Abstract
Introduction
Several results suggest that the frequency of dream recall is positively correlated with personality traits such as creativity and openness to experience. In addition, neuroimaging results have evidenced different neurophysiological profiles in high dream recallers (HR) and low dream recallers (LR) during both sleep and wakefulness, specifically within regions of the default mode network (DMN). These findings are consistent with the emerging view that dreaming and mind wandering pertain to the same family of spontaneous mental processes, subserved by the DMN.
Methods
To further test this hypothesis, we measured the DMN functional connectivity during resting wakefulness, together with personality and cognitive abilities (including creativity) in 28 HR and 27 LR.
Results
As expected, HR demonstrated a greater DMN connectivity than LR, higher scores of creativity, and no significant difference in memory abilities. However, there was no significant correlation between creativity scores and DMN connectivity.
Discussion
These results further demonstrate that there are trait neurophysiological and psychological differences between individuals who frequently recall their dreams and those who do not. They support the forebrain and the DMN hypotheses of dreaming and leave open the possibility that increased activity in the DMN promotes creative-thinking during both wakefulness and sleep. Further work is needed to test whether activity in the DMN is causally associated with creative-thinking.
Introduction
While dreaming has long been equated with rapid eye movement (REM) sleep, it is now well established that dreaming can occur in any sleep stage and is therefore not exclusive to a specific global vigilance state.Citation1–Citation3 As of today, no (neuro)physiological correlates of dreaming are known, which means that one cannot know for sure whether someone is actually dreaming or not at a specific moment of sleep. Consequently, most empirical investigations of dreaming have been based on dream reports collected after the awakening of the dreamer. Even then, a fundamental limitation of dream research is that the absence of a dream recall does not necessarily mean the absence of dreaming.Citation4 Dream recall is driven by both state and trait components, as described in recent integrative models of dream recall.Citation5,Citation6 Examples of state components include the sleep stage and arousal levels prior to awakening, as well as the salience of the dream, while trait components refer to person-specific factors that are associated with a higher or lower ability to recall dreams (eg, personality traits).
Focusing on the latter, prior works have highlighted several neurophysiological differences between high dream recallers (HR) and low dream recallers (LR), not only during sleep but also during wakefulness.Citation7–Citation10 A PET study from our team showed an increased regional cerebral blood flow in HR as compared to LR in the temporo-parietal junction (TPJ) and in the medial prefrontal cortex (MPFC) during REM sleep, N3 sleep and wakefulness.Citation7 In a second study conducted on an independent set of participants, we also observed an increased fMRI functional connectivity between the nodes of the DMN and memory regions 5 minutes post awakening in HR as compared to LR.Citation10 Consistent with these findings, lesions of these same areas (TPJ and MPFC) are associated with a global or partial cessation of dream recall, even in absence of any concurrent sleep disturbances.Citation11 These converging results suggest that TPJ and MPFC play a key role in dream production or recall. These two regions and the posterior cingulate cortex/precuneus (PCC) are core nodes of the default mode network (DMN), a set of functionally coupled brain regions involved in mind wandering, self-related thoughts, inferential reasoning, future envisioning and episodic memory;Citation12,Citation13 for a meta-analysis see.Citation14
The finding of a higher spontaneous activity within the DMN, along with a higher functional connectivity at awakening between its core regions in HR compared to LRCitation7,Citation10 support the hypothesis of a global differential cognitive and brain functioning between high and low dream recallers. This idea was first put forward by Schonbar,Citation15 who postulated that high dream recall is part of a general lifestyle characterized by creativity, divergent thinking and introspection. Several subsequent studies have supported this hypothesis by showing significant correlations between DRF and creativity,Citation16–Citation18 and between DRF and personality traits such as openness to experience and thin boundary structureCitation18–Citation21, with the strength of these correlations ranging from r=0.10 to r=0.40 depending on the study. These observations fit well with the emerging view that dreaming and creative-thinking both pertain to the same family of spontaneous mental processes.Citation22
Indeed, several works indicate that dreaming and creativity may both involve regions of the DMN, and especially the prefrontal areas.Citation23–Citation28 With regards to sleep, studies have found that DMN functional connectivity changes across the sleep stages,Citation29 severely weakening during deep NREM sleep (which is typically associated with no or little dream recall) and conversely increasing to a hyper-associated state during REM sleep – the sleep stage from which the proportion of dream recall upon awakening is highest. These findings have led to the hypothesis that DMN activity during sleep may be the substrate of dreaming.Citation30 Regarding creativity, a growing body of evidence suggests that creativity scores (in particular divergent thinking) positively correlate with DMN activation,Citation31,Citation32 as well as with the functional coupling between the DMN and executive regions.Citation26
In sum, the available data suggests that high dream recall frequency is related on the one hand to the activity of the DMN, and on the other hand to an increased creativity. To further test this hypothesis, we assessed DMN functional connectivity and psychological factors (personality traits, cognitive abilities and creativity) in HR and LR participants. As primary predictions, we expected to observe 1) a higher functional connectivity within the DMN in HR compared to LR, 2) higher creativity scores in HR and 3) a significant correlation between DMN connectivity and creativity. Second, based on prior results, we also expected to observe significant differences in personality between HR and LR,Citation16,Citation18 yet no difference in cognitive abilities.Citation3
Methods
This study is a reanalysis of the data published in Vallat et alCitation10,Citation33 to assess the links between DRF, DMN functional connectivity and creativity.
