Abstract
Background
Associations between subjective sleep quality and stage-specific heart rate (HR) may have important clinical relevance when aiming to optimize sleep and overall health. The majority of previously studies have been performed during short periods under laboratory-based conditions. The aim of this study was to investigate the associations of subjective sleep quality with heart rate during REM sleep (HR REMS) and non-REM sleep (HR NREMS) using a wearable device (Fitbit Versa).
Methods
This is a secondary analysis of data from the intervention group of a randomized controlled trial (RCT) performed between December 3, 2018, and March 2, 2019, in Tokyo, Japan. The intervention group consisted of 179 Japanese office workers with metabolic syndrome (MetS), Pre-MetS or a high risk of developing MetS. HR was collected with a wearable device and sleep quality was assessed with a mobile application where participants answered The St. Mary’s Hospital Sleep Questionnaire. Both HR and sleep quality was collected daily for a period of 90 days. Associations of between-individual and within-individual sleep quality with HR REMS and HR NREMS were analyzed with multi-level model regression in 3 multivariate models.
Results
The cohort consisted of 92.6% men (n=151) with a mean age (± standard deviation) of 44.1 (±7.5) years. A non-significant inverse between-individual association was observed for sleep quality with HR REMS (HR REMS −0.18; 95% CI −0.61, 0.24) and HR NREMS (HR NREMS −0.23; 95% CI −0.66, 0.21), in the final multivariable adjusted models; a statistically significant inverse within-individual association was observed for sleep quality with HR REMS (HR REMS −0.21 95% CI −0.27, −0.15) and HR NREMS (HR NREMS −0.21 95% CI −0.27, −0.14) after final adjustments for covariates.
Conclusion
The present study shows a statistically significant within-individual association of subjective sleep quality with HR REMS and HR NREMS. These findings emphasize the importance of considering sleep quality on the individual level. The results may contribute to early detection and prevention of diseases associated with sleep quality which may have important implications on public health given the high prevalence of sleep disturbances in the population.
Introduction
Sleep is a vital process for human life and is maintained through various complex physiological systems working symbiotically under regulation of the circadian rhythm.Citation1 Sleep is generally divided into 2 states: rapid eye movement sleep (REMS), and non-rapid eye movement sleep (NREMS). This distinction is established due to physiological differences observed during these states. NREMS is further divided into 2 groups: light sleep and deep sleep, where light sleep includes sleep stages 1 and 2, whilst deep sleep includes subsequent stages 3 and 4. During REMS, desynchronized EEG, rapid eye movements and muscle atonia are present.Citation2 NREMS, on the contrary, shows synchronized EEG waves and lacks rapid eye movements or muscle atonia. Measurement of different physiological functions are common in sleep research, and polysomnography (PSG) has long been the golden standard for registration.Citation3 However, in the past years, several wearable devices have been introduced as a promising method for measuring physiological parameters during both sleep and wakefulness.Citation4–6
It is well known that autonomic tone varies depending on sleep stage, and each sleep stage has a specific heart rate (HR) pattern.Citation7 During REMS, sympathetic-nerve activity dominates, whilst during NREMS, this activity is lower than in wakefulness. A high frequency of high-amplitude sympathetic bursts has also been observed during REMS compared to NREMS.Citation8 In contrast, NREMS is primarily dominated by parasympathetic activity.Citation9 Two frequently used parameters for measuring autonomic function and balance between sympathetic and parasympathetic activity are HR and heart rate variability (HRV). Both decrease during NREMS and increase during REMS.Citation7 Additionally, there are specific differences in HR within NREMS. During light sleep, there is a decrease in HR with an irregular slow HR; however, during deep sleep, there is a decrease in HR without marked slow HR waves.Citation7
Dysregulated sleeping patterns with sleep loss and reduced sleep quality have multifactorial causes. Several primary sleep disorders such as insomnia, sleep-disordered breathing and restless legs syndrome, as well as other medical conditions, affect sleep negatively.Citation10 However, lifestyle and environmental factors such as room temperature, work-related stress, and shift work may also decrease sleep quality. It has been shown how current work patterns and electronic device-use affect sleeping quality and quantity.Citation11 Long-term sleep loss has been associated with risk of cardiovascular diseases such as hypertension and myocardial infarction.Citation12–14 Additionally, studies have shown how dysregulated sleeping patterns increase long-term risk of metabolic disorders,Citation15 certain types of cancer,Citation16,Citation17 and all-cause mortality.Citation18 As sleep loss is often associated with a decrease in sleep quality, subjective sleep quality may have a potential impact on autonomic functions and thus an increased risk of long-term morbidity and mortality.
Studies on subjective sleep quality and its association with HR during specific sleep stages are few and mainly observe HRV as a measurement of autonomic tone. One study presented an association between REMS deprivation (REMSD) and an increase in HR during all sleep stages. The increase in HR was most prominent in REMS compared to NREMS.Citation19 An additional study suggested REMS as a predictive readout of sleep quality.Citation2 Specific REMSD is also common in many disorders and can probably be assumed to correspond with low sleep quality. The fundamentals of sleep stage order in sleep cycles present limitations for studying NREMS deprivation specifically.Citation2
This secondary analysis is, to our knowledge, the first study to specifically investigate associations between subjective sleep quality and average heart rate during specific REMS and NREMS.
The main objective of the present study is to investigate the within-individual and between-individual associations of subjective sleep quality with average HR during REMS and NREMS. HR data was collected with a wearable device in a previously conducted RCT.
We hypothesize that a higher subjective sleep quality will be associated with a lower HR during both REMS and NREMS. Additionally, we hypothesize that a potential increase in HR during REMS may be more significant than an increase in HR during NREMS, as has been observed when studying REMSD specifically.Citation19
Material and Methods
Study Design
The present study is a secondary analysis of data from the interventional group of a randomized controlled trial (RCT) studying full-time office workers in Japan. The main objective of the RCT was to investigate if participants with metabolic syndrome (MetS), Pre-MetS or a high risk of developing MetS could improve their metabolic status. A total of 272 participants were recruited and randomized into two groups (2:1, intervention: control). Daily physiological data, including HR, was collected from the intervention group with a wearable device, provided at the beginning of the study. The intervention group also answered daily questionnaires through a mobile application. Daily data was not collected from the control group. Both groups filled out an extensive questionnaire at the beginning and at the end of the study. The study was performed for 90 days between December 3, 2018, and March 2, 2019.
Study Participants
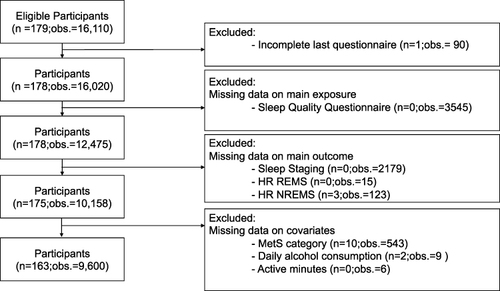
Study participants were full-time office-workers recruited from 5 companies in the metropolitan area of Tokyo, Japan. All participants were full-time managerial, professional, or clerical workers from companies with over 1000 employees. Office workers were considered eligible if they had completed the Japanese Annual Health Check-up (AHC) and, based on these measures, were categorized as having or at risk of developing MetS. A total of 272 participants were recruited from 7437 eligible office workers. Two participants later declined participation. After randomization, the intervention group consisted of 179 participants and the control group of 91 participants. One participant in the intervention group did not complete the final questionnaire and was therefore excluded from the present study. Further, participants and observations were excluded due to missing values on main exposure sleep quality (n=0; o=3545), sleep staging (n=0; observations=2179), and the main outcomes; HR REMS (n=0; observations=15), and HR NREMS (n=3; o=123). Information on sleep stages is a prerequisite to obtain the outcome measures. Additionally, observations, participants, or both were excluded due to missing values in the covariates MetS (n=10; observations=543), average daily alcohol consumption (n=2; observations=9), and active minutes (n=0; observations = 6). Missing data were likely the result of incorrect use of the wearable device or daily questionnaires left unresponded. After final exclusion, 163 eligible participants with 9600 observations were included in the analysis. The majority of excluded data was due to incomplete daily sleep questionnaires (o=3545) and missing sleep staging (o=2179) ().
Figure 1 Flowchart illustrating the inclusion and exclusion of participants (n) and observations (obs.) in the present study. Daily observations within individuals were excluded due to missing data from either St. Mary’s Hospital Sleep Questionnaire, Sleep staging, HR REMS, HR NREMS, MetS, alcohol consumption or active minutes from one specific day.
All participants provided written consent upon inclusion in the study. Information about the study, its purpose and ethical considerations were given in both written and oral form. The ability to discontinue the study at any time and without any penalty was clearly stated in the information and well understood by all participants before initiation of the study. All participants provided written informed consent. The original RCT was approved by The Ethics Committee of the School of Engineering, the University of Tokyo (approval number: KE18-44).
Data Collection
Wearable Devices
Daily heart rate, step count, physical activity and sleep staging was continuously collected from the intervention group with wearable device Fitbit Versa Classic (Fitbit Inc. San Francisco, CA, USA), details at https://help.fitbit.com/manuals/manual_versa_en_US.pdf (accessed on December 1, 2023). Participants were instructed to wear the device at all hours during the study period of 90 days, except if they were bathing or when it was charging. Data was transferred to a mobile application via Bluetooth. Inaccurate sleep stage registration commonly occurs when there is an error or difficulty measuring the heart rate, such as when the device sits to loosely on the wrist. In these cases, the device collects information in classic mode, which divides sleep into two groups: asleep and awake.
Upon completion of the final questionnaire, participants from both study groups were gifted the wearable device.
Questionnaires
All participants filled out extensive questionnaires at the beginning and at the end of the study. These questionnaires assessed demographics, socioeconomical status, medical and family history, lifestyle, including alcohol and caffeine consumption, smoking, exercise, psychological stress, sleep, and working conditions. Further, each participant of the intervention group received daily questionnaires assessing corresponding topics through a mobile application during each day of the study. Daily questionnaires were filled out twice a day, in the morning and in the afternoon.
Annual Health Check-Up
All full-time workers in Japan are offered an AHC, as part of a primary prevention program initiated by the Japanese government. The check-up includes measurements of height, weight and waist circumference, blood tests for anemia, lipids, glucose and liver disease, measurement of blood pressure, chest X-ray, ECG, urine sampling and hearing tests.Citation20 Data for this study was collected from each participant’s most recent AHC.
Main Exposure - Sleep Quality
Sleep quality was determined using 4 daily questions (A-D) derived from “St. Mary’s Hospital Sleep Questionnaire:Citation21 (A) “How was your depth of sleep last night?” (“1 = Very light”, “2 = Light”, “3 = Fairly light”, “4 = Light average”, “5 = Deep average”, “6 = Fairly deep”, “7= Deep”, “8 = Very deep”). (B) “How well did you sleep yesterday?” (“1 = Very badly”, “2 = Badly”, “3 = Fairly badly”, “4 = Fairly well”, “5 = Well”, “6 = Very well”). (C) “How clear was your head after getting up this morning?” (“1 = Still very drowsy indeed”, “2 = Still moderately drowsy”, “3 =Still slightly drowsy”, “4 = Fairly clear-headed”, “5 = Alert”, “6 = Very alert”). (D) “How satisfied were you with last night’s sleep?” (“1 = Very unsatisfied”, “2 = Moderately unsatisfied”, “3 = Slightly unsatisfied”, “4 = Fairly satisfied”, “5 = Completely satisfied”). An aggregate sleep quality score (4–25 points) was constructed for the 4 questions. A higher score indicated a higher level of sleep quality during the preceding night. A cluster mean sleep quality score (CMSQ) was created for each participant based on all available observations, where each individual was treated as a separate cluster. Further, a variable representing the deviation from the cluster mean sleep quality score (DCMSQ) was calculated as [individual daily sleep quality score - individual cluster mean]. CMSQ and DCMSQ allow for the study of between-individual variability and within-individual variability, respectively, in the investigation of the association for sleep quality and HR REMS and HR NREMS.
Main Outcome – HR REMS and HR NREMS
This study had 2 main outcomes: HR REMS and HR NREMS. Data was collected from each specific sleep stage with the wearable device through measurements of stage-specific heart rate patterns. The device provides information about specific sleep stages (wake, light sleep, deep sleep, and REM sleep). The timing of each sleep stage was merged with Fitbit intraday minute-by-minute HR data. For each sleep period, an average HR was calculated for NREM (light and deep sleep) and REM sleep, respectively. Consequently, all sleep data collected with classic mode was excluded due to lack of stage-specific information.
Covariates
Covariates were chosen based on previously known or assumed association with the main exposure or outcome. Information about age (continuous in years), sex, smoking, caffeine intake, stress levels and work overtime was collected from the start questionnaire. Four groups were constructed for smoking data: non-smoker, past smoker, current smoker < 20 cigarettes per day, and current smoker ≥ 20 cigarettes per day. Similarly, caffeine intake was categorized into 3 groups based on the number of caffeinated drinks per day: 0, 1–2, or ≥ 3. Stress levels were assessed with Perceived Stress Scale 4 (PSS-4)Citation22 consisting of 4 questions: (1)“In the past month, how often have you felt that you were unable to control the important things in your life?”, (2)“In the last month, how often have you felt confident about your ability to handle your personal problems?”, (3)“In the past month, how often have you felt that things were going your way?”, (4)“In the past month, how often have you felt difficulties were piling up so high that you could not overcome them?”. Answers were provided on a Likert scale: “Never”, “Almost never”, “Sometimes”, “Fairly often”, and “Very often”. Questions 2 and 3 were reverse scored, where a higher score indicated a lower stress level. Data of monthly work overtime was categorized into 2 groups based on the Japanese legal regulations of maximum work overtime: ≥ 45 hours a month, < 45 hours a month. Individuals were categorized by metabolic status (no MetS, pre-MetS and MetS) based on measurements from the AHC, in accordance with the Japanese definitions of MetS.Citation23 Information on alcohol consumption was collected from daily questionnaires. Based on number of glasses per day of specific alcoholic beverages, beer (5%), sake (5%), shochu (25%), chu-high (7%), cocktail (5%), wine (12%), whiskey (40%), and plum wine (15%), an average alcohol intake (g/day) was calculated with participants categorized into three groups, 0 g/day, < 20 g/day, and ≥ 20 g/day. Daily physical activity was collected in minutes by the wearable device and classified into two intensity categories based on metabolic equivalents (METs): Fairly active minutes (3–6 METs) and Very active minutes (≥ 6 METs).Citation24 The Exercise and Physical Activity Reference for Health Promotion (EPAR) issued 2013 by The Ministry of Health, Labour, and Welfare of Japan (MHLW) states a daily minimum of 60 minutes of moderate-to-vigorous intensity physical activity (MVPA) (23 metabolic equivalents h/week) for people aged 18–64.Citation25 Fairly active minutes and very active minutes were combined to create the category active minutes, which comprises the definition of MHLW (>3 METs).Citation24 A binary variable was created based on the cutoff of EPAR recommendation; ≥ 1 h active minutes/day and < 1 h active minutes/day. Total sleep time for each night was collected in minutes, and later converted into hours as a continuous variable.
Statistical Analyses
Baseline and sample characteristics of the cohort are presented with descriptive statistics. A mixed multi-level model regression was performed as the main analysis to investigate the association of subjective sleep quality with HR REMS and HR NREMS. Associations were investigated on between-individual (CMSQ) and within-individual (DCMSQ) level. The analyses were performed by using 3 models. Model 1 was adjusted for age and sex. Model 2 was additionally adjusted for weekday/weekend or holiday, stress-levels, MetS, work overtime. Model 3 was additionally adjusted for smoking, caffeine intake, mean alcohol intake, active minutes, and total sleep time. The analyses were performed separately for HR REMS and HR NREMS, using identical models and covariates. P-values <0.05 were considered as statistically significant. Statistical analyses were performed using Stata/MP Statistical Software Version 18 (StataCorp LLC (College Station, TX, USA)).
Results
Baseline and Sample Characteristics
Baseline and sample characteristics of the cohort are presented in . The participant group consisted of 92.6% men (n=151) and had a mean (± standard deviation (SD)) age of 44.1 (±7.5) years. The majority of participants were categorized as Pre-Mets (36.8%) and non-smokers (45.5%). Most participants reported a consumption of 1–2 cups of caffeinated drinks per day (61.4%), followed in size by the group who reported a consumption of ≥ 3 cups/day (36.1%). The mean PSS-4 score for the study population at baseline was 9.9 (SD ± 2.5) on a 20-point scale. Most participants reported a work overtime amount of < 45 hours/month (58.9%). When observing sample characteristics, the largest mean alcohol consumption was in the category ≥ 20 g alcohol/day (53.4%), followed in size by the category < 20 g/day (41.7%). The majority of observations of active minutes (obs.=5500, 57.3%) were in accordance with EPAR recommendations of MVPA. The mean total sleep time was 5.6 (±1.3) hours.
Table 1 Baseline and Sample Characteristics
Main Analyses
Associations of between-individual sleep quality (CMSQ) and within-individual sleep quality (DCMSQ) with HR REMS are presented in . In Model 1, CMSQ showed an inverse association with HR REMS without statistical significance (−0.19; 95% CI −0.61, 0.24). Results remained insignificant after further inclusion of covariates in Model 2 (−0.20; 95% CI −0.64, 0.24) and Model 3 (−0.18; 95% CI −0.61, 0.24). DCMSQ showed a statistically significant inverse association with HR REMS (−0.27; 95% CI −0.33, −0.21) in Model 1. The effect size was marginally increased after adjusting for covariates in Model 2 (−0.29; 95% CI −0.35, −0.23) and, although remaining statistically significant, was noticeably attenuated after adjustments in Model 3 (−0.21; 95% CI −0.27, −0.15).
Table 2a The Association of Between-Individual (CMSQ) and Within-Individual (DCMSQ) Sleep Quality with HR REMS
Corresponding results for HR NREMS are presented in . In Model 1, CMSQ, had an inverse association with HR NREMS without statistical significance (−0.19; 95% CI −0.63, 0.25). Results remained insignificant after further inclusion of covariates in Model 2 (−0.21; 95% CI −0.66, 0.24) and Model 3 (−0.23; 95% CI −0.66, 0.21). DCMSQ showed a statistically significant inverse association with HR NREMS (−0.24; 95% CI −0.30, −0.17) in Model 1. The effect size was marginally increased after adjusting for covariates in Model 2 (−0.25; 95% CI −0.32, −0.19) and, although remaining statistically significant, was moderately attenuated after further adjustments in Model 3 (−0.21; 95% CI −0.27, −0.14).
Table 2b The Association of Between-Individual (CMSQ) and Within-Individual (DCMSQ) Sleep Quality with HR NREMS
Discussion
The main objective of the present study was to investigate between-individual (CMSQ) and within-individual (DCMSQ) associations of subjective sleep quality with average heart rate during REM sleep (HR REMS) and average heart rate during non-REM sleep (HR NREMS). While an inverse association could be observed, no statistical significance was attained between individuals. Conversely, a statistically significant inverse association was observed within individuals. This partly confirms our main hypothesis that an increase in sleep quality would be associated with a decrease in HR in both sleep states. We further hypothesized that larger changes in HR would be observed in REMS than in NREMS. However, the results did not support this hypothesis and showed nearly identical effect sizes (beta coefficient: −0.21) in the final multivariable models of HR REMS and HR NREMS. When observing the individual effect of specific covariates, adjustments for total sleep time in the final multivariable model noticeably attenuated effect size. Nevertheless, results indicate a relationship between an individual’s variation in sleep quality and corresponding changes in heart rate that are independent of the corresponding night’s total sleep time.
The results may be explained by several factors. Individual physiology and autonomic responses may vary between individuals due to genetics, general health status, and lifestyle factors. This may possibly account for variations in the amplitude of autonomic responses between individuals after being exposed to similar sleep circumstances. Subjective sleep quality is additionally a challenging variable to measure.Citation26 The self-assessed subjective sleep quality may surely allow for discrepancy in individuals’ experience of identical sleeping conditions. Some participants may additionally have a long-term decreased sleep quality at baseline which they have developed a tolerance against over time. These factors, combined with a small study cohort, may account for the results reaching significance within individuals, but not between individuals.
Previous studies observing subjective sleep quality and its association with HR during sleep are limited and show ambiguous results. The variation in results may be due to a tangible difference in study design, as only one relevant studyCitation27 was found where data collection was performed with a similar wearable device. The majority of previous studies have used PSGCitation28–31 for physiological data collection, reflecting how wearable devices are currently still in an introductory phase. Additionally, several studies have not assessed subjective sleep quality specifically, but instead use variables, which may correspond to a decreased sleep quality. For example, the study by Faust et al investigated associations between variance in bedtime habits and changes in heart rate in a group of college students for up to 4 years using Fitbit Charge.Citation27 Results indicated a significant increase in heart rate after deviations from normal bedtime habits. Although this study’s main exposure differs from ours, results may be comparable as additional studies have observed an association between variability in bedtime habits with decreased sleep quality.Citation32
In one comparative study, HR was monitored during specific sleep stages (1–4 and REM), in 47 university students divided into either good or poor sleepers. Results showed a relative increase in HR during all stages except for stage 1 in the group of poor sleepers.Citation28 Another similar study found no statistically significant differences in HR between good and poor sleepers, in a participant group consisting of mainly workers with a mean age of 37.5 years.Citation33 These two studies differ from our model as they only observed sleep quality as a baseline measure and collected HR data for a maximum of two nights. Another study stated the inability to predict subjective sleep quality when comparing HR patterns during sleep and subjective sleep quality the following day, when studying community-dwelling older men.Citation29 This study differs from ours with regards to participant characteristics and study period as HR was measured during one night, whilst our study provides up to 90 observations of both HR and subjective sleep quality for each individual. An additional study on subjectively reported insomniacs, with no additional medical history, provided no significant associations when comparing with controlsCitation30 whilst one study in contrast, observed a significant increase in HR in a group of insomniacs.Citation31 It may, however, be challenging to compare insomnia with decreased sleep quality as it is a diagnosis that includes a severe and complex type of reduced sleep quality.
Unlike the present study, several previous studies have mainly focused on nighttime or daytime HRV as a measure of autonomic tone related to sleep quality or dysregulated sleep patterns.Citation34–38 Albeit not directly corresponding to our study design, the results primarily support an association between decreased sleep quality and sympathetic overactivity.
The insignificant results observed when comparing participants’ means imply that changes in HR may not follow the same pattern between individuals within a population. However, on the individual level, our results reflect how an individual’s daily deviations from their own mean sleep quality are associated with their average heart rate during sleep. These findings have important clinical relevance as a short-term decrease in sleep quality may be a risk of increased HR. While a slight increase in HR after a short-term decrease in sleep quality may not be clinically significant, it may be harmful if remained persistent over years. Indeed, resting HR has been suggested as an essential indicator of cardiovascular risk.Citation39–41 This emphasizes the relevance of early personalized interventions when detecting unhealthy sleeping patterns within an individual.When interpreting the results, a few limitations should be taken into consideration. Due to a rather small sample size, there may be a loss of statistical power, possibly restricting the ability to reach statistical significance between individuals. We therefore suggest that future studies aiming to investigate this specific association should include a larger number of individuals. Further, there may limited generalizability, as this occupational cohort consists of 92.6% Japanese office-working men with similar socioeconomic status and education. One can assume that workload as well as working hours may differ depending on nationality, which may in turn affect individual sleeping patterns. Additionally, there may be a participation bias, as there is a possibility that office workers who chose to participate were more health conscious compared to those who declined participation. The risk of an overrepresentation of orthosomnic participants, who meticulously control and optimize their sleeping patterns, was minimized by the randomization in the study design. There is a possibility that participants became more aware of their sleeping patterns during the study period. However, this would not influence a metric such as HR obtained during sleep which is the result of autonomic nervous system activity and therefore cannot be voluntarily controlled.
Nonetheless, the present study provides many strengths in the aspect of study design. Although studying a small sample, daily repeated measures collected with a wearable device provided a large amount of data for analysis. Further, this large number of repeated daily measures allow for analysis where each specific night’s HR was compared with the corresponding subjective sleep quality experienced the following day. The repeated measures additionally allowed for a within-individual analysis, which is not possible when collecting data from one single occasion. The study period is long when compared to previous studies on sleep quality and HR. This difference in the length of assessment is probably due in part to many previous studies using PSG for measurements of sleep physiology, which provides higher accuracy, but is a less flexible and more expensive method for collecting data. In contrast, data collection with wearable devices is under naturalistic conditions, where the participants are observed at home under normal sleep circumstances.
Conclusion
The present study was conducted with wearable devices, which is an innovative and accessible method for future data collection. It contributes to a limited body of evidence on the association between subjective sleep quality and average HR during REMS and NREMS. Our main findings present a within-individual association between subjective sleep quality and average heart rate during REMS and NREMS. Our results may contribute to the development of methods for early detection of unhealthy sleeping patterns and the implementation of precision health procedures for optimizing sleep and overall health. However, more studies with a similar monitoring method should be implemented to reach insight in the associations between sleep quality and HR during different sleep stages.
Abbreviations
AHC, Annual Health Check-up; CMSQ, Cluster Mean Sleep Quality; CI, Confidence Interval; DCMSQ, Deviation from Cluster Mean Sleep Quality; ECG, Electrocardiography; EEG, Electroencephalography; EPAR, Physical Activity Reference for Health Promotion; HR, Heart Rate; HR NREMS, Average Heart Rate during non-REM Sleep; HR REMS, Average Heart Rate during REM Sleep; HRV, Heart Rate Variability; METs, Metabolic Equivalents; MHLW, The Ministry of Health, Labour, and Welfare; MVPA, Moderate-to-Vigorous Intensity Physical Activity; MetS, Metabolic syndrome; NREMS, non-REM sleep; PSG, Polysomnography; PSS-4, Perceived Stress Scale 4; Pre-MetS, Pre-Metabolic Syndrome; RCT, Randomized Controlled Trial; REMS, REM Sleep; REMSD, Rem Sleep Deprivation; SD, Standard Deviation.
Availability of data and materials
We cannot provide public access to individual data due to participant privacy stipulations in accordance with ethical guidelines. Additionally, the written informed consent we obtained from study participants does not include a provision for publicly sharing data. Qualifying researchers may, upon reasonable request, apply to access an aggregated dataset by contacting the corresponding author.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Graduate School of Engineering, The University of Tokyo (approval number: KE18-44). All methods were performed in accordance with relevant guidelines and regulations.
Informed consent: All participants provided written informed consent. The nature of the informed consent complies with the current version of the Declaration of Helsinki, and the current requirements of Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan, whichever provided the greater protections to the participant.Registry and Registration No. of the study/Trial: Not applicable. Non-invasive trials conducted in Japan do not require registration according to Japanese Ethical Guidelines for Medical and Biological Research Involving Human Subjects.Animal Studies: Not applicable.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We thank all staff members at the Center of Innovation, The University of Tokyo for their extensive efforts and support to conduct the study. We would also like to thank all members of Precision Health, The University of Tokyo, for their invaluable assistance, in particular Kayoko Yajima for her help with preparing the dataset.
Additional information
Funding
References
- Roth T. Characteristics and determinants of normal sleep. J Clin Psychiatry. 2004;65(Suppl 16):8–11.
- Mehta R, Giri S, Mallick BN. REM sleep loss-induced elevated noradrenaline could predispose an individual to psychosomatic disorders: a review focused on proposal for prediction, prevention, and personalized treatment. Epma j. 2020;11(4):529–549. doi:10.1007/s13167-020-00222-1
- de Zambotti M, Trinder J, Silvani A, Colrain IM, Baker FC. Dynamic coupling between the central and autonomic nervous systems during sleep: a review. Neurosci Biobehav Rev. 2018;90:84–103. doi:10.1016/j.neubiorev.2018.03.027
- Hoang NH, Liang Z. Knowledge discovery in ubiquitous and personal sleep tracking: scoping review. JMIR mHealth uHealth. 2023. 11:e42750. 10.2196/42750
- Miller DJ, Sargent C, Roach GD. A validation of six wearable devices for estimating sleep, heart rate and heart rate variability in healthy adults. Sensors, 2022 22 16 6317 doi: 10.3390/s22166317.
- Lee T, Cho Y, Cha KS, et al.. Accuracy of 11 wearable, nearable, and airable consumer sleep trackers: prospective multicenter validation study. JMIR mHealth uHealth. 2023;11:e50983. doi:10.2196/50983.
- Zemaityte D, Varoneckas G, Sokolov E. Heart rhythm control during sleep. Psychophysiology. 1984;21(3):279–289. doi:10.1111/j.1469-8986.1984.tb02935.x
- Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993; 328(5):303–307. doi:10.1056/nejm199302043280502
- Cabiddu R, Cerutti S, Viardot G, Werner S, Bianchi AM. Modulation of the sympatho-vagal balance during sleep: frequency domain study of heart rate variability and respiration. Front Physiol. 2012;3:45. doi:10.3389/fphys.2012.00045
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151–161. doi:10.2147/nss.S134864
- Institute of Medicine Committee on. Sleep M. Research. the national academies collection: reports funded by national institutes of health. Colten HR, Altevogt BM, editors. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. National Academies Press (US)Copyright © 2006, National Academy of Sciences., 2006
- Phillips B, Mannino DM. Do insomnia complaints cause hypertension or cardiovascular disease?. J Clin Sleep Med, 2007 3 5 489–494 doi: 10.5664/jcsm.26913.
- Meisinger C, Heier M, Löwel H, Schneider A, Döring A. Sleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population: the MONICA/KORA Augsburg cohort study. Sleep. 2007;30(9):1121–1127. doi:10.1093/sleep/30.9.1121
- Meng L, Zheng Y, Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta-analysis of prospective cohort studies. Hypertens Res. 2013;36(11):985–995. doi:10.1038/hr.2013.70
- Schmid SM, Hallschmid M, Schultes B. The metabolic burden of sleep loss. Lancet Diabetes Endocrinol. 2015;3(1):52–62. doi:10.1016/s2213-8587(14)70012-9
- Sigurdardottir LG, Valdimarsdottir UA, Mucci LA, et al. Sleep disruption among older men and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(5):872–879. doi:10.1158/1055-9965.Epi-12-1227-t
- Schernhammer ES, Laden F, Speizer FE, et al.. Night-shift work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Inst. 2003;95(11):825–828. 10.1093/jnci/95.11.825.
- Garfield V, Joshi R, Garcia-Hernandez J, Tillin T, Chaturvedi N. The relationship between sleep quality and all-cause, CVD and cancer mortality: the Southall and Brent revisited study (SABRE). Sleep Med. 2019;60:230–235. doi:10.1016/j.sleep.2019.03.012
- Karadzic V, Dement WC. Heart rate changes following selective deprivation of rapid eye movement (REM) sleep. Brain Res. 1967;6(4):786–788. doi:10.1016/0006-8993(67)90138-2
- OECD. OECD Reviews of Public Health: Japan; 2019.
- Ellis BW, Johns MW, Lancaster R, Raptopoulos P, Angelopoulos N, Priest RG. The St. Mary’s Hospital sleep questionnaire: a study of reliability. Sleep. 1981;4(1):93–97. doi:10.1093/sleep/4.1.93
- Cohen S, Spacapan S, Oskamp S. Perceived stress in a probability sample of the United States. The social psychology of health. In: The Claremont Symposium on Applied Social Psychology. Sage Publications, Inc; 1988:31–67.
- Teramoto T, Sasaki J, Ueshima H, et al. Metabolic Syndrome. J Atheros Throm. 2008;15(1):1–5. doi:10.5551/jat.E580
- Semanik P, Lee J, Pellegrini CA, Song J, Dunlop DD, Chang RW. Comparison of physical activity measures derived from the fitbit flex and the actigraph GT3X+ in an employee population with chronic knee symptoms. ACR Open Rheumatol. 2020;2(1):48–52. doi:10.1002/acr2.11099
- Exercise and Physical Activity Reference for Health Promotion (EPAR) (2013).
- Fabbri M, Beracci A, Martoni M, Meneo D, Tonetti L, Natale V. Measuring subjective sleep quality: a review. Int J Environ Res Public Health, 2021 18 3 1082 doi: 10.3390/ijerph18031082.
- Faust L, Feldman K, Mattingly SM, Hachen DV, Chawla N. Deviations from normal bedtimes are associated with short-term increases in resting heart rate. Npj Digital Med. 2020;3(1):39. doi:10.1038/s41746-020-0250-6
- Cosgrave J, Phillips J, Haines R, Foster RG, Steinsaltz D, Wulff K. Revisiting nocturnal heart rate and heart rate variability in insomnia: a polysomnography-based comparison of young self-reported good and poor sleepers. J Sleep Res. 2021;30(4):e13278. doi:10.1111/jsr.13278
- Faerman A, Kaplan KA, Zeitzer JM. Subjective sleep quality is poorly associated with actigraphy and heart rate measures in community-dwelling older men. Sleep Med. 2020;73:154–161. doi:10.1016/j.sleep.2020.04.012
- Spiegelhalder K, Fuchs L, Ladwig J, et al. Heart rate and heart rate variability in subjectively reported insomnia. J Sleep Res. 2011;20(1 Pt 2):137–145. doi:10.1111/j.1365-2869.2010.00863.x
- Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60(5):610–615. doi:10.1097/00006842-199809000-00017
- Yang CM, Lin SC, Hsu SC, Cheng CP. Maladaptive sleep hygiene practices in good sleepers and patients with insomnia. J Health Psychol. 2010;15(1):147–155. doi:10.1177/1359105309346342
- Thielmann B, Schierholz RS, Böckelmann I. Subjective and objective consequences of stress in subjects with subjectively different sleep quality-a cross-sectional study. Int J Environ Res Public Health, 2021 18 19 9990 doi: 10.3390/ijerph18199990.
- Werner GG, Ford BQ, Mauss IB, Schabus M, Blechert J, Wilhelm FH. High cardiac vagal control is related to better subjective and objective sleep quality. Biol Psychol. 2015;106:79–85. doi:10.1016/j.biopsycho.2015.02.004
- Sajjadieh A, Shahsavari A, Safaei A, et al. The association of sleep duration and quality with heart rate variability and blood pressure. Tanaffos. 2020;19(2):135–143.
- Kageyama T, Nishikido N, Kobayashi T, Kurokawa Y, Kaneko T, Kabuto M. Self-reported sleep quality, job stress, and daytime autonomic activities assessed in terms of short-term heart rate variability among male white-collar workers. Ind Health. 1998;36(3):263–272. doi:10.2486/indhealth.36.263
- Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. Am J Hypertens. 1999;12(1):63–68. doi:10.1016/s0895-7061(98)00200-3
- Jackowska M, Dockray S, Endrighi R, Hendrickx H, Steptoe A. Sleep problems and heart rate variability over the working day. J Sleep Res. 2012;21(4):434–440. doi:10.1111/j.1365-2869.2012.00996.x
- Böhm M, Reil JC, Deedwania P, Kim JB, Borer JS. Resting heart rate: risk indicator and emerging risk factor in cardiovascular disease. Am J Med. 2015;128(3):219–228. doi:10.1016/j.amjmed.2014.09.016
- Fox K, Borer JS, Camm AJ, et al.. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50(9):823–830. 10.1016/j.jacc.2007.04.079.
- Cooney MT, Vartiainen E, Laatikainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. 2010;159(4):612–619.e3. doi:10.1016/j.ahj.2009.12.029
