Abstract
Obstructive sleep apnea (OSA) in children is a highly prevalent disorder caused by a conglomeration of complex pathophysiological processes, leading to recurrent upper airway dysfunction during sleep. The clinical relevance of OSA resides in its association with significant morbidities that affect the cardiovascular, neurocognitive, and metabolic systems. The American Academy of Pediatrics recently reiterated its recommendations that children with symptoms and signs suggestive of OSA should be investigated with polysomnography (PSG), and treated accordingly. However, treatment decisions should not only be guided by PSG results, but should also integrate the magnitude of symptoms and the presence or absence of risk factors and signs of OSA morbidity. The first-line therapy in children with adenotonsillar hypertrophy is adenotonsillectomy, although there is increasing evidence that medical therapy, in the form of intranasal steroids or montelukast, may be considered in mild OSA. In this review, we delineate the major concepts regarding the pathophysiology of OSA, its morbidity, diagnosis, and treatment.
Introduction
Children with obstructive sleep apnea (OSA) experience partial or complete obstruction of the upper airway during sleep, with resultant oxygen desaturation and hypercapnia, leading to increasing respiratory effort and attendant changes in intrathoracic pressures, ultimately culminating in subcortical or cortical arousals. Following such arousals, the child will typically drift back to sleep, and the cycle then repeats itself, with similar episodes occurring throughout the night and resulting in sleep fragmentation and nonrestorative sleep.Citation1 It is now recognized that pediatric OSA is not only a common health problem, but also one that can result in significant morbidity. Estimates of prevalence vary, depending on the populations studied and on the stringency of the diagnostic criteria but are traditionally reported to range between 1%–5%, with the peak prevalence occurring at 2–8 years of age.Citation1–Citation5
The distinctive symptoms of OSA in children are remarkably scarce and usually require a high level of suspicion or alternatively, require systematic implementation of explorative screening questions to enable their detection. Common nighttime symptoms include snoring, excessive sweating, restless sleep, mouth breathing, apneas, gasping, labored or paradoxical breathing, and hyperextension of the neck during sleep. Daytime symptoms most commonly include difficulty concentrating, behavioral and mood problems, morning headaches, excessive daytime sleepiness (EDS), and failure to thrive.Citation1
In this review, we will selectively identify issues of contention in pediatric OSA that address aspects of the pathophysiology, morbidity, diagnosis, and treatment of the disease. As such, this work is not meant to serve as a comprehensive and exhaustive overview, but rather, to illustrate and invigorate the debate on issues for which either conclusive evidence is lacking or a substantial level of conflictive evidence is present.
Pathophysiology of OSA
Although interactively related, the pathophysiological factors involved in OSA can be broadly and arbitrarily divided into anatomical factors that effectively reduce airway caliber and those that promote increased upper airway collapsibility (). Examples of the former include craniofacial factors, eg, a small or retropositioned mandible, a large or retropositioned tongue, increased pharyngeal fat pads, and hypertrophic upper airway lymphoid tissues (particularly of the adenoids and tonsils). Among the factors underlying collapsibility, the presence of upper airway inflammation and altered neurological reflexes involving respiratory control of upper airway muscles emerge as the most prominent. These elements readily explain why children at higher risk of OSA include those with craniofacial syndromes (eg, Treacher Collins syndrome, Crouzon syndrome, Apert syndrome, and Pierre Robin sequence), achondroplasia, cerebral palsy, neuromuscular disorders, myelomeningocele, sickle cell disease, trisomy 21, allergic rhinitis, asthma, micrognathia, mucopolysaccharidoses, macroglossia, Afro Caribbean race, and those who are obese.Citation6 Notwithstanding, the fundamental understanding of the individual mechanistic factors explaining why children with similar degrees of adenotonsillar hypertrophy may either suffer from OSA or not even snore during sleep remains somewhat elusive.
Figure 1 Pathophysiological factors involved in pediatric OSA.
