnoea: data from 4 randomised controlled CPAP withdrawal trialsRespiration201793531131828351060
- UlasliSSSarıaydınMGunayEEffects of nondipping pattern on systemic inflammation in obstructive sleep apneaSleep Breath20151941185119025724552
- IshikawaJHoshideSEguchiKIncreased low-grade inflammation and plasminogen-activator inhibitor-1 level in nondippers with sleep apnea syndromeJ Hypertens20082661181118718475156
- LeeSThomasRJKimHAssociation between high nocturnal blood pressure and white matter change and its interaction by obstructive sleep apnoea among normotensive adultsJ Hypertens201432102005201225023151
- SasakiNOzonoREdahiroYImpact of non-dipping on cardiovascular outcomes in patients with obstructive sleep apnea syndromeClin Exp Hypertens201537644945326395950
- GamiASHowardDEOlsonEJSomersVKDay-night pattern of sudden death in obstructive sleep apneaN Engl J Med2005352121206121415788497
- MartinsEFMartinezDda SilvaFADisrupted day-night pattern of cardiovascular death in obstructive sleep apneaSleep Med20173814415028807565
- KuniyoshiFHGarcia-TouchardAGamiASDay-night variation of acute myocardial infarction in obstructive sleep apneaJ Am Coll Cardiol200852534334618652941
- IshibashiYOsadaNSekidukaHPeak time of acute coronary syndrome in patients with sleep disordered breathingJ Cardiol200953216417019304118
- GarcíaMABlancartRGSaltLCCataluñaJJEscamillaTSánchezPRPrevalencia de trastornos respiratorios durante el sueño en pacientes con ictus isquémico agudo: influencia del momento de aparición del ictus [Prevalence of sleep-disordered breathing in patients with acute ischemic stroke: influence of onset time of stroke]Arch Bronconeumol200440519620215117618
- HsiehSWLaiCLLiuCKHsiehCFHsuCYObstructive sleep apnea linked to wake-up strokesJ Neurol201225971433143922215237
- WeaverTEMaislinGDingesDFRelationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioningSleep200730671171917580592
- HermidaRCAyalaDESmolenskyMHFernándezJRMojónAPortaluppiFSleep-time blood pressure: unique sensitive prognostic marker of vascular risk and therapeutic target for preventionSleep Med Rev201733172727316324
- FavaCDorigoniSVedoveFDEffect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta-analysisChest2014145476277124077181
- ScheinASKerkhoffACCoronelCCPlentzRDSbruzziGContinuous positive airway pressure reduces blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis with 1000 patientsJ Hypertens20143291762177324979300
- HuXFanJChenSYinYZrennerBThe role of continuous positive airway pressure in blood pressure control for patients with obstructive sleep apnea and hypertension: a meta-analysis of randomized controlled trialsJ Clin Hypertens (Greenwich)201517321522225582849
- SunYHuangZYSunQRQiuLPZhouTTZhouGHCPAP therapy reduces blood pressure for patients with obstructive sleep apnoea: an update meta-analysis of randomized clinical trialsActa Cardiol201671327528027594122
- PépinJLTamisierRBarone-RochetteGLaunoisSHLévyPBaguetJPComparison of continuous positive airway pressure and valsartan in hypertensive patients with sleep apneaAm J Respir Crit Care Med2010182795496020522795
- SerinelYYeeBJGrunsteinRRChronotherapy for hypertension in obstructive sleep apnoea (CHOSA): a randomised, double-blind, placebo-controlled crossover trialThorax201772655055827974526
- KarioKKuwabaraMHoshideSNagaiMShimpoMEffects of nighttime single-dose administration of vasodilating vs sympatholytic antihypertensive agents on sleep blood pressure in hypertensive patients with sleep apnea syndromeJ Clin Hypertens (Greenwich)201416645946624798657
- YoshidaTKuwabaraMHoshideSKarioKThe effect of the bedtime-dosing doxazosin on nocturnal hypoxia-triggered blood pressure surge in a young adult man with severe obstructive sleep apnea syndrome and a history of three recurrent sleep-onset strokesBlood Press Monit201722317317428459764
- KasiakogiasATsioufisCThomopoulosCEvening versus morning dosing of antihypertensive drugs in hypertensive patients with sleep apnoea: a cross-over studyJ Hypertens201533239340025318654
- BonsignoreMRParatiGInsalacoGBaroreflex control of heart rate during sleep in severe obstructive sleep apnoea: effects of acute CPAPEur Respir J200627112813516387945
- CarterJRFonkoueITGrimaldiDPositive airway pressure improves nocturnal beat-to-beat blood pressure surges in obesity hypoventilation syndrome with obstructive sleep apneaAm J Physiol Regul Integr Comp Physiol20163107R602R61126818059
- BonsignoreMRParatiGInsalacoGContinuous positive airway pressure treatment improves baroreflex control of heart rate during sleep in severe obstructive sleep apnea syndromeAm J Respir Crit Care Med2002166327928612153958
- EnglemanHMGoughKMartinSEKingshottRNPadfieldPLDouglasNJAmbulatory blood pressure on and off continuous positive airway pressure therapy for the sleep apnea/hypopnea syndrome: effects in “non-dippers”Sleep19961953783818843528
- DursunoğluNDursunoğluDCuhadaroğluCKiliçaslanZAcute effects of automated continuous positive airway pressure on blood pressure in patients with sleep apnea and hypertensionRespiration200572215015515824524
- Campos-RodriguezFGrilo-ReinaAPerez-RonchelJEffect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trialChest200612961459146716778262
- WangHLWangYZhangYChanges in plasma angiotensin II and circadian rhythm of blood pressure in hypertensive patients with sleep apnea syndrome before and after treatmentChin Med Sci J201126191321496417
- Martínez-GarcíaMACapoteFCampos-RodríguezFEffect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trialJAMA2013310222407241524327037
- MuxfeldtESMargalloVCostaLMEffects of continuous positive airway pressure treatment on clinic and ambulatory blood pressures in patients with obstructive sleep apnea and resistant hypertension: a randomized controlled trialHypertension201565473674225601933
- LemmerBScholtzeJSchmittJCircadian rhythms in blood pressure, heart rate, hormones, and on polysomnographic parameters in severe obstructive sleep apnea syndrome patients: effect of continuous positive airway pressureBlood Press Monit201621313614326683380
- PengoMFRatneswaranCBerryMEffect of continuous positive airway pressure on blood pressure variability in patients with obstructive sleep apneaJ Clin Hypertens (Greenwich)201618111180118427251875
- PennestriMHMontplaisirJFradetteLLavigneGColomboRLanfranchiPABlood pressure changes associated with periodic leg movements during sleep in healthy subjectsSleep Med201314655556123643655
- MatsuiYIshikawaJEguchiKShibasakiSShimadaKKarioKMaximum value of home blood pressure: a novel indicator of target organ damage in hypertensionHypertension20115761087109321536993
- KarioKObstructive sleep apnea syndrome and hypertension: mechanism of the linkage and 24-h blood pressure controlHypertens Res200932753754119461649
- ParatiGLombardiCHednerJRecommendations for the management of patients with obstructive sleep apnoea and hypertensionEur Respir J201341352353823397300
- AbuzaidASal AshryHSElbadawiAMeta-analysis of cardiovascular outcomes with continuous positive airway pressure therapy in patients with obstructive sleep apneaAm J Cardiol2017120469369928651851
- BonsignoreMRGironMCMarroneOCastrogiovanniAMontserratJMPersonalised medicine in sleep respiratory disorders: focus on obstructive sleep apnoea diagnosis and treatmentEur Respir Rev20172617006929070581