Participants
Behavioral and neurophysiological data were acquired from 55 healthy subjects (27 females, mean age = 22.55, standard deviation = 2.41, range = 19–29) having normal sleep characteristics and body mass index (habitual sleep time per night, 7.7 ± 0.9 hours; body mass index, 22.1 ± 2.6 kg/m2).Citation34 The subjects were informed of the study through an announcement sent to the mailing list of Lyon University, which briefly described the study and included a link to a questionnaire concerning sleep and dream habits.Citation34 Subjects were selected if they reported and subsequently confirmed during a phone interview: 1) having a high or low DRF (DRF superior to five dream recalls per week and inferior to two dream recalls per month respectively) 2) having a regular sleep-wake schedule, no difficulty to fall asleep, being occasional or frequent nappers and having preferentially already done an MRI brain scan in the past few years. Importantly, the subjects were unaware that DRF was the main criterion for inclusion in the study. Among the 55 participants, 28 of them were high dream recallers (HR; mean DRF = 6.6 ± 0.7 dream reports per week) and 27 were low dream recallers (LR; mean DRF = 0.2 ± 0.1 dream reports per week). Apart from the DRF (p < 0.001), the two groups did not significantly differ in age, sex ratio, body mass index (BMI), habitual sleep duration and education.Citation10 Participants had no history of neurological and psychiatric disorders, and had no sleep disturbances. They provided written informed consent according to the Declaration of Helsinki and received monetary compensation for their participation. The Lyon Neuroscience Research Centre (main affiliation of the authors) does not have an ethics committee, and therefore the protocol was reviewed by an independent ethics committee from the nearby cancer research and medical center (CCPPRB, Centre Leon Berard, Lyon, France). Sample size was not based on an explicit power analysis but instead on previous neuroimaging works from our group that have shown significant physiological differences between HR and LR using a smaller or similar sample size.Citation7,Citation8
Procedure
Participants arrived in the sleep unit of Le Vinatier Hospital at 8 pm the night before the scanning session. Participants had to put on an actigraphic watch (Actigraph, Pensacola, USA) upon arrival and could freely read and watch movies after the behavioral tests described below were administered by R.V. They were allowed to sleep for 3 hours between 5 and 8 am in a bed of the sleep unit. Actigraphic measures allowed us to assess time asleep and ensure that the average sleep duration of the two groups did not differ. Indeed, no between-group difference in actigraphy-derived sleep duration was observed during the 3 hours sleep slot (HR = 149.9 ± 19.2 min; LR = 155.6 ± 9.3; p = 0.164). This partial sleep deprivation was done to increase the likelihood of sleep in the MR scanner in the subsequent afternoon nap. In the morning, the participants read or were on the internet. The scanning session started after an early lunch (around 11.30 am) at the neuroimaging center (CERMEP). During the first half hour, experimenters installed an MRI compatible EEG cap (EASYCAP®) on the participant’s head. Participants were then settled in the MRI scanner at about 1.20 pm (1.17 pm ± 13 min). During the eye tracker calibration, a comic was presented. Prior to the first resting state scan, participants performed the first descending subtraction task (DST). The instructions for the resting state acquisition were to remain awake and look at a central fixation cross on the screen. At the end of the scan, participants were informed that they would be left alone without stimulation in the dark for the next 45 min and that they could sleep if they wanted to. At the end of the nap slot, participants were awakened, if they were sleeping, by calling their first name. Then, the second resting state scan was acquired just before the second DST. During the following 10 minutes, subjects were asked to recall the dream they may have had during the nap and the comic they read during the eye tracking calibration. Then, the third resting state scan and DST was performed about 25 min after awakening. Finally, an 8-min T1 anatomical scan was acquired. For more details on the experimental procedure, please refer to.Citation10,Citation33
Data Collection
MRI Acquisition
MRI scans were obtained from a MAGNETOM Prisma 3.0 T scanner (Siemens Healthcare, Erlangen, Germany) at the Primage neuroimaging platform (CERMEP). Structural MRIs were acquired with a T1-weighted (0.9-mm isotropic resolution) MPRAGE sequence and functional MRI data with a T2*-weighted 2D gradient echo planar imaging sequence (EPI) with 180 volumes (TR/TE: 2000/25 ms; flip angle: 80°; voxel size: 2.68 × 2.68 × 3 mm; slices: 40, duration: 6 minutes). Functional and anatomical scans were performed using a 20-channel head coil. The coil was foam-padded to improve subject comfort and restrict head motion. Furthermore, to ensure that the participants were not closing their eyes during the resting state scans, eye movements were monitored during the experiment using an EyeLink 1000 fMRI eye tracking system (SR Research Ontario, Canada). Eye position was calibrated at the beginning of the experiment and monitored throughout.