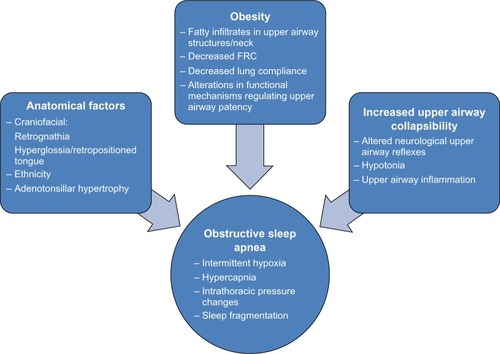
To resolve some of these issues, Arens et al used magnetic resonance imaging (MRI) techniques to delineate in detail the upper airway and surrounding tissues as well as the lower facial skeletal structure, in children with OSA.Citation7 The volumetric measurements obtained indicated that the adenoids and tonsils were significantly increased in children with OSA compared with matched controls, concomitant with smaller upper airway volumes. Furthermore, in this case-control study, when the percent difference of the combined tonsil and adenoidal volume between each subject and his/her corresponding age-, height-, weight-, gender-, and ethnicity-matched control was plotted against the apnea hypopnea index, a positive correlation was seen. The volume of the soft palate was also noted to be on average 30% larger in subjects with OSA, adding additional restriction to the airway lumen size. However, no study has addressed the major question as to what factors differ between children with OSA and no OSA, when matched for the degree of adenotonsillar tissues, or alternatively, why in otherwise similar children with OSA, adenotonsillectomy (AT) may yield different surgical outcomes as far as the degree of resolution of OSA. Furthermore, the interactions between obesity and adenotonsillar hypertrophy are only now being elucidated.Citation8–Citation10
In addition to enlarged adenoids and tonsils, children with OSA have recently been shown to also have hypertrophy/hyperplasia of the lymphoid tissues in other regions of the airway as well, such as in the deep cervical lymph nodes (ie, those outside Waldeyer’s ring).Citation11 Upper respiratory tract perturbations, such as prominence of the inferior nasal turbinates, deviation of the nasal septum, middle ear effusions, and opacification of the sinuses have also been described.Citation12 These observations raise the question as to whether there is in fact, a broader disorder affecting the airway as a whole, mediated by inflammatory processes, chronic or recurrent infectious processes, or a combination thereof.Citation13 In other words, are there unique mechanisms that underlie the lymphoid tissue proliferation that leads to OSA as compared with mechanisms that lead to such proliferation but that do not elicit OSA?
The exact mechanisms underlying follicular lymphoid proliferation and hyperplasia of the tonsils and adenoids remain poorly understood. When tonsillar tissues from children with OSA were placed in an in vitro culture system, the proliferative rates of the associated cluster of differentiation (CD)3, CD4, and CD8 cells were higher compared with tonsillar tissues from children with recurrent tonsillitis.Citation14,Citation15 Furthermore, the proinflammatory cytokines tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1α were more highly expressed in the OSA-derived tonsils. It is postulated that respiratory viruses and possibly recurrent vibration of the upper airway wall may promote localized inflammation. Exhaled breath condensate levels of leukotriene B4 and cysteinyl leukotrienes have been reported to be higher in children with OSA.Citation16 Similarly, induced sputum from children with OSA has been shown to exhibit increased neutrophilia compared with controls.Citation17
As indicated above, anatomical factors are clearly not the whole story for OSA in children: we have all seen children with large kissing tonsils who do not have OSA. Furthermore, the apnea–hypopnea index (AHI) has not been shown to directly correlate with airway volume,Citation7 and the coefficients of correlation between AHI and adenotonsillar size have been found to be usually relatively weak, albeit statistically significant,Citation9,Citation18 suggesting that other factors feature in the pathophysiology of pediatric OSA. The concept of airway collapsibility has thus been proposed to provide a more comprehensive and unified approach to this issue.
One of the earliest studies of airway collapsibility in children with OSA was by Marcus et al.Citation19 In the Starling resistor modelCitation20 of the upper airway, under conditions of flow limitation, the maximum inspiratory airflow is determined by the pressure changes upstream (nasal) to a collapsible locus of the upper airway and is independent of the downstream (tracheal) pressure generated by the diaphragm. The pressure at which the upper airway collapses has been termed the critical closing pressure (Pcrit). Pcrit is thus, an objective measure of airway collapsibility and was found to be higher (ie, less negative) in children with OSA than in those with primary snoring and correlated with the AHI. In other words, children with OSA had demonstrably more collapsible upper airways during sleep. However, during wakefulness, active neural processes preserve upper airway patency and make it difficult to recognize such increased collapsibility. Interestingly, the surgical treatment of OSA was associated with a decline in Pcrit, ie, the upper airways became less collapsible post-AT.Citation19
Gozal and Burnside therefore postulated that measurement of upper airway dynamics using acoustic pharyngometry in the awake child, before and after application of local anesthetic to the pharyngeal introitus, may provide a useful clinical adjunct to the diagnosis of OSA.Citation21 Upper airway collapsibility was determined from the percentage change in cross-sectional area before and after topical anesthesia, and an upper airway collapsibility less than or equal to −30% proved to be highly sensitive and specific in the identification of children with an AHI > 5/hour of total sleep time (TST). In agreement with these findings, another group also showed that children with OSA have a more collapsible upper airway than do normal children, even when awake, using the negative expiratory pressure (NEP) technique.Citation22 However, although this technique could distinguish between children with OSA and normal controls, it could not distinguish between children with OSA and primary snorers. Similar approaches using the Müller maneuver have also been proposed as potentially predictive of OSA severity.Citation23
Thus, it is probably safe to conclude that a multiplicity of causative factors appear to coexist in every child with OSA. As a corollary to such assumptions, children with sickle cell disease have, for example, not only reduced upper airway size due to overgrowth of the surrounding lymphoid tissues,Citation24 but they are also likely to be of African American ethnicity, which is also a risk factor for OSA. However, not all children with sickle cell disease have OSA, and those who do may have less prominent upper airway reflexes than those who do not.Citation25
Obesity
With the obesity epidemic that is sweeping through many of the developed countries, the demographics of childhood OSA appear to be changing. Compared with the classical presentation of a child with adenotonsillar hypertrophy and failure to thrive, as described in the not so distant past, nowadays, there are increasing numbers of children being diagnosed with OSA who are obese. Although all of the pathophysiological mechanisms described above for nonobese children would still remain applicable in the context of obesity, it is likely that other contributors may be operational in obese children as well. Indeed, not all obese children with OSA have adenotonsillar hypertrophy, often present at a slightly later age, and their clinical presentation is more likely to resemble the adult OSA phenotype.Citation26
Obesity has become one of the most significant risk factors for OSA in children.Citation27 Each 1 kg/m2 increment in body mass index above the 50th percentile (adjusted for gender and age) is associated with an increased risk of OSA by 12%. However, we should also point out that 45% of obese children with OSA also have evidence of adenotonsillar hypertrophy. When the upper airways of obese children with OSA were critically examined using MRI volumetric approaches, an increased size of upper airway lymphoid tissues, parapharyngeal fat pads, and abdominal visceral fat became apparent.Citation10 When obese and nonobese children with OSA were matched for age, gender, ethnicity, and obstructive AHI, the size of the adenotonsillar tissues in obese children was smaller, while their Mallampati scores were higher, indicating that the presence of obesity increases the risk for OSA, not only via increased lymphadenoid tissue proliferation, but also, by restricting the overall pharyngeal space.Citation9
Furthermore, the percentage of obese children who have residual OSA post-AT is significantly higher than in nonobese children.Citation28 It has thus been suggested that alterations in the functional mechanisms regulating upper airway patency may be present in obese children and promote increased airway collapsibility. The presence of a linear association between obesity and Pcrit values would then explain, at least in part, the increased propensity of these subjects to develop OSA. Fatty infiltrates within the compartments of the upper airway structures and the neck are likely to result in upper airway narrowing and increased pharyngeal collapsibility. Central obesity also reduces the functional residual capacity of the lungs, due to abdominal visceral fat impinging on the chest cavity limiting diaphragmatic descent, particularly when lying in the supine position;Citation29 in addition, thoracic fat weighing on the chest wall can effectively decrease lung compliance, leading to hypoventilation, atelectasis, and ventilation–perfusion mismatch. The reduced lung volumes may decrease airway stiffness by reducing the tracheal tethering effect, further increasing the risk of upper airway collapse during sleep. In a recent study, in which MRI scans were performed both pre- and post-AT in obese children with OSA, there was not only increased residual adenoidal tissue, but also the volume of the tongue and soft palate were greater after AT.Citation8 Taken together, all of these factors could be operational contributors to the low success rate of AT reported in obese children with OSA Citation28,Citation30–Citation32
A reciprocal interaction between obesity and OSA has also been suggested whereby in addition to the contributions of obesity to OSA discussed above, OSA may also be contributing to the pathogenesis of obesity. Leptin is a key hormonal regulator of appetite and metabolism, mainly secreted by adipocytes, that promotes satiety and reduces food intake. In contrast, ghrelin is an orexigenic hormone secreted in the gut. OSA can induce leptin resistance and increase ghrelin levels, both of which can potentiate obesogenic behaviors, in particular, the intake of high-calorie comfort foods.Citation33 OSA can also cause EDS and fatigue, both of which are likely to reduce the commitment to and engagement in physical activity.Citation33 The low-grade inflammatory responses induced by OSA could further interact and potentiate the underlying inflammatory responses, due to obesity, and exacerbate the morbid phenotypes associated with either obesity or OSA.Citation34
OSA and inflammation
It is now recognized that OSA can promote the activation and propagation of systemic inflammatory responses.Citation35 Elevation of proinflammatory cytokines such as IL-6, interferon (IFN)-γ and TNF-α have all been reported,Citation36–Citation38 albeit inconsistently, in children with OSA, while levels of the anti-inflammatory cytokine IL-10 were reduced.Citation39 Microarray analyses of ribonucleic acid (RNA) from peripheral leukocytes has revealed the coordinated recruitment of functionally relevant gene clusters involved in the regulation and propagation of inflammatory pathways and the inflammasome.Citation40 One of the potential mechanisms responsible for the initiation of inflammation may reside in molecular events in specific genes. For example, the promoter region of the FOXP3 gene, which controls the transcriptional fate and differentiation of lymphocytes into regulatory T lymphocytes (Tregs), exhibits severity-dependent increases in methylation in pediatric OSA.Citation41 Such epigenetic alterations were subsequently linked to the presence of reduced counts of Tregs in the peripheral blood of children with OSA,Citation42 a significant finding considering the major role that Tregs play in the suppression of inflammation. It is therefore possible that differentially orchestrated responses of various tissues to the presence of OSA-induced perturbations may further interact with environmental factors and intrinsic genetic variance to elicit a wide spectrum of inflammatory phenotypes that is linked to end-organ morbidities.Citation43
Morbidity of OSA
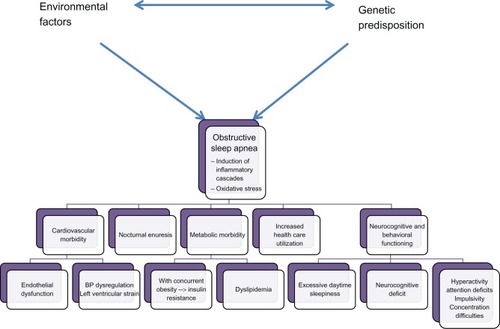
The foremost relevance of OSA relates to the fact that it imposes a vast array of morbidities. It has been proposed that such morbidities result from the combination of the activation of inflammatory cascades alluded to above and the induction of oxidative stress mechanisms, collectively leading to cellular injury and dysfunction, senescence, and various forms of cell death. Although these mechanisms are probably universal across the various targeted organs, we will describe each of the morbid consequences in greater detail below. They are also summarized in .
Figure 2 Morbidity of pediatric OSA.
