Abstract
Introduction
Obstructive sleep apnea (OSA) is a respiratory disorder characterized by chronic intermittent hypoxia and fragmented sleep, leading to inflammatory response and oxidative stress. However, the differences in immune inflammatory response in OSA patients with different severity remain unclear.
Purpose
This study aims to examine the differences in peripheral blood immune cells and their risk factors in OSA patients.
Patients and Methods
A total of 277 snoring patients from the Sleep Respiratory Disorder Monitoring Center of Zhongnan Hospital of Wuhan University were recruited in this study. According to the diagnosis and severity criteria of OSA, the included patients were further divided into simple snoring, mild, moderate, and severe groups. Peripheral blood immune cell counts including white blood cells, neutrophils, lymphocytes, monocytes, eosinophils, basophils, red blood cells, platelets, and polysomnography indicators were collected from the patients.
Results
Compared with simple snoring patients, the OSA patients had increased circular monocyte and basophil count levels. In addition, correlation analysis results indicated that monocyte count was positively associated with chronic obstructive pulmonary disease (COPD), smoking, apnea-hypopnea index (AHI), the longest apnea duration, and Oxygen desaturation index (ODI), and negatively correlated with average SpO2 in snoring patients. Finally, multiple linear regression analysis revealed that AHI, COPD, smoking, and maximum heart rate were independent predictors of monocyte count.
Conclusion
OSA patients had a significant increase in their peripheral blood monocyte count. AHI, COPD, smoking, and maximum heart rate were risk factors for increased peripheral blood monocyte count in OSA patients. These findings suggest that peripheral blood monocytes can be considered an inflammatory biomarker of OSA.
Introduction
Obstructive sleep apnea (OSA) is characterized by upper respiratory collapse, intermittent hypoxia, recurrent arousals, and sleep fragmentation. This results in secondary sympathetic nerve activation, oxidative stress, and systemic inflammation.Citation1 Inflammatory response and oxidative stress in OSA patients are risk factors for the occurrence and development of various diseases, including cardiovascular and cerebrovascular diseases, metabolic diseases, cognitive impairment, and tumors.Citation2,Citation3 It is reported that recurrent intermittent hypoxia and increased oxidative stress during sleep play an important role in immune dysfunction in OSA patients.Citation4–6
Previous studies found that peripheral blood cells can reflect the immune inflammatory response.Citation7,Citation8 They often were regarded as easily measurable biomarkers of inflammation and oxidative stress in OSA, such as neutrophils, lymphocytes, monocytes, hemoglobin, and hematocrit, as well as new biomarkers including neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and systemic inflammatory index (SII).Citation9–11 In addition, immune cell-driven inflammation also plays a crucial role in the pathogenesis of OSA. Some pro-inflammatory markers released by peripheral blood cells such as C-reactive protein (CRP), interleukin (IL)‑6, tumor necrosis factor-alpha (TNF-α), erythrocyte sedimentation rate (ESR) were also strongly associated with OSA severity.Citation12,Citation13 It is reported that long-term exposure to intermittent hypoxia promotes neutrophil and monocyte infiltration into the liver, leading to strong inflammatory pathways including IL-16 and TNF-α.Citation14 Polymorphonuclear neutrophils (PMN) are also key cells in the inflammatory process. The increased number of PMN has been found to play a potential role in the inflammation associated with OSA patients. IL-6, β2-adrenergic receptor (ADRB2), and IL-8 may contribute to regulating the activation of PMN, including migration, recruitment, degranulation, exocytosis, and respiratory burst.Citation15
Therefore, it is crucial to explore the differences in peripheral blood immune cells in patients with obstructive sleep apnea. We assume that peripheral blood cell parameters could indicate the inflammation degree and be a predictive tool for evaluating hypoxia severity in OSA. However, the results from previous studies have been inconsistent, and the reasons for the differences in peripheral blood cells remain unclear. As a cross-sectional study, this study aimed to analyze differences in immune cells in OSA patients of different severity and identify associated risk factors.
Materials and Methods
Study Population
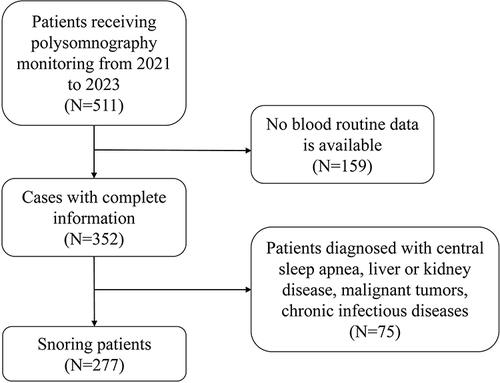
This retrospective study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University (No.2023297K, December 27, 2023) and waived written informed consent from all patients. This study collected 277 snoring patients receiving polysomnography (PSG) monitoring at the Sleep Respiratory Disorder Monitoring Center of Zhongnan Hospital of Wuhan University from 2021 to 2023. The screening process for 277 patients is shown in .
Diagnosis and disease severity criteria for OSA were defined according to the guidelines.Citation16 The Apnea hypopnea index (AHI) was calculated as the average number of apnea and hypopnea events per hour. Snoring patients were divided into the simple snoring group (AHI <5) and the OSA group (AHI ≥5). The severity of OSA patients was further classified into subgroups of mild (5 ≤ AHI<15), moderate (15 ≤ AHI<30), and severe (AHI ≥30).Citation17 Patients diagnosed with central sleep apnea, liver or kidney disease, malignant tumors, chronic infectious diseases, inflammatory bowel disease, and hematological disorders such as leukemia, anemia, or myelodysplastic syndrome were excluded from this study.
Data Collection
Data on the patient characteristics collected in this study included age, sex, body mass index (BMI), smoking status, and comorbidities. Peripheral blood immune cell was evaluated by complete blood count (CBC), including white blood cell (WBC), monocytes, lymphocytes, neutrophils, platelets, eosinophils, and basophils, hemoglobin, red blood cell count (RBC), NLR, PLR, systemic inflammation index (SII: neutrophils × platelets/lymphocytes) were recorded. Additionally, AHI, Oxygen desaturation index (ODI, number of oxygen desaturations per hour of sleep), the sum of all desaturations, the longest apnea duration, the minimum and average pulse oxygen saturation (SpO2), the minimum, average, and maximum heart rate during PSG were collected. Apnea was defined as a more than 90% decrease in airflow for 10 seconds. Hypopneas was defined as a reduction in airflow of at least 30% lasting for 10 seconds or more, accompanied by oxygen desaturation of 3% or more, or an electroencephalogram (EEG) awakening.Citation18
Statistical Analysis
Continuous variables were presented as median with interquartile range (IQR). Student’s t-test was performed for normally distributed variables, and the Mann–Whitney test was employed for non-normally distributed variables between two groups. The one-way analysis of variance (ANOVA) test was used to compare normally distributed variables, and the Kruskal–Wallis H-test was used for non-normally distributed variables between OSA subgroups. Categorical variables were expressed as numbers (percentages). The chi-square or Fisher’s exact test was conducted for two groups, and the Kruskal–Wallis H-test was used for multiple groups. The Bonferroni test was conducted as a post-test for multiple comparisons. The correlation between variables was examined using Spearman’s or Pearson’s test. The point-biserial correlation, a special form of Pearson’s test was used to assess correlation between continuous variables and dichotomous chronic disease variables. Multiple linear regression analysis was conducted to assess the independent associations between biochemical, clinical, and polysomnography variables. Data analysis used SPSS software (version 25.0, IBM Corporation). Statistical significance was set at P <0.05.
Results
Demographic and Clinical Profiles
After the screening, 277 snoring patients (196 with OSA and 81 simple snoring) were included in this study. The median age of OSA patients was 45 (34–61) years, with a body mass index (BMI) of 29 (25.4–33.2) kg/m2 and 117 (59.7%) being male. The proportion of smokers, hypertension, diabetes, chronic obstructive pulmonary disease (COPD), and asthma in OSA patients was 21.3%, 39.8%, 15.8%, 8.2%, and 7.7%, respectively. There were significant differences in BMI (P = 0.009), age (P < 0.001), sex (P < 0.001), hypertension (P < 0.001), and smoking (P = 0.004) between the simple snoring and OSA group. Furthermore, significant differences were shown in age (P < 0.001), hypertension (P < 0.001), and smoking (P = 0.017) between subgroups. The detailed demographic and clinical data are shown in .
Table 1 Clinical Characteristics of Snoring Patients
Differences in Blood Immune Cells in Snoring Patients
The hematological profiles are shown in . Compared with the simple snoring group, the OSA patients had increased levels of peripheral blood monocyte (P < 0.001) and basophil (P = 0.012) counts. After conducting a post hoc analysis, there was a significant difference in the monocyte count between the simple snoring group and the severe OSA group (P = 0.001). Additionally, the post-test results showed a statistically significant difference in basophil count between the simple snoring group and the moderate OSA group (P = 0.036). No significant differences were observed in other peripheral blood parameters ().
Table 2 Peripheral Blood Immune Cells Expression in Snoring Patients
Polysomnographic Parameters in Snoring Patients
The polysomnography indicators in snoring patients are presented in . There were significant differences in AHI (P < 0.001), the longest apnea duration (P < 0.001), the sum of all desaturations (P < 0.001), ODI (P < 0.001), minimum SpO2 (P < 0.001), average SpO2 (P < 0.001), and minimum heart rate (P < 0.001) between OSA patients and simple snoring patients. Moreover, significant differences were observed in AHI, the longest apnea duration, the sum of all desaturations, and ODI between four subgroups. However, no significant difference was observed in maximum and average heart rate between OSA and simple snoring patients.
Table 3 Polysomnographic Parameters in Snoring Patients
Correlation Between Peripheral Blood Cells with Clinical and Polysomnographic Parameters in Snoring Patients
Spearman or Pearson test was conducted to investigate the relationship between blood immune cells with clinical and polysomnographic parameters (). Monocytes were associated positively with COPD (r = 0.224, P < 0.001), smoking (r = 0.261, P < 0.001), AHI (r = 0.261, P < 0.001), the longest apnea duration (r = 0.181, P = 0.002), the sum of all desaturations (r = 0.169, P = 0.005), ODI (r = 0.194, P = 0.001) respectively, and negatively with average SpO2 (r = −0.179, P = 0.003). Moreover, a significant correlation was observed between basophils and AHI (r = 0.12, P = 0.046) and ODI (r = 0.136, P = 0.024). The correlation analysis results for other blood parameters including WBC, neutrophils, lymphocytes, eosinophils, RBC, hemoglobin, platelets, NLR, PLR, and SII are presented in . The differences in peripheral blood immune cells in snoring patients with or without hypertension, COPD, and smoking are shown in .
Table 4 Correlation Between Blood Immune Cells with Clinical and Polysomnographic Parameters in Snoring Patients
Table 5 Peripheral Blood Immune Cell Expression in Snoring Patients with and without Clinical Comorbidities
Risk Factors for Increased Peripheral Blood Monocyte and Basophil Count in Snoring Patients
In multiple linear regression, monocytes were independently associated with AHI (β = 0.314, P = 0.005), COPD (β = 0.15, P = 0.029), smoking (β = 0.189, P = 0.004), and maximum heart rate (β = 0.161, P = 0.015) (). However, there was no significance of the basophil’s regression model ().
Table 6 Multiple Linear Regression Analysis for Peripheral Blood Monocyte Count in Snoring Patients
Table 7 Multiple Linear Regression Analysis for Peripheral Blood Basophil Count in Snoring Patients
Discussion
OSA leads to various pathophysiological processes in the body such as sympathetic nervous system hyperactivity, oxidative stress, metabolic disorders, endothelial dysfunction, and airway inflammation, which causes systemic damage to multiple organs including immune dysfunction.Citation19 In our study, we investigated the differences in all peripheral blood immune cells and further analyzed risk factors for these differences in snoring patients of different severity. We found that the OSA patients had increased peripheral blood monocyte and basophil counts compared with simple snoring patients. Moreover, they increased gradually with the OSA severity. In addition, multiple linear regression analysis identified AHI, COPD, smoking, and maximum heart rate as independent predictors of monocyte count in OSA patients.
The level of blood monocytes is an indicator of the body’s inflammatory state.Citation20 Sleep deprivation or restriction increases circulating monocytes and inflammatory cytokine production.Citation21,Citation22 It was reported that the two fundamental pathophysiological mechanisms of OSA characterized by chronic intermittent hypoxia and sleep fragmentation interact with the immune system triggered by monocytes.Citation23 In addition, monocytes increase NLRP3 (NLR family pyrin domain containing 3) signaling under intermittent hypoxia conditions, leading to systemic inflammatory response.Citation24 Consistent with our results, a previous study demonstrated a positive association between monocytes and the severity of OSA measured by AHI.Citation25 At the same time, SpO2 which considers the duration of intermittent oxygen desaturations was strongly and independently associated with specific inflammatory parameters including monocytes.Citation26 Besides, studies have demonstrated a positive correlation between basophils and the severity of OSA, which is consistent with our findings.Citation25,Citation27 However, we did not find any risk factors for elevated basophils in OSA patients, possibly due to insufficient sample size and data to analyze and draw correct conclusions. Thus, larger detailed investigations are needed to reveal the relationship between basophils and OSA in the future. Our findings suggest that intermittent hypoxia triggers systemic inflammation, leading to an increase in circulating monocytes. Therefore, peripheral monocyte count may be considered a valuable assessment tool for intermittent hypoxia in OSA.
COPD is a chronic airway inflammatory disease with persistent airflow obstruction due to exposure to inhaled particulate matter such as cigarettes and air pollutants.Citation28 COPD and OSA coexist and are accompanied by episodes in the same patient, defined as the “overlap syndrome”.Citation23 Patients with overlap syndrome experience a more significant reduction in nocturnal oxygen saturation and increased daytime hypercapnia compared to patients with either COPD or OSA alone.Citation29 These patients exhibit elevated levels of pro-inflammatory cytokines and reduced levels of anti-inflammatory cytokines resulting in a sustained state of chronic inflammation.Citation30 Therefore, more severe airway inflammatory states were observed in patients with overlap syndrome compared to those with COPD alone.Citation31 Likewise, a clinical study indicated that patients with overlap syndrome exhibit more severe endothelial damage, stronger inflammatory response, and lower cellular immune function.Citation32 Smoking can mobilize an increase in blood monocytes, which exacerbate inflammation in various diseases including chronic lung injury, tuberculosis, and ischemic brain injuries.Citation33–35 A systematic review showed that smokers have elevated levels of AHI and reduced minimum SaO2 levels in OSA patients.Citation36 In brief, smoking leads to OSA development through various mechanisms, including airway inflammation, changes in sleep structure, unstable awakening mechanisms, and alterations in upper airway neuromuscular function.Citation37,Citation38 Further, smoking increases systemic oxidative stress and inflammatory response.Citation39 In our study, COPD and smoking were independently associated with peripheral monocyte count, suggesting concomitant COPD exacerbates inflammation levels in OSA patients. Therefore, assessing COPD comorbidities and smoking status might be important for OSA patients’ inflammation levels in clinical practice.
In addition, our results showed that the maximum heart rate was associated with the monocytes. Hypoxemia and hypercapnia act on oxygen and carbon dioxide through peripheral and central chemoreceptors to increase the activity of the sympathetic nervous system in OSA.Citation1 Moreover, OSA patients experience elevated heart rate and muscle sympathetic activity during wakefulness, with further increases in blood pressure and sympathetic activity during sleep.Citation40 The heart rate also increases during inflammation situations. Thus, the maximum heart rate in OSA patients may also be related to systematic inflammation conditions. OSA patients can conveniently monitor their sympathetic nervous activity and inflammatory response during sleep by measuring their heart rate.
Lymphocytes also play an important role in inflammation and immune disease as executors of immune function.Citation41 Vicente E et al found increased CD4+ T cells in pharyngeal lavage of OSA patients than those in controls.Citation42 In a previous study investigating phenotypic changes of various peripheral blood immune cells in patients with sleep apnea/hypopnea syndrome (OSAHS), significant differences were found in CD4+ and CD8+ T lymphocytes, CD19+ B cells, and natural killer (NK) like T cells between OSAHS patients and control group. The authors also found the CD4+/CD8+ T lymphocyte ratio is positively correlated with AHI and negatively correlated with the lowest SaO2. However, no significant difference was observed in the percentage of CD3+ total T cells and dendritic cells between the OSAHS and control groups.Citation43 Consistent with our results, no significant differences were observed in peripheral blood total lymphocyte count. We will analyze the differential expression of peripheral blood lymphocyte subsets in OSA patients in further studies.
In addition, immune cell-driven inflammation is also associated with OSA and its severity. Increased IL-8 levels were observed in peripheral blood mononuclear cells supernatant of OSA children than in controls.Citation44 Likewise, a recent study involving different severe patients with OSA found that serum markers of high-sensitivity (hs)-CRP, TGF-β1, TNF-α, and IL-6 in OSA patients were significantly increased compared with normal controls.Citation45 However, Guasti L et al found that there was no difference in TNF-α releasing from peripheral blood mononuclear cells (PBMCs) and IL-8 produced by PMNs between OSA and control groups when similar prevalence of cardiovascular risk factors and cardio-metabolic therapies.Citation46 These differences may have occurred because only moderate and severe OSA patients with AHI greater than 20 were included in this study. Besides, the different statistical power and designs of these researches may also have influenced the final results. This may explain the differences in results between these studies and also highlights that future studies may take into account cardiovascular risk factors and cardiovascular treatments when exploring the role of cytokine imbalance and inflammation in OSA patients.
It was found that systemic inflammatory markers CRP, ESR, and NLR were correlated with OSA severity.Citation47 The SII, NLR, and PLR reflect the body’s extensive immune and inflammatory states and have been used as new white blood-cell-based inflammatory indices in OSA. A cross-sectional study consistent with our results demonstrated that no significant difference was observed in the SII, NLR, and PLR between the different severe OSA subgroups. The further subgroup analysis showed a significant positive correlation between AHI and SII in the severe OSA subgroup.Citation9
Our research has some limitations. Firstly, this study was a retrospective data analysis from a single center. The co-location of data collection and laboratory testing in a single hospital setting may lead to selection bias. Secondly, the sample size of OSA patients in our study was relatively small, which may limit the analysis and interpretation of the results. Thirdly, peripheral blood immune cell counts and polysomnography parameters from healthy subjects were absent. Therefore, further studies are required to validate the results in a more diverse and larger population.
Conclusion
Our study showed that increased levels of blood monocyte and basophil counts were found in OSA patients compared with simple snorers. AHI, COPD, smoking, and maximum heart rate were risk factors for elevated peripheral blood monocyte count in OSA patients. These findings suggest that monocytes in peripheral blood could serve as an inflammatory biomarker for OSA, which contributes to a better understanding of hypoxia and systemic inflammation levels in OSA patients.
Statement of Ethics
This study adhered to the Guidelines for Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) and complied with the tenets underlying the Declaration of Helsinki. This retrospective study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University (No.2023297K) and waived written informed consent from all patients. We have de-identified all patient information to protect the participants’ privacy. The date of approval by the Ethics Committee was December 27, 2023.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
Additional information
Funding
References
- Lévy P, Kohler M, McNicholas WT, et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers. 2015;1:15015. doi:10.1038/nrdp.2015.15
- Redline S, Azarbarzin A, Peker Y. Obstructive sleep apnoea heterogeneity and cardiovascular disease. Nat Rev Cardiol. 2023;20(8):560–573. doi:10.1038/s41569-023-00846-6
- Gleeson M, McNicholas WT. Bidirectional relationships of comorbidity with obstructive sleep apnoea. Eur Respir Rev. 2022;31(164):210256. doi:10.1183/16000617.0256-2021
- Kim DK, Lee BC, Park KJ, Son GM. Effect of obstructive sleep apnea on immunity in cases of chronic rhinosinusitis with nasal polyps. Clin Exp Otorhinolaryngol. 2021;14(4):390–398. doi:10.21053/ceo.2020.02250
- Geovanini GR, Wang R, Weng J, et al. Elevations in neutrophils with obstructive sleep apnea: the Multi-Ethnic Study of Atherosclerosis (Mesa). Int J Cardiol. 2018;257:318–323. doi:10.1016/j.ijcard.2017.10.121
- Said EA, Al-Abri MA, Al-Saidi I, et al. Altered blood cytokines, CD4 T cells, NK and neutrophils in patients with obstructive sleep apnea. Immunol Lett. 2017;190:272–278. doi:10.1016/j.imlet.2017.08.009
- Nøst TH, Alcala K, Urbarova I, et al. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur J Epidemiol. 2021;36(8):841–848. doi:10.1007/s10654-021-00752-6
- Luo J, Thomassen JQ, Nordestgaard BG, Tybjaerg-Hansen A, Frikke-Schmidt R. Neutrophil counts and cardiovascular disease. Eur Heart J. 2023;44(47):4953–4964. doi:10.1093/eurheartj/ehad649
- Kim M, Cho SW, Won TB, Rhee CS, Kim JW. Associations between systemic inflammatory markers based on blood cells and polysomnographic factors in obstructive sleep apnea. Clin Exp Otorhinolaryngol. 2023;16(2):159–164. doi:10.21053/ceo.2022.01368
- Zorlu D, Ozyurt S, Bırcan HA, Erturk A. Do complete blood count parameters predict diagnosis and disease severity in obstructive sleep apnea syndrome? Eur Rev Med Pharmacol Sci. 2021;25(11):4027–4036. doi:10.26355/eurrev_202106_26044
- Deniz Doğan 1 RÖ. Possible changes in hematological parameters of the patients with obstructive sleep apnea. Gulhane Med J. 2019;61:97–102.
- Yi M, Zhao W, Tan Y, et al. The causal relationships between obstructive sleep apnea and elevated CRP and TNF-α protein levels. Ann Med. 2022;54(1):1578–1589. doi:10.1080/07853890.2022.2081873
- Popadic V, Brajkovic M, Klasnja S, et al. Correlation of dyslipidemia and inflammation with obstructive sleep apnea severity. Front Pharmacol. 2022;13:897279. doi:10.3389/fphar.2022.897279
- Gaucher J, Montellier E, Vial G, et al. Long-term intermittent hypoxia in mice induces inflammatory pathways implicated in sleep apnea and steatohepatitis in humans. iScience. 2024;27(2):108837. doi:10.1016/j.isci.2024.108837
- Ferrari M, Sica E, De Bernardi F, et al. Reduction of IL-6, IL-8 and β2-ADRENOCEPTOR mRNA levels in circulating polymorphonuclear leukocytes after adenotonsillectomy in children with obstructive sleep apnea syndrome. Sleep Med. 2024;114:82–85. doi:10.1016/j.sleep.2023.12.017
- Berry RB, Brooks R, Gamaldo CE, et al; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.2. Darien, Illinois: American Academy of Sleep Medicine; 2015.
- American Academy of Sleep Medicine. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of sleep medicine task force. Sleep. 1999;22(5):667–689.
- Gottlieb DJ, Punjabi NM. Diagnosis and Management of Obstructive Sleep Apnea: a Review. JAMA. 2020;323(14):1389–1400. doi:10.1001/jama.2020.3514
- Drager LF, Togeiro SM, Polotsky VY, Lorenzi G. Obstructive sleep apnea a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62(7):569–576. doi:10.1016/j.jacc.2013.05.045
- Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11(11):762–774. doi:10.1038/nri3070
- Carroll JE, Carrillo C, Olmstead R, et al. Sleep deprivation and divergent toll-like receptor-4 activation of cellular inflammation in aging. Sleep. 2015;38(2):205–211. doi:10.5665/sleep.4398
- Lasselin J, Rehman JU, Åkerstedt T, Lekander M, Axelsson J. Effect of long-term sleep restriction and subsequent recovery sleep on the diurnal rhythms of white blood cell subpopulations. Brain Behav Immun. 2015;47:93–99. doi:10.1016/j.bbi.2014.10.004
- Maniaci A, Iannella G, Cocuzza S, et al. Oxidative stress and inflammation biomarker expression in obstructive sleep apnea patients. J Clin Med. 2021;10(2):277. doi:10.3390/jcm10020277
- Díaz-García E, García-Tovar S, Alfaro E, et al. Inflammasome activation: a keystone of proinflammatory response in obstructive sleep apnea. Am J Respir Crit Care Med. 2022;205(11):1337–1348. doi:10.1164/rccm.202106-1445OC
- Fan Z, Lu X, Long H, Li T, Zhang Y. The association of hemocyte profile and obstructive sleep apnea. J Clin Lab Anal. 2019;33(2):e22680. doi:10.1002/jcla.22680
- Pau MC, Zinellu A, Mangoni AA, et al. Evaluation of inflammation and oxidative stress markers in patients with Obstructive Sleep Apnea (OSA). J Clin Med. 2023;12(12):3935. doi:10.3390/jcm12123935
- Cummins E, Waseem R, Piyasena D, et al. Can the complete blood count be used as a reliable screening tool for obstructive sleep apnea? Sleep Breath. 2022;26(2):613–620. doi:10.1007/s11325-021-02383-3
- Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet. 2022;399(10342):2227–2242. doi:10.1016/s0140-6736(22)00470-6
- McNicholas WT. Chronic obstructive pulmonary disease and obstructive sleep apnea: overlaps in pathophysiology, systemic inflammation, and cardiovascular disease. Am J Respir Crit Care Med. 2009;180(8):692–700. doi:10.1164/rccm.200903-0347PP
- Chaszczewska-Markowska M, Górna K, Bogunia-Kubik K, Brzecka A, Kosacka M. The influence of comorbidities on chemokine and cytokine profile in obstructive sleep apnea patients: preliminary results. J Clin Med. 2023;12(3):801. doi:10.3390/jcm12030801
- Wang Y, Hu K, Liu K, et al. Obstructive sleep apnea exacerbates airway inflammation in patients with chronic obstructive pulmonary disease. Sleep Med. 2015;16(9):1123–1130. doi:10.1016/j.sleep.2015.04.019
- Wang J, Li X, Hou WJ, Dong LX, Cao J. Endothelial function and T-lymphocyte subsets in patients with overlap syndrome of chronic obstructive pulmonary disease and obstructive sleep apnea. Chin Med J. 2019;132(14):1654–1659. doi:10.1097/cm9.0000000000000312
- Sangani RG, Deepak V, Anwar J, Patel Z, Ghio AJ. Cigarette smoking, and blood monocyte count correlate with chronic lung injuries and mortality. Int J Chronic Obstr Pulm Dis. 2023;18:431–446. doi:10.2147/copd.S397667
- Baluku JB, Nabwana M, Kansiime G, Nuwagira E. Cigarette smoking is associated with an increase in blood monocytes in people with tuberculosis: a cross-sectional study. Medicine. 2022;101(37):e30737. doi:10.1097/md.0000000000030737
- Li HD, Li XP, Gao SM, et al. Exposure to cigarette smoke augments post-ischemic brain injury and inflammation via mobilization of neutrophils and monocytes. Front Immunol. 2019;10:2576. doi:10.3389/fimmu.2019.02576
- Zeng X, Ren Y, Wu K, et al. Association between smoking behavior and obstructive sleep apnea: a systematic review and meta-analysis. Nicotine Tob Res. 2023;25(3):364–371. doi:10.1093/ntr/ntac126
- Pataka A, Kotoulas S, Kalamaras G, et al. Does Smoking Affect OSA? What about smoking cessation? J Clin Med. 2022;11(17):5164. doi:10.3390/jcm11175164
- McNicholas WT, Hansson D, Schiza S, Grote L. Sleep in chronic respiratory disease: COPD and hypoventilation disorders. Eur Respir Rev. 2019;28(153):190064. doi:10.1183/16000617.0064-2019
- Caliri AW, Tommasi S, Besaratinia A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat Res Rev Mutat Res. 2021;787:108365. doi:10.1016/j.mrrev.2021.108365
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. doi:10.1172/jci118235
- Saigusa R, Winkels H, Ley K. T cell subsets and functions in atherosclerosis. Nat Rev Cardiol. 2020;17(7):387–401. doi:10.1038/s41569-020-0352-5
- Vicente E, Marin JM, Carrizo SJ, et al. Upper airway and systemic inflammation in obstructive sleep apnoea. Eur Respir J. 2016;48(4):1108–1117. doi:10.1183/13993003.00234-2016
- Xie H, Yin J, Bai Y, Peng H, Zhou X, Bai J. Differential expression of immune markers in the patients with obstructive sleep apnea/hypopnea syndrome. Eur Arch Otorhinolaryngol. 2019;276(3):735–744. doi:10.1007/s00405-018-5219-6
- Ke D, Kitamura Y, Lejtenyi D, Mazer B, Brouillette RT, Brown K. Enhanced interleukin-8 production in mononuclear cells in severe pediatric obstructive sleep apnea. Allergy Asthma Clin Immunol. 2019;15:23. doi:10.1186/s13223-019-0338-1
- Tosun F, Babayigit C, Dikmen N, Dogan S, Dirican E. The effect of continuous positive airway pressure treatment on inflammatory parameters and periostin levels in patients with obstructive sleep apnea syndrome. Sleep Breath. 2023;27(1):275–282. doi:10.1007/s11325-022-02616-z
- Guasti L, Marino F, Cosentino M, et al. Cytokine production from peripheral blood mononuclear cells and polymorphonuclear leukocytes in patients studied for suspected obstructive sleep apnea. Sleep Breath. 2011;15(1):3–11. doi:10.1007/s11325-009-0315-x
- Zota IM, Adam CA, Marcu DTM, et al. CPAP influence on readily available inflammatory markers in OSA-A Pilot study. Int J Mol Sci. 2022;23(20):12431. doi:10.3390/ijms232012431