Behavioral Tests
BFI
The Big Five Inventory (BFI) is a self-report inventory designed to measure the Big Five personality dimensions,Citation35 which have been typically labelled as O (Openness to experience), C (Conscientiousness), E (Extraversion), A (Agreeableness), N (Neuroticism). We used the validated French version (BFI-FrCitation36); which includes 45 items presenting a collection of statements concerning interpersonal relationships and personality. Each item is scored on a 5-point Likert-type response scale, ranging from “strongly disagree” (1) to “strongly agree” (5).
STAI
The State-Trait Anxiety Inventory (STAI) is a self-report inventory consisting of 40 items pertaining to anxiety affect.Citation37 The STAI purports to measure one’s conscious awareness at two extremes of anxiety affect, labeled state anxiety (A-state), and trait anxiety (A-trait), respectively. The A-Trait and A-State scales comprise 20 items each, scored on a 4-point Likert-type response scale. Scores range from 20 to 80, with higher scores suggesting greater levels of anxiety.
PQSI
The Pittsburgh Sleep Quality Index (PQSI) is a self-rated questionnaire, which assesses sleep quality and disturbances over a one-month time interval.Citation38 It comprises 19 individual items concerning among others subjective sleep quality, sleep latency, sleep duration and daytime dysfunction. Higher scores at the PQSI indicate poorer sleep quality.
MEM-III
The MEM-III is the validated French version of the Wechsler Memory Scale (WMS-third edition, WMS-III).Citation39 We used a subtest to assess immediate and delayed memory. Participants were read two stories and asked, after each story, to say out loud everything they could remember about the story. The experimenter rated how many items the participants were able to recall (maximum 25). Twenty-five minutes later, the subjects were asked again to recall the two stories (delayed memory). The subjects were not aware that they would have to recall the stories at any point after the initial recall. For both immediate and delayed recall, scores were summed across the two stories and therefore range from 0 to 50, with higher scores reflecting greater memory performances.
Digit Span
Individual memory abilities were also assessed using a digit span task, which measures the working memory’s number storage capacity. Participants were asked to repeat a sequence of numerical digits, the length of which increases at each trial. Digit span was assessed first forwards (maximum score 16) and then backwards (maximum score 14). The digit span index was obtained by summing the two scores. Higher scores (maximum 30) indicate higher working memory abilities.
Guildford’s Alternative Uses Task
The Guildford Uses TaskCitation40 is a creativity test in which participants are asked to list as many alternative uses as possible for an everyday object. Participants were shown images of three objects (a pen, a mug, a chair) in a randomized order and subsequently asked to enumerate for 2 minutes as many alternative or unusual uses they could think of for this object. The fluency index is the total number of responses averaged across the three items. Higher scores indicate higher creativity. Additionally, we computed for each subject and each object the number of rare uses (top 10% rarest uses: uses found by 5 or less participants, top 20% rarest uses: uses found by 10 or less participants). The rare uses index is the total number of rare uses averaged across the three items. Here again, higher scores indicate higher creativity.
fMRI Data Analysis
EEG and eye tracking data were used to make sure that the participants did not sleep during any of the three resting state fMRI scans. One subject (HR) was excluded from the MRI analysis because of a technical failure during MRI acquisition, leading to a total of 27 HR and 27 LR. As the failure only concerned MRI acquisition, the behavioral and cognitive measures of this participant were still included in the analysis. For the remaining subjects, preprocessing and quality check were performed using standard routine in SPM12 software (Wellcome Department of Imaging Neuroscience). Preprocessing included functional realignment, slice-time correction, coregistration to structural scan, spatial normalization and spatial smoothing using a 6 mm full-width at half-maximum isotropic Gaussian kernel filter. Individual T1 images were segmented into gray matter, white matter and cerebrospinal fluid tissue maps. Functional and structural images were then normalized to MNI152 space (Montreal Neurological Institute). Functional images underwent artifact and motion regression in the Artifact Detection Toolbox using the following criteria to define outliers: global signal intensity changes greater than 9 standard deviations and movement exceeding 2 mm. SPM motions parameters and outliers were subsequently included as covariates in connectivity analyses.
Connectivity analyses were performed on the concatenated resting-state scans (3 scans × 6 minutes = 18 minutes resting-state data) using the CONN toolbox version 17f.Citation41 As the aim of this study was to compare the DMN connectivity during resting-state between HR and LR, we selected four main regions of interest (ROIs) corresponding to the core nodes of the DMN (see ). These include the Posterior Cingulate Cortex (PCC; center of mass in MNI coordinates: 1, −61, 38), the Medial Prefrontal Cortex (MPFC; 1, 55, −3) and bilateral Lateral Parietal cortices (LP; left: −39, −77, 33, right: 47, −67, 29). These regions are implemented within the CONN Toolbox version 17f as part of a parcellation atlas of the main brain networks, obtained by applying an independent component analysis on 467 subjects from the Human Connectome Project.
Figure 1 Increased default mode network connectivity in high dream recallers (HR) compared to low dream recallers (LR). (A) Schematic illustration of the four main nodes of the default mode network (DMN) included in the functional connectivity analysis. (B) Mean pairwise connectivity of the DMN for HR (red) and LR (black), obtained by averaging for each subject all the pairwise correlation values within the default network. The average DMN connectivity was significantly higher in HR than in LR. Error bars represent the 95% confidence intervals. *p < 0.05. (C) Left grey panel. Functional connectivity matrix representing the mean pairwise correlation coefficient between regions of the DMN in HR and LR. Right. Between-group statistical comparison (two-sided t-test corrected for multiple comparisons using the false discovery rate). The connectivity between the right lateral parietal and medial prefrontal cortex was significantly higher in HR than in LR.
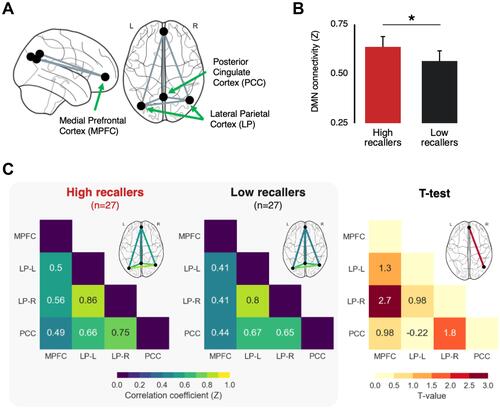
The connectivity analysis included the following steps: first, we performed a denoising step including a regression of the six motion correction parameters and their corresponding first-order temporal derivatives, as well as a component-based strategy (aCompCorCitation42 to identify and remove physiological confounds that are unlikely to be related to neural activity). The resulting BOLD time series were band-pass filtered (0.008–0.09 Hz) to further reduce noise and increase sensitivity.Citation43 Then, Pearson’s correlation coefficients were calculated for each pairwise connection across the four nodes of the DMN, resulting in a single skew-symmetric connectivity matrix with six correlation coefficients for each subject. These values were normalized using a Fisher’s r-to-Z transformation and then compared between HR and LR (two-sided t-tests corrected for multiple comparisons using the Benjamini–Hochberg method to decrease the false discovery rate (FDR, p<0.05). Finally, the average within-DMN connectivity (average of all pairwise correlations within the DMN) was calculated and compared between groups.
Statistics
As several studies reported a higher creativity in HR than in LR, between-group comparisons of the fluency index and rare uses index were achieved using one-tailed t-test (p<0.05). Similarly, as we expected HR to demonstrate a higher DMN functional connectivity than LR based on previous findings from our team, between-group comparison of the average DMN functional connectivity was achieved using a one-tailed t-test. All the other comparisons were achieved using two-sided t-tests. All statistical analyses were performed in the Pingouin 0.5.0 package for Python.Citation44
Results
Behavioral Tests
Results of the cognitive and personality tests are reported in . First, we did not find any significant differences in the memory abilities of HR and LR, as measured by the MEM-III scale, the digit span task and the recall of the comics presented in the scanner (number of words reported during free recall: HR, 230 ± 118, LR, 180 ± 98, p = 0.09; answer to the questions asking about the content of the story, on a scale from 0 to 10: HR, 5.7 ± 1.9, LR, 5.2 ± 2.5, p = 0.42). Second, there was no significant difference in the PSQI score. Third, there was no difference in the state and trait anxiety levels, as measured by the STAI self-report scale. Fourth, the Big Five personality dimensions were not significantly different between the two groups; however, there was a tendency (p=0.07) for a higher agreeableness score in HR than in LR, a dimension related to the tendency to be compassionate and cooperative rather than suspicious and antagonistic towards others.
Table 1 Between-Group Differences in Cognitive and Personality Assessments
Finally, we observed significant between-group differences in creativity scores. HR had a higher fluency index at the Guildford’s alternate uses task (HR = 8.2 ± 2.4 uses per object, LR = 7.2 ± 2.3, T(53) = 1.68, p = 0.049, Cohen’s d = 0.45; ). Furthermore, HR also reported a significantly greater number of rare uses, both when considering the 10% rarest uses (HR = 3.1 ± 1.6 rare uses per object, LR = 2.3 ± 1.5, T(53) = 1.77, p = 0.041, d = 0.48) and the 20% rarest uses (HR = 4.4 ± 1.9 rare uses per object, LR = 3.5 ± 1.8, T(53) = 1.88, p = 0.033, d = 0.51; ).
Figure 2 Creativity score in high dream recallers (HR) and low dream recallers (LR). (A) Box plot of the average number of uses per object found by HR (red) and LR (black) during the Guildford’s alternate uses task (also referred to as the fluency index). HR reported significantly more uses than LR. (B) Box plot of the average number of uses reported by 10 or less participants per object (ie top 20%) found by HR (red) and LR (black). HR reported significantly more rare uses than LR. (C) Significant correlation between the fluency index at the Guildford’s task and the openness to experience personality dimension measured using the BFI questionnaire. All plots share the same y-ticks, ranging from 0 to 16. *p<0.05.
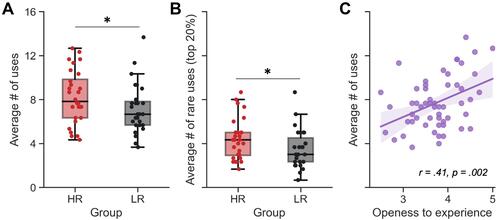
As could be expected from previous results,Citation45 creativity was also positively correlated with the “openness to experience” personality dimension of the BFI questionnaire (fluency index: r = 0.41, p = 0.002, ; top 20% rarest uses: r = 0.42, p = 0.001; top 10% rarest uses: r = 0.33, p = 0.014; Supplementary Figure 1). Noteworthy, these significant associations were mostly driven by the HR group. Indeed, the correlations between openness to experience and creativity measures were significant in the HR group (all ps <0.017), but not in the LR group (all ps >0.14; Supplementary Figure 1).
Functional Connectivity
The mean DMN functional connectivity in the scans of the 3 time points considered was higher in HR than in LR (HR = 0.64 ± 0.14, LR = 0.56 ± 0.16, T(52) = 1.81, p = 0.038; ). The DMN connectivity matrices for each group with pairwise connectivity color coded by strength are presented in . Between-group comparisons of the connectivity matrices indicated a greater connectivity in HR between the MPFC and right LP (T(52) = 2.70, p-FDR = 0.028, p-unc = 0.009).
We did a second analysis using only the resting state scan 1 and 3 to test that the group effect was not driven by the scan acquired at awakening during possible sleep inertia (resting state scan 2, see).Citation10 We found that the between-group difference in average DMN connectivity was no longer significant (p=0.24), but the functional connectivity between the MPFC-LP was very close to significance (p-FDR=0.0537).
We then tested whether increased DMN connectivity predicts higher creativity scores. There was no significant correlation between the average DMN connectivity and any of the creativity scores (fluency index, r = −0.002, p = 0.99; top 20% rarest uses: r = 0.036, p = 0.080, top 10% rarest uses: r = 0.11, p = 0.45). This was also true when looking separately at HR and LR (all p’s > 0.325).
Discussion
Consistent with our predictions, HR demonstrated an increased functional connectivity within the DMN – specifically between the MPFC and TPJ – and scored higher than LR on measures of creative-idea generation. Furthermore, no other between-group differences in cognitive abilities (including memory) were observed. However, contrary to our third prediction, there was no significant correlation between DMN connectivity and creativity scores.
With regards to functional connectivity, our results are consistent with a previous PET study from our team that showed, in an unrelated sample of 41 participants, a higher cerebral blood flow in HR compared to LR in these two same regions during both sleep and wakefulness.Citation7 Both these studies are also in line with clinical reports showing that lesions in the TPJ and MPFC lead to a cessation of dream recall.Citation11 These converging results provide therefore strong evidence that the ability to recall dreams is linked to the activity within these two brain regions. In line with these results, several other studies of our team evidenced that HR and LR have a differential neurophysiological profile. We showed that HR have a larger amplitude of brain responses to auditory novel stimuli during both sleep and wakefulness, as well as an increased duration of intra-sleep wakefulness and nocturnal awakenings, regardless of sleep stage, and without any other differences in the micro- or macro-structure of sleep.Citation8,Citation9 We also recently evidenced an increase of neurophysiological markers of bottom-up and top-down attentional processes in HR during wakefulness.Citation46
Our findings also confirm previous studies reporting an association between creativity and DRF.Citation16–Citation18 The effect size that we observed in this sample (Cohen’s d ≈ 0.50, which translates to a correlation coefficient of r=0.24) is consistent with prior reports (ranging from r=0.10 to r=0.40). This association between creativity and dream recall is of particular interest given that the generation of creative ideas is thought to be supported by a preferential recruitment of regions of the DMN, and particularly of the MPFC.Citation24–Citation28,Citation47 This large overlap of brain regions involved in dreaming and creativity was noticed by ChristoffCitation22 who proposed that creative thought and dreaming are part of the same “family of spontaneous-thought processes”. In the same direction, BarrettCitation48 recalled a long-standing hypothesis that “dreaming is essentially our brain thinking in another neurophysiologic state – and therefore it is likely to solve some problems on which our waking minds have become stuck”. Several historical anecdotes and studies have indeed reported that dream content per se often contains solutions of unsolved problems and can be a source of insight.Citation49–Citation52
Our results strengthen previous findings showing for the first time in the same study increased creativity and DMN connectivity in HR vs LR. Building on these results and previous ones, we argue here that high dream recallers have a specific cognitive and brain functioning profile, involving greater baseline activity and functional connectivity in regions of the DMN, which might in turn promote creativity and dreaming abilities in these individuals.
Along with the consistent positive association between DRF and creativity, studies have often reported a substantial correlation between creativity and openness to experienceCitation45 and between DRF and several personality traits, including openness to experience,Citation21 thin boundaries (ie, propensity to being more open, trustworthy, vulnerable, and sensitive;Citation19,Citation20 and anxiety).Citation53,Citation54 While we could replicate the correlation between creativity and openness to experience in our sample, we did not observe a statistically significant difference in personality traits between HR and LR. It may be explained by the fact that the association between DRF and personality traits is not ubiquitous in the literature (eg,Citation55), and, when present, is often very weak (r≈0.1–0.2).Citation21 Therefore, our study may have been underpowered to detect such subtle personality differences between HR and LR.
The general idea that differential DRF is linked to traits factors was first introduced by SchonbarCitation15 in her so-called lifestyle hypothesis. While she did not explain the underlying mechanisms, she postulated that high dream recall is part of a general lifestyle characterized by “creativity, rich fantasy, introversion, introspection, field independence and divergent thinking”.Citation56 Our findings of a higher creativity and DMN connectivity in HR compared to LR argue in favor of this model. However, and contrary to our initial hypothesis, we did not find a significant correlation between creativity and DMN connectivity. Such a null finding does not prove the definite lack of an association between DMN and creativity. Our study may have been underpowered to evidence such an effect, especially if the latter is only present in HR, as observed for the correlation between openness to experience and creativity measures. Further research is therefore needed to confirm or refute the theory that increased basal DMN connectivity in HR may promote creativity (for example, with a larger sample size and/or with other measures of creativity). Moreover, future works could test whether DRF-enhancing methods (such as keeping a dream diary;Citation57) would result in increased creativity scores and DMN functional connectivity in a pre/post design within the same individuals, preferentially low dream recallers. If confirmed, DRF-enhancement methods could potentially become a creativity-enhancement method.
Limitations
First, an important limitation of this study is that the participants were partially sleep-deprived during all the resting-state fMRI scans, and possibly in a severe state of sleep inertia during the second resting-state scan. A previously published analysis of the same dataset has shown that HR have increased DMN functional connectivity compared to LR during the second resting-state scan.Citation10 We therefore ran a second analysis excluding the second resting state scan, which revealed that the group difference in average DMN functional connectivity was not significant anymore – although the pairwise connectivity between the MPFC and LP was close to significance. This indicates that the main effect of a higher DMN connectivity in HR is only present when including the second resting-state scan, which likely contains a high carry-over effect from prior sleep. This would suggest that the main effect of higher DMN activity in HR is more pronounced during sleep and/or the awakening process rather than during fully installed wakefulness. However, a previous study from our team on an unrelated sample has found a higher PET activation in the DMN in HR compared to LR during both sleep and resting wakefulness,Citation7 which supports the idea of a higher basal DMN activity in HR regardless of the vigilance state. Still, it remains to be tested whether the observed between-group differences in fMRI DMN connectivity replicate when participants are not sleep deprived during the resting-state scans. According to the available data, it may be hypothesized that DMN connectivity is increased in HR, and that this effect is maximum during sleep and/or when sleep intrudes into wakefulness.
Second, creativity was assessed using the well-validated task of divergent thinking (the Alternate Use Task [AUT],Citation40). Prior works on DRF and creativity have used different tasks or questionnaires,Citation16,Citation17 and one strength of this study was thus to replicate previous results with a new/different measure of creativity. However, using several tasks to assess creativity (eg, Remote Associates Test to measure convergent thinking, or questionnaires) may have led to a fuller picture of the association between DMN connectivity and creativity. Indeed, creativity is certainly a complex and multifaceted ability that involves several cognitive processes and traits (eg, memory, associative reasoning, flexibility, etc) combined in various proportions across individuals. The simple task that was used in this study may thus not have captured the proper/pertinent variable related to DMN connectivity.
Abbreviations
REM, rapid eye movement; HR, high dream recallers; LR, low dream recallers; DRF, dream recall frequency; DMN, default mode network; MPFC, medial prefrontal cortex; TPJ, temporo-parietal junction; LP, lateral parietal; PCC, posterior cingulate cortex.
Data Sharing Statement
Data and Python scripts are available from the corresponding author upon request.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank Morgane Hamon, Franck Lamberton and Danielle Ibarrola for substantial help in data collection and analysis, as well as Jamila Lagha for her help in administrative work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Funding
References
- Solms M. Dreaming and REM sleep are controlled by different brain mechanisms. Behav Brain Sci. 2000;23(6):843–850;discussion 904–1121. doi:10.1017/S0140525X00003988
- Schwartz S, Dang-Vu TT, Ponz A, Duhoux S, Maquet P. Dreaming: a neuropsychological view. Schweizer Archiv Neurologie Psychiatrie. 2005;156(8):426–439.
- Ruby PM. Experimental research on dreaming: state of the art and neuropsychoanalytic perspectives. Front Psychol. 2011;2:286. doi:10.3389/fpsyg.2011.00286
- Ruby P. The neural correlates of dreaming have not been identified yet. Commentary on “The neural correlates of dreaming. Nat Neurosci. 2017.” Front Neurosci. 2020;14:1109. doi:10.3389/fnins.2020.585470
- Schredl M. Researching Dreams: The Fundamentals. Springer International Publishing; 2018.
- Vallat R. Content and Frequency of Dream Reports: Psychological and Neurophysiological Correlates. Université de Lyon; 2017.
- Eichenlaub J-B, Nicolas A, Daltrozzo J, Redouté J, Costes N, Ruby P. Resting brain activity varies with dream recall frequency between subjects. Neuropsychopharmacology. 2014;39(7):1594–1602. doi:10.1038/npp.2014.6
- Eichenlaub J-B, Bertrand O, Morlet D, Ruby P. Brain reactivity differentiates subjects with high and low dream recall frequencies during both sleep and wakefulness. Cereb Cortex. 2014;24(5):1206–1215. doi:10.1093/cercor/bhs388
- Vallat R, Lajnef T, Eichenlaub J-B, et al. Increased evoked potentials to arousing auditory stimuli during sleep: implication for the understanding of dream recall. Front Hum Neurosci. 2017;11(132):132. doi:10.3389/fnhum.2017.00132
- Vallat R, Nicolas A, Ruby P. Brain functional connectivity upon awakening from sleep predicts interindividual differences in dream recall frequency. Sleep. 2020;43:12. doi:10.1093/sleep/zsaa116
- Solms M. The Neuropsychology of Dreams: A Clinico-Anatomical Study. L. Erlbaum Associates; 1997.
- Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi:10.1038/35094500
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi:10.1073/pnas.98.2.676
- Legrand D, Ruby P. What is self-specific? Theoretical investigation and critical review of neuroimaging results. Psychol Rev. 2009;116(1):252–282. doi:10.1037/a0014172
- Schonbar RA. Differential dream recall frequency as a component of“ life style”. J Consult Psychol. 1965;29(5):468. doi:10.1037/h0022464
- Fitch T, Armitage R. Variations in cognitive style among high and low frequency dream recallers. Pers Individ Dif. 1989;10(8):869–875. doi:10.1016/0191-8869(89)90022-6
- Schredl M. Creativity and dream recall. J Creat Behav. 1995;29(1):16–24. doi:10.1002/j.2162-6057.1995.tb01420.x
- Schredl M, Wittmann L, Ciric P, Götz S. Factors of home dream recall: a structural equation model. J Sleep Res. 2003;12(2):133–141. doi:10.1046/j.1365-2869.2003.00344.x
- Hartmann E. Boundaries of dreams, boundaries of dreamers: thin and thick boundaries as a new personality measure. Psychiatr J Univ Ott. 1989;14(4):557–560.
- Schredl M, Kleinferchner P, Gell T. Dreaming and personality: thick vs. thin boundaries. Dreaming. 1996;6(3):219–223. doi:10.1037/h0094456
- Schredl M, Ciric P, Götz S, Wittmann L. Dream recall frequency, attitude towards dreams and openness to experience. Dreaming. 2003;13(3):145–153. doi:10.1023/A:1025369311813
- Christoff K, Irving ZC, Fox KCR, Spreng RN, Andrews-Hanna JR. Mind-wandering as spontaneous thought: a dynamic framework. Nat Rev Neurosci. 2016;17(11):718–731. doi:10.1038/nrn.2016.113
- William domhoff G. The neural substrate for dreaming: is it a subsystem of the default network? Conscious Cogn. 2011;20(4):1163–1174. doi:10.1016/j.concog.2011.03.001
- Ellamil M, Dobson C, Beeman M, Christoff K. Evaluative and generative modes of thought during the creative process. Neuroimage. 2012;59(2):1783–1794. doi:10.1016/j.neuroimage.2011.08.008
- Jung RE, Mead BS, Carrasco J, Flores RA. The structure of creative cognition in the human brain. Front Hum Neurosci. 2013;7:330. doi:10.3389/fnhum.2013.00330
- Beaty RE, Benedek M, Wilkins RW, et al. Creativity and the default network: a functional connectivity analysis of the creative brain at rest. Neuropsychologia. 2014;64:92–98. doi:10.1016/j.neuropsychologia.2014.09.019
- Mok LW. The interplay between spontaneous and controlled processing in creative cognition. Front Hum Neurosci. 2014;8:663. doi:10.3389/fnhum.2014.00663
- Beaty RE, Benedek M, Kaufman SB, Silvia PJ. Default and executive network coupling supports creative idea production. Sci Rep. 2015;5(1):10964. doi:10.1038/srep10964
- Picchioni D, Duyn JH, Horovitz SG. Sleep and the functional connectome. Neuroimage. 2013;80:387–396. doi:10.1016/j.neuroimage.2013.05.067
- Domhoff GW, Fox KCR. Dreaming and the default network: a review, synthesis, and counterintuitive research proposal. Conscious Cogn. 2015;33:342–353. doi:10.1016/j.concog.2015.01.019
- Wei D, Yang J, Li W, Wang K, Zhang Q, Qiu J. Increased resting functional connectivity of the medial prefrontal cortex in creativity by means of cognitive stimulation. Cortex. 2014;51:92–102. doi:10.1016/j.cortex.2013.09.004
- Takeuchi H, Taki Y, Hashizume H, et al. The association between resting functional connectivity and creativity. Cereb Cortex. 2012;22(12):2921–2929. doi:10.1093/cercor/bhr371
- Vallat R, Meunier D, Nicolas A, Ruby P. Hard to wake up? The cerebral correlates of sleep inertia assessed using combined behavioral, EEG and fMRI measures. Neuroimage. 2019;184:266–278. doi:10.1016/j.neuroimage.2018.09.033
- Vallat R, Eskinazi M, Nicolas A, Ruby P. Sleep and dream habits in a sample of French college students who report no sleep disorders. J Sleep Res. 2018;27(5):e12659. doi:10.1111/jsr.12659
- John OP, Srivastava S. The Big Five trait taxonomy: history, measurement, and theoretical perspectives. Handbook Personal. 1999;2(1999):102–138.
- Plaisant O, Courtois R, Réveillère C, Mendelsohn GA, John OP. Factor structure and internal reliability of the French Big Five Inventory (BFI-Fr). Convergent and discriminant validation with the NEO-PI-R. In: Annales Médico-Psychologiques, Revue Psychiatrique. Vol. 168. Elsevier; 2010:97–106.
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970.
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
- Wechsler D. Wechsler Memory Scale (WMS-third edition, WMS-III). MEM-III: Échelle Clinique de Mémoire de Wechsler. Éditions du Centre de psychologie appliquée; 2001.
- Guildford JP, Christensen PR, Merrifield PR, Wilson RC. Alternate Uses: Manual of Instructions and Interpretation. Orange, CA: Sheridan Psychological Services; 1978.
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi:10.1089/brain.2012.0073
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi:10.1016/j.neuroimage.2007.04.042
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47(4):1408–1416. doi:10.1016/j.neuroimage.2009.05.005
- Vallat R. Pingouin: statistics in Python. JOSS. 2018;3(31):1026. doi:10.21105/joss.01026
- Vartanian O, Wertz CJ, Flores RA, et al. Structural correlates of openness and intellect: implications for the contribution of personality to creativity. Hum Brain Mapp. 2018;39(7):2987–2996. doi:10.1002/hbm.24054
- Ruby P, Masson R, Chatard B, et al. High dream recall frequency is associated with an increase of both bottom-up and top-down attentional processes. Cereb Cortex. 2021. doi:10.1093/cercor/bhab445
- Dietrich A, Kanso R. A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychol Bull. 2010;136(5):822–848. doi:10.1037/a0019749
- Barrett D. Dreams and creative problem-solving. Ann NY Acad Sci. 2017;1406(1):64–67. doi:10.1111/nyas.13412
- Dement WC. Some Must Watch While Some Must Sleep. Vol. 37. San Francisco Book Co.; 1976.
- Barrett D. The “committee of sleep”: a study of dream incubation for problem solving. Dreaming. 1993;3(2):115–122. doi:10.1037/h0094375
- Maquet P, Ruby P. Psychology: insight and the sleep committee. Nature. 2004;427(6972):304–305. doi:10.1038/427304a
- Edwards CL, Ruby PM, Malinowski JE, Bennett PD, Blagrove MT. Dreaming and insight. Front Psychol. 2013;4:979. doi:10.3389/fpsyg.2013.00979
- Schonbar RA. Some manifest characteristics of recallers and nonrecallers of dreams. J Consult Psychol. 1959;23(5):414. doi:10.1037/h0047400
- Tart CT. Frequency of dream recall and some personality measures. J Consult Psychol. 1962;26(5):467–470. doi:10.1037/h0046056
- Schredl M. Dream recall frequency and openness to experience: a negative finding. Pers Individ Dif. 2002;33(8):1285–1289. doi:10.1016/S0191-8869(02)00013-2
- Schredl M. Dream recall: research, clinical implications and future directions. Sleep Hypn. 1999;1(2):72–81.
- Schredl M. Questionnaires and diaries as research instruments in dream research: methodological issues. Dreaming. 2002;12(1):17–26. doi:10.1023/A:1013890421674