Cardiovascular system
Arguably, one of the most serious complications of severe OSA is pulmonary hypertension, and the resultant cor pulmonale and right-sided heart failure if left untreated.Citation44–Citation46 Fortunately, with increased awareness and earlier diagnosis, the frequency of such cases is now much less commonly seen. However, it is unclear whether pulmonary hypertension will develop only in the severest cases, such that increased recognition and awareness of sleep disordered breathing in children would lead to a reduced likelihood of long-standing disease and concurrent pulmonary hypertension, or whether the current techniques for noninvasive assessment of the pulmonary circulation in children are insufficiently sensitive to detect much milder involvement of the pulmonary vasculature. Furthermore, it is also unclear whether chronic mild intermittent hypoxia may result in lesser recruitment of the pulmonary vascular network than following exposures to sustained hypoxia of similar duration.Citation47,Citation48 This issue merits further research, particularly considering that the occurrence of intermittent hypoxia and mobilization of the lung capillary endothelial network may promote long-term susceptibility to pulmonary hypertension even during adulthood.Citation49 Notwithstanding, there is increasing evidence that OSA can impose even “subclinical” effects on the autonomic and cardiovascular systems and promote cardiovascular disturbances in blood pressure (BP) regulation, ventricular remodeling, and endothelial dysfunction, all of which can lead to far-reaching detrimental consequences if left unattended.Citation51–Citation66
One of the earliest studies exploring the relationship between BP and OSA in children found that children with OSA tend to have higher diastolic BP during sleep compared with children with primary snoring.Citation50 The degree of increase in BP during rapid eye movement (REM) sleep has also been shown to correlate with the severity of OSA,Citation51 which is perhaps unsurprising considering that OSA, in the majority of children, tends to occur during REM sleep and that surges in arterial BP have been shown to occur after respiratory event termination.Citation52 Amin et al studied slightly older children, and found that children with OSA had evidence of BP deregulation: they had significantly greater mean BP variability during wakefulness and sleep, a higher night-to-day systolic BP, and marked reductions in nocturnal dipping of the mean BP.Citation53 In fact, children with OSA had night-to-day systolic BP ratios that surpassed the established cutoff ratios of 0.899 for females and 0.9009 for males, both of which are known to increase the risk for cardiovascular morbidity in adults.Citation54 A few years later, the same group of investigators reported that children with OSA exhibit increases in morning BP surges, BP load, and 24-hour ambulatory BPs compared with healthy controls.Citation55 Furthermore, these differences were associated with left ventricular remodeling, and the effects were apparent even in children with mild OSA. Interestingly, even habitually snoring children without evidence of OSA, ie, “primary snorers,” were also found to be at higher risk for elevations in systemic BP.Citation56–Citation58
The evidence of left ventricular strain and reduced contractility reported by Amin et alCitation55 led to exploration of potential mechanisms that may account for the left ventricular involvement in OSA. Among the various putative factors, assessment of changes in brain natriuretic peptide (BNP) seemed logical, considering that this 32 amino acid peptide is released by cardiac myocytes in response to cardiac wall distension. Indeed, overnight changes in BNP levels were found to be greater in children with moderate/severe OSA compared with mild OSA and controls,Citation59 most likely related to the increased and more frequent negative intrathoracic pressure swings seen in more severe OSA, further supporting the presence of nocturnal cardiac strain in children who have moderate to severe OSA. It remains unknown whether the left ventricular changes associated with pediatric OSA are completely reversible or indicate a group of susceptible individuals at risk for more adverse cardiovascular disease during adulthood.
Endothelial dysfunction is thought to be an early precursor to atherosclerosis. Assessment of postocclusive hyperemic responses in children, using various methodologies, such as flow-mediated dilation, pulse arterial tonometry, and laser Doppler reperfusion kinetics,Citation60–Citation63 has revealed significant impairment in endothelial function among children with OSA compared with controls. Furthermore, although the majority of these children demonstrated resolution of the endothelial dysfunction following treatment with AT,Citation60 a subgroup who also had a strong family history of cardiovascular disease did not show the anticipated improvements in endothelial function, suggesting that the effects of OSA in a genetically susceptible subset of children may persist for unknown periods of time, potentially into adulthood. As anticipated, in children with concurrent obesity and OSA, the magnitude of endothelial dysfunction was greater than when either condition was present in isolation, suggesting the convergence of the deleterious consequences of obesity and OSA.Citation26,Citation34,Citation64–Citation65 The evidence for severity-dependent alterations in endothelial function was confirmed in children with OSA, using pulse arterial tonometry to derive the reactive hyperemia index, whereby significant differences in evening-to-morning changes of endothelial function emerged in children with OSA, and such overnight changes in endothelial function were closely associated with the severity of the disease.Citation62 It should be stressed, however, that not every child with OSA manifests endothelial dysfunction.Citation64 One potential explanation for the variance in endothelial phenotype of pediatric OSA may well reside in the intrinsic ability to recruit endothelial progenitors to the circulation via the release of stromal derived factor 1 (SDF-1).Citation61
C-reactive protein (CRP), an acute-phase reaction protein produced in the liver, has emerged as a robust and independent predictor of cardiovascular morbidity and is extensively used to stratify the risk for ischemic heart disease.Citation66 It has been postulated that CRP may even participate directly in atheromatous lesion formation, through reduction of nitric oxide synthesis and induction of the expression of adhesion molecules in endothelial cells.Citation67,Citation68 Increased CRP levels have been demonstrated in children with OSA, correlate with severity of the disease, and decrease following effective treatment.Citation69–Citation72 It should be stressed that not all children with OSA have elevated CRP levels, as the interplay of genetic variants in IL-6 and CRP genes as well as environmental factors plays an important role (Gozal et alCitation72). However, we would surmise that the children in whom CRP levels are elevated constitute a higher-risk group for the development of long-term cardiovascular complications. Indeed, markers of vascular injury and endothelial activation, such as adhesion molecules, myeloid-related protein 8/14, fatty acid-binding protein, and circulating microparticles have all been shown to be elevated in children with OSA and are associated with the presence of endothelial dysfunction.Citation73–Citation76 Efforts are now ongoing to develop a panel of biomarkers that may identify those children at risk for cardiovascular morbidity as a result of OSA.
Metabolic system
Although in adult cohorts OSA has been identified as an important risk factor for insulin resistance and diabetes,Citation77–Citation83 the presence of this association in children is not as clear cut and may well depend on factors such age, ethnicity, pubertal status, the degree of inflammatory response, and the concurrent presence of obesity.
In a community-based study of predominantly postpubertal adolescents, strong associations were demonstrated between OSA and the metabolic syndrome, as well as with individual metabolic parameters, such as fasting insulin and homeostatic model assessment (HOMA).Citation29,Citation84,Citation85 Sleep fragmentation and intermittent hypoxia were also found to be associated with decreased insulin sensitivity in obese adolescent boys.Citation86 In younger children, OSA was associated with reduced insulin sensitivity only when obesity was concurrently present,Citation87,Citation88 and effective treatment of OSA ameliorated HOMA in these children.Citation89 However, when highly sensitive bioinformatics approaches and pathway analyses were employed, in conjunction with transcriptome microarray analyses, in children with primary snoring, alterations in insulin homeostatic mechanisms emerged and were further confirmed with HOMA in such children, suggesting that even mild perturbations in sleep may impose subclinical changes in peripheral tissue insulin receptor sensitivity.Citation90
OSA has also been implicated in rises in low-density lipoprotein (LDL) cholesterol with concomitant decreases in high-density lipoprotein (HDL) cholesterol, in both obese and nonobese children.Citation89,Citation91 Significant improvements in lipid profile were observed in all children after treatment of their OSA.
Even in childhood, evidence of end-organ morbidity, in the form of fatty liver disease, has been demonstrated in obese children with OSA.Citation92–Citation94 Of note, the treatment of OSA, usually with AT followed by continuous positive airway pressure (CPAP), in a large subset of these obese children resulted in improved liver serum aminotransferases in the majority of the patients.Citation92
Neurocognitive and behavioral functioning
The first seminal paper to highlight the potential causative links between OSA and its detrimental consequences on academic performance was published by Gozal in 1998.Citation95 In a prospective study looking at 297 first-grade children whose school performance was in the lowest tenth percentile of their class, screening for OSA revealed a marked elevation in the prevalence of OSA. Furthermore, the children who were treated for OSA showed significant academic improvements in their school grades the subsequent year, while children who had OSA but who were not treated failed to improve academically. