Abstract
Purpose
Heart rate variability (HRV) indices have been used as stress indicators. Rare studies investigated the associations of circadian rhythms of the HRV indices with the stress, mood, and sleep conditions in populations under stress.
Methods
In total 257 female participants (203 shift workers and 54 non-shift workers) were included. All the participants completed a structured questionnaire to assess the stress, mood, and sleep conditions and performed 24-hour Holter electrocardiogram monitoring on the day away from shifts. Using epochs of 1-min or 5-min beat-to-beat intervals, the HRV indices (SDNN, RMSSD, LF, HF, LF/HF, and LFnu, SD1, SD2, SD1/SD2) were plotted as a function of time and fitted into cosine periodic curves, respectively. Three mathematical parameters based on the cosine periodic curves were extracted, MESOR (M, overall averages of the cosine curve), amplitude (A, amplitude of the peak of the cosine curve), and acrophase (θ, latency to the peak) to quantify the circadian rhythms of the HRV indices. Multivariable linear regression models were used to reveal the associations of these parameters with the clinical assessments of stress, mood, or sleep conditions, as well as with the 24-h averages of the HRV indices.
Results
The parameters M and A of SDNN, RMSSD, LF, and HF, and θ of LF/HF and LFnu significantly differ between shift and non-shift workers. The parameter θ of LF/HF positively correlates with the severity of stress and anxiety. The parameter A of LF/HF and LFnu also positively correlates with daytime sleepiness and sleep fragmentation. In addition, the parameters M and A instead of θ of SDNN, RMSSD, LF, LF/HF, and LFnu significantly correlate with the 24-h averages of HRV indices.
Conclusion
The circadian rhythms of the HRV indices over 24 hours can, to some extent, predict the severity of stress, emotion and sleep conditions in female populations under stress.
Introduction
Medical workers are exposed to constant work-related stressors, especially those with night shifts. Chronic stress could induce serious health issues, such as insomnia, mood disorders, sleep disturbances, cognitive decline, cardiovascular diseases, and metabolic disturbances.Citation1–4 Shift work impairs the physiological circadian rhythms of metabolisms, the release of hormones and neurotransmitters.Citation5–7 Heart rate variability (HRV) quantifies the variation of the cardiac beat-to-beat (RR) intervals and reflects the autonomic nervous system function.Citation8 Numerous studies have reported the tight connections of the HRV indices and stress, although the conclusions were diversified.Citation9–11 For instance, the HRV indices, low frequency (LF) power and high frequency (HF) power, were both found lower in young populations under short-term stress.Citation12 However, another study reported the acute stress increases LF power and decreases HF power.Citation13 The discrepancies could be due to multiple factors, such as sex, age, the time of day or the conditions when conducting cardiac signals monitoring that can instantly affect the HRV indices.
The physiological function of autonomic nervous system (ANS) fluctuates across a circadian day, the vagal nerve activity usually peaks at the late phase of night-time sleep and is suppressed during wakefulness due to external stimuli.Citation14 By plotting the HRV indices over 24-hour recording in 5-min epochs, HF and the LF/HF ratio both exhibited circadian rhythms.Citation14 Researchers fit the data points of the HRV indices distributing over 24 hours with a cosine periodic curve to exemplify their circadian features. The cosine curves for HF and RMSSD peak during the midnight, while the cosine curve for LF/HF peaks in the afternoon.Citation15 HF is a widely used index of cardiac vagal modulation.Citation16 If breathing at normal rates, the natural log transformed HF power (Ln HF) can be used to estimate putative vagal tone under certain conditions.Citation17,Citation18 HF decreases encountering tasks in need of sustained attention to perceived stress and recovers to the normal level after the tasks were over.Citation19 It makes it is reasonable that HF strongly follows circadian rhythms under physiological conditions.
In the current study, we were interested to analyze the circadian rhythms of the HRV indices following the published methods.Citation15,Citation20 For the individual HRV index, three mathematical parameters were extracted from the fitted cosine periodic curve. MESOR (M, overall averages of the cosine curves of HRV indices), amplitude (A, amplitude of the peak of the cosine curve), and acrophase (θ, latency to the peak from the reference timepoint) to quantify the circadian rhythms of the HRV indices.Citation21,Citation22
Systemic inflammation was found to strongly correlate with the parameters M and A of the HRV indices (HF, LF, RMSSD, SDNN).Citation23 In addition, the presence of type 2 diabetes correlates with the parameter θ of the HRV index RMSSD.Citation24 However, rare studies have explored the association of these parameters with the stress, mood, and sleep status. In this study, we applied the multivariable linear regression models to reveal the associations of the parameters (M, A, θ) of the HRV indices with the subjectively evaluated stress, mood, or sleep conditions. At the meantime, we also tested the correlations between these parameters resulted from the two-dimensional analysis of the HRV indices and the 24-hour averages of the HRV indices. We hope this can help to develop the novel approaches of validating the subjective evaluations of the stress, emotion and sleep conditions of the stress populations.
Participants and Methods
Participants
Data were collected in the autumn of 2021 at two large general hospitals in Wuhan City, China. We targeted hospital staff working night shifts. Potential participants, mainly from clinical units (intensive care units, emergency units, and night urgency operating rooms), were informed about this study through staff meetings and flyers. The inclusion criteria for this study were as follows: (1) working night shift; (2) willingness to participate in this study and sign the consent form; (3) age < 50 years; (4) body mass index (BMI) < 30 kg/m2; (5) no medical conditions that may affect cardiac pulse, such as hypertension, diabetes, asthma, cardiovascular disease, cancer, and thyroid disease; and (6) history of medication that does include drugs that may affect cardiac function or mental status, such as β-blockers. Finally, a total of 203 women with night shifts were included into the study analysis. Meanwhile, a small pilot study of 54 women without night shifts was also conducted. Demographic characteristics, including age, marital status, educational levels, work experience, jobs, family income, weekly working hours, subjective health status, and medical diagnosis, were self-reported in both shift workers and non-shift workers.
Clinical Assessment of Stress, Depression and Anxiety
The conditions of stress, anxiety, and depression, were evaluated with two clinical scales, the Perceived Stress Scale-14(PSS-14) and Depression-Anxiety-Stress Scale-21 (DASS-21) (Chinese versions).Citation25,Citation26 The PSS-14 measures the level of perceived stress from two aspects: the feeling of tension and the sense of lack of control. Each item of the in-total 14 items in PSS-14 is scored from 0 to 4, and the total score ranges from 0 to 56. Higher scores of PSS-14 indicate the greater levels of the perceived stress. According to the previous literature, the median PSS score was used as the criteria for defining the elevated perceived stress levels (PSS ≥ 30 points in the study).Citation25
The DASS-21 contains 21 items that can be clustered into three subscales to evaluate depression, anxiety, and stress, respectively.Citation26 The stress subscale (DASS-S) of DASS-21, contains 7 items. Each item is rated on a 4-point Likert scale and the sum score is multiplied by two, with the final score ranging from 0 to 42. A total score of 10-point was considered the upper limit of normal stress.Citation27
The depression subscale (DASS-D) of DASS-21, containing 7 items, assesses the depression levels. A total score of 6-point was the upper limit of the normal range.Citation27 The anxiety subscale (DASS-A) of DASS-21, containing 7 items, assesses the severity of anxiety. A total score of 9-point was considered the upper limit of the normal level.Citation27
Assessment of Sleep Status
Sleep condition was evaluated using the Excessive Sleep Scale (ESS) score, Fatigue Assessment Scale (FAS) score, and self-reported sleep quality in the structured questionnaire. The ESS is used to evaluate daytime sleepiness.Citation28 This scale consists of eight items, and each item is rated on a 4-point Likert scale. A total score higher than 10-point (>10 points) was usually considered to be abnormal.Citation28 The FAS, containing ten items that are rated on a 5-point Likert scale, was adopted to measure the levels of fatigue.Citation29 The FAS scores higher than 21 (>21 points) indicated mild-to-severe fatigue. In the structured questionnaire, the participants were asked to report the self-evaluated sleep quality, which was encoded with 1–5 points: poor=1, relatively poor=2, tolerable =3, relatively good=4, and good=5. The score less than 3-point was considered as poor sleep quality.
A subgroup of 80 participants (all working night shifts) wore Huawei smart devices to collect photoplethysmography (PPG) signals. The PPG signals contain details of beat-to-beat variability and breath-to-breath dynamics.Citation30 The cardiopulmonary coupling (CPC) algorithm was applied for sleep scoring according to the previous literature.Citation31 The data processing and sleep scoring was performed by the HUAWEI Research team. The CPC algorithm combines respiratory signals and R-R intervals (the time between ventricular depolarizations), and calculates the product of the cross-spectral power of the two signals. The CPC algorithm has been proved to have a high interscorer reliability (kappa > 0.75) in cyclic alternating pattern detection which is a measure of sleep instability.Citation31 Episodes with high-frequency coupling (HFC, 0.1–0.4 Hz) are defined as stable sleep (non-CAP), episodes with low-frequency coupling (LFC, 0.01–0.1 Hz) are defined as unstable sleep, and episodes with very low-frequency coupling (<0.01 Hz) are defined as wakefulness or rapid eye movement (REM) sleep.Citation31 The sleep bouts is the summed episodes of stable sleep, unstable sleep and wakefulness or REM sleep, indicating the severity of sleep fragmentation.
Continuous Holter ECG Recording and HRV Indices Analysis
Twenty-four-hour Holter ECG recordings were obtained using an ambulatory ECG device (DMS300-4, USA). ECG signals were visually inspected by two experts in the field using the DMS software to manually remove artifacts. Thereafter, the processed 24-h ECG signals were then used for a frequency power analysis (LF, low-frequency power, 0.04–0.15 Hz; HF, high-frequency power, 0.15–0.40 Hz), or time-domain analysis (SDNN, standard deviation of all normal intervals; RMSSD, the square root of the mean of the sum of the squares of differences between adjacent normal intervals). The frequency power analysis was based on the fast Fourier transform (FFT) method.Citation32 The LF/HF ratio and normalized LF power (LFnu, LF/(LF+HF)) were also calculated. In addition, nonlinear HRV indices of SD1, SD2, and SD1/SD2 derived from Poincare plots were also extracted, as previously described.Citation33 Poincare plots are a kind of scatter plots, which are graphed by plotting every R–R interval (time period between successive heartbeats) against the prior interval. SD1 (minor axis of the cloud) is the standard deviation of short-term R-R-interval variability, SD2 (major axis of the cloud) is the standard deviation of long-term R-R-interval variability, and SD1/SD2 is the axis ratio.Citation34
The Circadian Rhythm Analysis of the HRV Indices
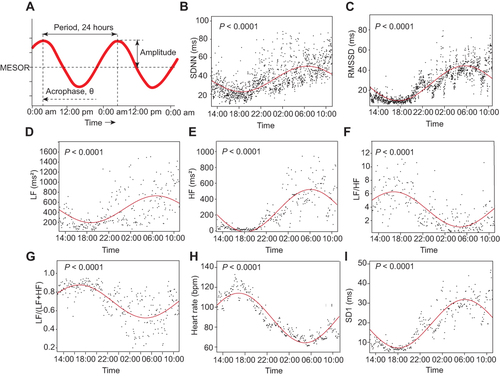
The entire 24-h normal beat-to-beat RR interval data were divided into 1-min or 5-min epochs of RR data for further circadian rhythm analysis.Citation15 The epoch of 5-min was applied for frequency power analysis (LF, HF) and non-linear HRV indices analysis (SD1, SD2, SD1/SD2) (288 epochs over 24 hours), and the epoch of 1-min was used for time-domain measures analysis (SDNN, RMSSD) (1440 epochs over 24 hours). The LF/HF ratio, LFnu, and heart rates per minute were also extracted. The distribution of the epoch-based data points of above HRV indices over 24 hours were then fitted to the cosine curves based on a single-component cosinor model: y=M+A1*sin (t P/i · 2π) + A2*con (t P/i · 2π).Citation35 From the cosine curves, three parameters were extracted, including MESOR (M, Midline Estimating Statistic OF Rhythm), amplitude (A) and acrophase (θ), as shown in . M, MESOR, is a rhythm-adjusted mean that reflects the overall average of the HRV index; A, amplitude, the height of the maximum oscillation to the midline, and double amplitude represents the extent of rhythmic fluctuation within a cycle; acrophase, θ, is the timing of the zenith occurring in each cycle (the reference time point 12:00 AM was set as the value of 0), namely the time lag.Citation21,Citation22 A F-test based on the model sum of squares and sum of square residuals and a zero amplitude test were performed to detect whether the cosine periodic regression curve was significant.Citation15,Citation21,Citation35 Non-significant circadian curves of individual participants were excluded from further analysis. Approximately 67 of the 257 cosine periodic regression curves of the nonlinear HRV index, SD2, showed no significance. Consequently, we did not perform further analysis with SD2 and SD1/SD2.
Figure 1 HRV circadian rhythm analysis. Definition of circadian rhythm parameters (A). The 1440 or 288 data points (black dots) extracted from 1-min (SDNN, RMSSD) or 5-min (LF, HF, LF/HF ratio, and LFnu) epochs of 24-hour ECG signals. The red lines are the circadian rhythm fitting curves of SDNN (B), RMSSD(C) and LF(D), HF(E), LF/HF ratio(F), LFnu(G), heart rate(H) and SD1(I) from one individual, respectively. MESOR, M, is a rhythm-adjusted mean that reflects the overall average of the HRV index; A, amplitude, the height of the maximum oscillation to the midline, and double amplitude represents the extent of rhythmic fluctuation within a cycle; θ, acrophase, is the timing of the zenith occurring in each cycle (the reference time point 12:00 AM was set as the value of 0). The significance of the cosine periodic rhythm curves was tested, indicated by the P value (All P < 0.0001).
Based on the above cosinor model: y=M+A1*sin (t P/i · 2π) + A2*con (t P/i · 2π), the mathematical equations of calculating A and θ were listed below.
Statistical Analysis
We compared the baseline characteristics, the scores of PSS, DASS-21 subscales of stress, anxiety, and depression, ESS, FAS, 24-hour heart rate, and 24-hour HRV indices (SDNN, RMSSD, LF, HF, LF/HF, LFnu, SD1, SD2, and SD1/SD2) between the shift workers and non-shift workers using the Mann Whitney Wilcoxon test.
The three parameters (M, A, θ) of each HRV index (SDNN, RMSSD, LF, HF, LF/HF, LFnu, SD1) and heart rate were compared between shift workers and non-shift workers using the Mann Whitney Wilcoxon Test.
We used multivariable linear regression models to assess the associations of the severity of perceived stress with the circadian rhythm features (M, A, θ) of each HRV index (SDNN, RMSSD, LF, HF, LF/HF, LFnu, SD1), as well as the heart rate in shift workers. The shift workers with PSS score higher than 30 (including 30, ≥ 30 points) were classified as the stress group, while the ones with PSS score less than 30 points were classified into the reference group. Age (continuous), BMI (continuous), smoking status (yes or no), alcohol consumption (yes or no), and coffee consumption (yes or no) were also included as covariates in the model. The values of the three circadian pattern parameters (M, A, and θ) were log 10-transformed to improve the normality. The association of the severity of anxiety, depression, stress, sleepiness, fatigue, self-reported sleep quality, with the circadian patterns (M, A, θ) of HRV indices and the heart rate in shift workers was also examined using the same linear regression method, repsectively. The shift group with a DASS-D score ≤ 9 points, DASS-A score ≤ 6 points, DASS-S score ≤ 10 points, ESS score ≤ 10 points, FAS score ≤ 21 points, or sleep quality ≤ 2 points was treated as the reference group. Similarly, the association between the number of sleep bouts (continuous) and the circadian patterns (M, A, θ) of HRV indices alongside the heart rate in shift workers was examined. The acquired regression coefficients (β) and P-values are reported.
Daily HRV indices can reflect temporal ANS modulation; however, circadian pattern alteration is a long-term result. Herein, we examined the association of 24-hour LF, HF and the LF/HF ratio with features of HRV circadian patterns using a multivariable linear regression model. Age (continuous), BMI (continuous), smoking (yes or no), alcohol consumption (yes or no), coffee consumption (yes or no), HF (continuous), and LF (continuous) were all included as covariates. The acquired standardized regression coefficients (βs) and P values are reported.
All analyses were performed using the SPSS version 26 software (IBM, USA). Statistical significance was set at P < 0.05.
Results
Demographic Information of the Study Populations
Among the total number of the participants (n= 257), there were 203 female shift workers and 54 female non-shift workers (). In comparisons between the shift and non-shifter workers, the parameters, including age, PSS-14 score, DASS-A (anxiety) and DASS-S (stress), ESS score, FAS score showed significant difference (P < 0.05). The median ages of the 203 female participants with night shift work and 54 non-shift workers were 32 years (IQR = 6.0) and 37 years (IQR = 8.0), respectively. The shift workers exhibited abnormal levels of perceived stress, anxiety, excessive daytime sleepiness, and fatigue (). The parameters BMI, heart rate, DASS-D (depression), self-reported sleep quality were comparable between the two groups (P > 0.05).
Table 1 The Median (IQR) Values of Selected Characteristics of the Two Female Groups Stratified by Night Shift Work
Comparisons of the 24-hour Averages of HRV Indices Between Shift and Non-Shift Workers
The averaged HRV indices (SDNN, RMSSD, LF, HF, and LF/HF ratio and LFnu) and heart rate output from 24-hour Holter monitoring were compared between shift and non-shift workers. SDNN, standard deviation of NN intervals, a time-domain HRV index that indicates the short-term variability,Citation32 was significantly higher in shift workers compared to the non-shift workers () (P < 0.01). And both of the non-linear HRV indices (SD1 and SD2) were significantly higher in shift workers compared to the non-shift workers (P < 0.05, Supplementary Table 1). The 24-hour averages of RMSSD, LF, HF, LF/HF, LFnu were all comparable between the two groups ().
Circadian Rhythms of HRV Indices Were Altered in Night-Shift Workers
The linear HRV indices (SDNN, RMSSD, LF, HF, LF/HF, LFnu), the non-linear HRV index, SD1, as well as heart rate calculated in either 1-min or 5-min epoch, were plotted as scatter points over 24-hour time frame. The fitted cosine periodic curves of the respective HRV indices were displayed along with the scatter points (). The parameters, M, A, and θ, extracted from the fitted cosine periodic curves of the HRV indices were summarized in , and Supplementary Table 2. The parameter M reflects the overall averages of the HRV indices (). The M values of the HRV indices (SDNN, RMSSD, LF, HF, SD1) were significantly higher in shift workers compared to the non-shift workers ( and Supplementary Table 2). Similar changes were found in the parameter A for those HRV indices ( and Supplementary Table 2), which was also elevated in shift workers. In contrast, the parameter θ for the HRV indices (LF/HF and LFnu), was slightly longer in shift worker versus the non-shift workers (P < 0.05) (), indicating a delayed time point of reaching the peak of the cosine curve within one cycle. No significant difference was found for the comparisons of the parameters, M, A, θ of the fitted cosine curve for the heart rate between shift and non-shift workers (Supplementary Table 2).
Table 2 The Comparison of the Three Circadian Pattern Parameters (M, A, θ) for Each HRV Index Between the Two Female Groups Stratified by Night Shift Work
Correlations Between the Parameters (M, A, θ) of the HRV Indices and the Clinical Assessments of Stress, Depression, Anxiety
The relationship between circadian pattern features (M, A, and θ) and mental health (stress, anxiety, and depression) in night shift workers was examined using multivariable linear regression models and presented in . As shown in , a higher level of perceived stress, PSS score ≥ 30 points, was not only associated with the increased MESORs of SDNN, RMSSD, LF, and HF but also related to a delayed θ of the LF/HF ratio (β = 0.02, P = 0.03). The MESORs of heart rate and SD1 also increased in night shift workers with higher perceived stress (Supplementary Table 3). The abnormal stress level evaluated by the DASS-21 stress subscale was also associated with delayed θ of the LF/HF ratio (β = 0.02, P = 0.01) and LFnu (β = 0.02, P = 0.04), respectively. As shown in , abnormal depression levels were associated with a left-shifted θ of the LF (β = - 0.15, P = 0.04). Meanwhile, abnormal anxiety levels were related to a right-shifted θ of the LF/HF ratio (β = 0.02, P = 0.03).
Table 3 Association of the Severities of Mental Stress and the Circadian Pattern Parameters (M, A, θ) of Each HRV Index in Night-Shift Female Workers Based on Linear Regression Modelsa
Table 4 Association of the Severities of Anxiety, Depression and the Circadian Patterns Parameters (M, A, θ) of Each HRV Index in Night-Shift Female Workers Based on a Linear Regression Modela
Correlations Between the Parameters (M, A, θ) of the HRV Indices and the Clinical Assessments of Sleep
The relationship between sleep status (sleepiness, fatigue, and sleep quality) and the features of HRV circadian patterns (M, A, and θ) were summarized in . Mild to severe daytime sleepiness and fatigue were both associated with increased amplitudes of the LF/HF ratio and LFnu circadian oscillation. In addition, fatigue was related to an advanced θ of SDNN (β = −0.20, P = 0.04). While better self-reported sleep quality showed an opposite association with the θ of SDNN (β = 0.18, P = 0.01). Better sleep quality was also associated with an advanced θ of the LF/HF ratio and LFnu (β = - 0.02, P < 0.05). A higher number of the total sleep bouts scored by the smart device positively correlated with the parameter A of the HRV indices (LF/HF ratio and LFnu) (β = 0.01, P = 0.02 for both; ), indicating that the circadian pattern of LF/HF ratio was also sensitive in detecting sleep fragmentation.
Table 5 Association of the Severities of Sleepiness, Fatigue, Sleep Quality and the Circadian Patterns Parameters (M, A, θ) of Each HRV Indices in Night-Shift Female Workers Based on a Linear Regression Modela
Table 6 Association of the Number of Sleep Bouts and the Circadian Patterns Parameters (M, A, θ) of Each HRV Index in Night-Shift Female Workers Based on a Linear Regression Model a
Correlations Between the Circadian Patterns Parameters (M, A, θ) of the HRV Indices and the 24-Hour Averages of the HRV Indices
To explore whether average HRV indices could reflect the circadian rhythms of HRV, the association of 24-hour LF, HF and the LF/HF ratio with HRV circadian patterns was examined (). The 24-hour average of LF was positively correlated with the MESORs of SDNN, RMSSD, LF, LF/HF, and LFnu, as well as the amplitude of the LF circadian pattern; however, it was negatively correlated with the amplitude of the LF/HF ratio and LFnu (all P < 0.05). In contrast, the 24-hour average of HF showed reciprocal association with the MESORs and amplitudes of LF, LF/HF ratio, and LFnu. The 24-hour average of LF/HF ratio was negatively correlated with the MESORs and amplitudes of the HF and RMSSD circadian patterns. In addition, 24-hour LF, HF, and LF/HF ratio had almost no relationship with the parameter θ of their circadian parameters and the circadian pattern of heart rate; however, the LF/HF ratio was negatively correlated with the MESOR and amplitude of SD1 circadian rhythm, as shown in Supplementary Table 4.
Table 7 Combined Effects of Daily Mean LF, HF, LF/HF Ratio on the Circadian Pattern Parameters (M, A, θ) of Each HRV Index in Night-Shift Female Workers Based on a Linear Regression Model b
Discussion
In the current study, we plotted the 1-min or 5-min epoch-specific HRV indices over 24-hour Holter monitoring as a function of time and fitted these scatter data points to cosine periodic curves to characterize the circadian rhythms of the HRV indices. Subsequently, we calculated the mathematical parameters (M, A, θ) based on the cosine curves. The parameters M and A of the HRV indices (SDNN, RMSSD, LF, HF and SD1), and the parameter θ of the HRV indices (the LF/HF ratio, LFnu) significantly differ between shift and non-shift workers. The parameter θ of the LF/HF ratio positively correlates with the severity of stress and anxiety conditions, while the amplitude of the LF/HF ratio and LFnu and the parameter θ of SDNN, positively correlate with the severity of the sleep disturbance and daytime sleepiness. The parameters M and A of the HRV indices (RMSSD, LF, HF, LF/HF and LFnu) significantly correlate with the 24-hour averages of HRV frequency indices, while the θ shows no significant correlations.
Using the three parameters to quantify HRV circadian variations (M, the overall mean level; A, the peak of HRV oscillation; θ, the time lag to the peak from the reference timepoint), our data showed that RMSSD, SDNN, HF, LF and SD1 follow a very similar circadian pattern: the peak occurs in the early morning and the nadir occurs in the afternoon; while the LF/HF ratio, LFnu and heart rate show an opposed oscillation pattern: the HRV oscillation reaches the peak in the afternoon around 14:00~15:00 PM, and declines to the lowest level in the early morning, which is consistent with the previous studies.Citation15,Citation20 But, the non-linear HRV index SD2 of a quarter of the participants did not exhibit significant circadian rhythm. It was reported that SD2 is correlated with both LF and HF power. The complex regulation of SD2 may be one of the reason why a small portion of SD2 did not show significant circadian rhythm patterns.Citation34 The HRV indices fluctuate all the time rather than keep stable, for example there is an ultradian 80–120 min rhythm in the LF/(LF+HF) ratio,Citation36 instead, the circadian rhythms of HRV indices show a global trend of the entire day, which will not be affected by the local details and are less changeable in a period.
To the best of our knowledge, this is the first study to report the association of mental stress, mood, and sleep status with HRV circadian pattern features. The ANS and CNS interact with each other, while circadian is one of the ways for both to modulate reciprocally.Citation37 Circadian rhythm-regulated processes, like sleep, hormone secretion, body temperature, are driven by an endogenous central pacemaker in the suprachiasmatic nucleus (SCN) of the hypothalamus, and the SCN can also affect the hypothalamic-pituitary-adrenal axis and ANS.Citation38 According to Porges’ polyvagal theory, stress and emotional processing are related to the bidirectional and dynamical interplay of the ANS and the central nervous system (CNS) mediated by the vagal nerve.Citation39 Vagal nerves originating from the nucleus ambiguus (NA) act on the sinoatrial node and play a dominant role in controlling respiratory sinus arrhythmia (RSA, an index of cardiac vagal modulation, indexed by HF or RMSSD).Citation19 The higher the resting respiratory sinus arrhythmia (reflected by HF and RMSSD), the greater the withdrawal during stressors.Citation18 This may account for the higher MESORs and amplitudes of HRV frequency and time-domain measures observed in the night shift women in our study. Meanwhile, the neurovisceral theory proposed that prefrontal hypoactivity leading to amygdala excitation can depress vagal activity and result in anxiety, which can account for the higher anxiety score in the shift workers.Citation8,Citation40 Sleep is also closely linked to ANS activity and circadian rhythm regulation.Citation41 The LF/HF ratio was reported to progressively decline with deeper sleep stages, from the highest value during wake episodes to the lowest value during SWS.Citation42,Citation43 This supports our results that sleep quality and fatigue were associated with the θ of the LF/HF ratio (). Based on these theories, we speculate that nightshift work can prolong sympathetic activation due to work stress, physical activity and active electroencephalography (EEG) oscillation, and disinhibit GABAergic neurons in the amygdala which plays a role in the modulation of cardiovascular, autonomic, and endocrine responses.Citation8,Citation44 This may interrupt the brain–heart interaction mediated by vagal modulation. And the imbalance can suppress autonomic cortical control and lead to variable RAS responses to the stimuli that underlie anxiety or depression.Citation18,Citation44,Citation45 For example, a study found that the amplitude of RMSSD circadian oscillation was positively associated with mild depressive symptoms in healthy occupational women.Citation22 On the other hand, deprived sleep due to night shift work can exaggerate the disrupted circadian rhythm of biological process and homeostasis.
Our results indicate that 24-hour averages of HRV changes (LF, HF, and LF/HF ratio) can affect the MESORs and amplitudes of HRV circadian patterns, but not the θ (). However, many 24-hour HRV indices of night-shift female workers did not exhibit differences except SDNN, SD1 and SD2, while the HRV circadian patterns were distinctive when compared to those of non-shift workers. 24-hour averages of HRV are adaptive, reversible, and one-dimensional, while the alteration of the circadian patterns of HRV indices is a result of long-term accumulation, and the three parameters of circadian patterns are all significant to ANS physiological regulation and homeostasis. This may explain why 24-hour averages of mean HRV indices did not differ between night shift workers and non-shift workers while HRV circadian patterns did. The shift workers showed a slightly higher SDNN, SD1 and SD2 than the non-shift workers, which may result from the age difference between the two groups.Citation46,Citation47 The median age of the shift workers were five-year younger than that of non-shift group. Above results indicate that the HRV circadian patterns may be more sensitive in detecting chronic ANS function alteration than temporal 24-hour averages of HRV values.
Circadian rhythm changes in ANS are of great importance to cardiovascular health. It was reported that there is an increased shift towards sympathetic modulation of the heart in the transition from sleep to wakefulness during morning hours.Citation48 Meanwhile, there is a 40% increased risk of myocardial infarction and a 29% increased risk of cardiac death between 06:00 and 12:00 AM.Citation49 In particular, the circadian pattern features (M, A, and θ) of the LF/HF ratio and LFnu were found to be linked with multiple pathological indicators, such as anxiety, stress, sleepiness, fatigue, poor sleep quality, and sleep fragmentation. Therefore, we propose the use of two-dimensional HRV circadian patterns as indicators to detect long-term HRV function changes and reflect chronic abnormal mental stress, negative emotion disorders, poor sleep quality, and burnout syndrome. It is also important to help medical workers deal with negative moods and enjoy high-quality sleep to maintain good health.
Limitations
The current study unavoidably has the following limitations: 1) Only women medical workers were recruited in the study due to practical reasons. It made the significance of the study limited to the female gender. 2) Menstrual cycles might affect the ANS function and therefore the HRV indices.Citation50,Citation51 Herein, we did not analyze participants’ menstrual cycle. 3) Burnout is a work-related stress syndrome and depends on the length of service, which is common in medical workers. It would be better if taking the length of service into consideration.Citation52
Conclusion
Our study revealed that the circadian patterns of HRV indices, compared to 24-hour HRV values, changed profoundly in female medical workers working night shifts. Stress, mood, and sleep quality are all linked with HRV circadian pattern alterations, indicating a potential disturbance in brain-heart interplay in female night shift workers. We propose that HRV circadian patterns could be sensitive indicators of long-term ANS fluctuation changes, chronic mental stress, negative mood, and physical exhaustion.
Ethics Approval and Informed Consent
This study was performed in line with the principles of the Declaration of Helsinki, and approval was granted by the Ethics Committee of Tongji Medical School, Huazhong University of Science and Technology (IRB # 2021S141). Informed consents were obtained from all patients included in the study.
Disclosure
This project is an investigator-initiated study. The scientific question of the study was originated from Dr. Fengfei Ding and Dr. Wei Wang. No fee was paid personally. Runsen Wang, Yujia Han, Rong Sheng from HUAWEI Research platform provided technical supports in portable device raw data processing. The authors report no conflicts of interest in this work.
Acknowledgments
We thank Hui Wang, Sufang Huang, Daiqi Chen, Yaru Xiao, Miqi Li, Yanmei Xu, Danhui Zhou, Zhenni Wang, Na Shao, Chang Cheng, Renjie Chen, Caihong Hu, Ye Xia of Tongji hospital contributed in clinical data collections and interactions with participants. We thank Chong Liu of the Public Health school of Huazhong University of Science and Technology for statistical consultation.
Additional information
Funding
References
- Schneiderman N, Ironson G, Siegel SD. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2005;1:607–628. doi:10.1146/annurev.clinpsy.1.102803.144141
- Średniawa A, Drwiła D, Krotos A, Wojtaś D, Kostecka N, Tomasik T. Insomnia and the level of stress among students in Krakow, Poland. Trends Psychiatry Psychother. 2019;41(1):60–68. doi:10.1590/2237-6089-2017-0154
- Gallo LC, Roesch SC, Fortmann AL, et al. Associations of chronic stress burden, perceived stress, and traumatic stress with cardiovascular disease prevalence and risk factors in the Hispanic Community Health Study/Study of Latinos Sociocultural Ancillary Study. Psychosom Med. 2014;76(6):468–475. doi:10.1097/PSY.0000000000000069
- Turner AD, James BD, Capuano AW, Aggarwal NT, Barnes LL. Perceived Stress and Cognitive Decline in Different Cognitive Domains in a Cohort of Older African Americans. Am j Geriatric Psychiatry. 2017;25(1):25–34. doi:10.1016/j.jagp.2016.10.003
- Harris A, Waage S, Ursin H, Hansen AM, Bjorvatn B, Eriksen HR. Cortisol, reaction time test and health among offshore shift workers. Psychoneuroendocrinology. 2010;35(9):1339–1347. doi:10.1016/j.psyneuen.2010.03.006
- Pietroiusti A, Neri A, Somma G, et al. Incidence of metabolic syndrome among night-shift healthcare workers. Occupational and Environmental Medicine. 2010;67(1):54–57. doi:10.1136/oem.2009.046797
- Mirick DK, Bhatti P, Chen C, Nordt F, Stanczyk FZ, Davis S. Night Shift Work and Levels of 6-Sulfatoxymelatonin and Cortisol in Men. Cancer Epidemiol Biomarkers Prevention. 2013;22(6):1079–1087. doi:10.1158/1055-9965.EPI-12-1377
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33(2):81–88. doi:10.1016/j.neubiorev.2008.08.004
- Kim HG, Cheon EJ, Bai DS, Lee YH, Koo BH. Stress and Heart Rate Variability: a Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018;15(3):235–245. doi:10.30773/pi.2017.08.17
- Sin NL, Sloan RP, McKinley PS, Almeida DM. Linking Daily Stress Processes and Laboratory-Based Heart Rate Variability in a National Sample of Midlife and Older Adults. Psychosom Med. 2016;78(5):573–582. doi:10.1097/PSY.0000000000000306
- Schubert C, Lambertz M, Nelesen RA, Bardwell W, Choi JB, Dimsdale JE. Effects of stress on heart rate complexity—A comparison between short-term and chronic stress. Biol Psychol. 2009;80(3):325–332. doi:10.1016/j.biopsycho.2008.11.005
- Pushpanathan Punita KS, Chandrasekar M. Gender difference in heart rate variability in medical students and association with the level of stress. National J Physiol Pharmacy Pharmacol. 2016;6(5):437.
- Lucini D, Norbiato G, Clerici M, Pagani M. Hemodynamic and Autonomic Adjustments to Real Life Stress Conditions in Humans. Hypertension. 2002;39(1):184–188. doi:10.1161/hy0102.100784
- Sammito S, Sammito W, Böckelmann I. The circadian rhythm of heart rate variability. Biol Rhythm Res. 2016;47(5):717–730. doi:10.1080/09291016.2016.1183887
- Li X, Shaffer ML, Rodriguez-Colon S, et al. The circadian pattern of cardiac autonomic modulation in a middle-aged population. Clin Autonomic Res. 2011;21(3):143–150. doi:10.1007/s10286-010-0112-4
- Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17(3):354–381.
- Egizio VB, Eddy M, Robinson M, Jennings JR. Efficient and cost-effective estimation of the influence of respiratory variables on respiratory sinus arrhythmia. Psychophysiology. 2011;48(4):488–494. doi:10.1111/j.1469-8986.2010.01086.x
- Tonhajzerova I, Mestanik M, Mestanikova A, Jurko A. Respiratory sinus arrhythmia as a non-invasive index of ‘brain-heart’ interaction in stress. Indian J Med Res. 2016;144(6):815–822. doi:10.4103/ijmr.IJMR_1447_14
- Porges SW. The polyvagal perspective. Biol Psychol. 2007;74(2):116–143. doi:10.1016/j.biopsycho.2006.06.009
- Rodríguez-Colón S, He F, Bixler EO, et al. The circadian pattern of cardiac autonomic modulation and obesity in adolescents. Clin Autonomic Res. 2014;24(6):265–273. doi:10.1007/s10286-014-0257-7
- Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model. 2014;11:16. doi:10.1186/1742-4682-11-16
- Jarczok MN, Aguilar-Raab C, Koenig J, et al. The Heart´s rhythm ‘n’ blues: sex differences in circadian variation patterns of vagal activity vary by depressive symptoms in predominantly healthy employees. Chronobiol Int. 2018;35(7):896–909. doi:10.1080/07420528.2018.1439499
- Li X, Shaffer ML, Rodríguez-Colón SM, et al. Systemic inflammation and circadian rhythm of cardiac autonomic modulation. Autonomic Neurosci. 2011;162(1–2):72–76. doi:10.1016/j.autneu.2011.03.002
- Rodríguez-Colón SM, Li X, Shaffer ML, et al. Insulin resistance and circadian rhythm of cardiac autonomic modulation. Cardiovasc Diabetol. 2010;9:85. doi:10.1186/1475-2840-9-85
- Wang H, Huang D, Huang H, et al. The psychological impact of COVID-19 pandemic on medical staff in Guangdong, China: a cross-sectional study. Psychol Med. 2022;52(5):884–892. doi:10.1017/S0033291720002561
- Jiang LC, Yan YJ, Jin ZS, et al. The Depression Anxiety Stress Scale-21 in Chinese Hospital Workers: reliability, Latent Structure, and Measurement Invariance Across Genders. Front Psychol. 2020;11:247. doi:10.3389/fpsyg.2020.00247
- Wang C, Pan R, Wan X, et al. Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. Int J Environ Res Public Health. 2020;17(5):548.
- Surani S, Hesselbacher S, Guntupalli B, Surani S, Subramanian S. Sleep Quality and Vigilance Differ Among Inpatient Nurses Based on the Unit Setting and Shift Worked. J Patient Saf. 2015;11(4):215–220. doi:10.1097/PTS.0000000000000089
- Barker LM, Nussbaum MA. Fatigue, performance and the work environment: a survey of registered nurses. J Adv Nurs. 2011;67(6):1370–1382. doi:10.1111/j.1365-2648.2010.05597.x
- Nilsson LM. Respiration Signals from Photoplethysmography. Anesthesia and Analgesia. 2013;117(4):859–865. doi:10.1213/ANE.0b013e31828098b2
- Thomas RJ, Mietus JE, Peng CK, Goldberger AL. An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep. 2005;28(9):1151–1161. doi:10.1093/sleep/28.9.1151
- Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. doi:10.3389/fpubh.2017.00258
- Behbahani S, Dabanloo NJ, Nasrabadi AM, Teixeira CA, Dourado A. Pre-ictal heart rate variability assessment of epileptic seizures by means of linear and non-linear analyses. Anatolian j Cardiol. 2013;13(8):797–803. doi:10.5152/akd.2013.237
- Brennan M, Palaniswami M, Kamen P. Poincaré plot interpretation using a physiological model of HRV based on a network of oscillators. Am J Physiol Heart Circ Physiol. 2002;283(5):H1873–1886. doi:10.1152/ajpheart.00405.2000
- Moškon M. CosinorPy: a python package for cosinor-based rhythmometry. BMC Bioinform. 2020;21(1):485. doi:10.1186/s12859-020-03830-w
- Brandenberger G, Ehrhart J, Piquard F, Simon C. Inverse coupling between ultradian oscillations in delta wave activity and heart rate variability during sleep. Clin Neurophysiol. 2001;112(6):992–996. doi:10.1016/S1388-2457(01)00507-7
- Riganello F, Prada V, Soddu A, Di Perri C, Sannita WG. Circadian Rhythms and Measures of CNS/Autonomic Interaction. Int J Environ Res Public Health. 2019;16(13):2336. doi:10.3390/ijerph16132336
- Fishbein AB, Knutson KL, Zee PC. Circadian disruption and human health. J Clin Invest. 2021;131(19). doi:10.1172/JCI148286
- Porges SW. The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleve Clin J Med. 2009;76 Suppl 2(Suppl2):S86–90. doi:10.3949/ccjm.76.s2.17
- Thayer JF, Sternberg E. Beyond Heart Rate Variability. Annals of the New York Academy of Sciences. 2006;1088(1):361–372. doi:10.1196/annals.1366.014
- Borbély A. The two-process model of sleep regulation: beginnings and outlook. J Sleep Res. 2022;31(4):e13598. doi:10.1111/jsr.13598
- Boudreau P, Yeh WH, Dumont GA, Boivin DB. Circadian variation of heart rate variability across sleep stages. Sleep. 2013;36(12):1919–1928. doi:10.5665/sleep.3230
- Sforza E, Pichot V, Cervena K, Barthélémy JC, Roche F. Cardiac variability and heart-rate increment as a marker of sleep fragmentation in patients with a sleep disorder: a preliminary study. Sleep. 2007;30(1):43–51. doi:10.1093/sleep/30.1.43
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61(3):201–216. doi:10.1016/S0165-0327(00)00338-4
- Cattaneo LA, Franquillo AC, Grecucci A, Beccia L, Caretti V, Dadomo H. Is low heart rate variability associated with emotional dysregulation. Psychopathological Dimensions Prefrontal Dysfunctions. 2021;11(9):872.
- Abhishekh HA, Nisarga P, Kisan R, et al. Influence of age and gender on autonomic regulation of heart. J Clin Monit Comput. 2013;27(3):259–264. doi:10.1007/s10877-012-9424-3
- Almeida-Santos MA, Barreto-Filho JA, Oliveira JL, Reis FP, da Cunha Oliveira CC, Sousa AC. Aging, heart rate variability and patterns of autonomic regulation of the heart. Arch Gerontol Geriatr. 2016;63:1–8. doi:10.1016/j.archger.2015.11.011
- Boudreau P, Yeh WH, Dumont GA, Boivin DB, Circadian A. Rhythm in Heart Rate Variability Contributes to the Increased Cardiac Sympathovagal Response to Awakening in the Morning. Chronobiol Int. 2012;29(6):757–768. doi:10.3109/07420528.2012.674592
- Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA. Meta-Analysis of the Morning Excess of Acute Myocardial Infarction and Sudden Cardiac Death. Am J Cardiol. 1997;79(11):1512–1516. doi:10.1016/S0002-9149(97)00181-1
- Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8(6):613–622. doi:10.1016/j.sleep.2006.09.011
- Kiselev AR, Neufeld IW, Bobyleva IV, Prokhorov MD, Karavaev AS. Interaction between cardiovascular autonomic control and sex hormones in perimenopausal women under menopausal hormone therapy. Cardiovascular Endocrinology Metab. 2018;7(3):58–63. doi:10.1097/XCE.0000000000000153
- De Hert S. Burnout in healthcare workers: prevalence, impact and preventative strategies. Local Reg Anesth. 2020;13:171–183. doi:10.2147/LRA.S240564
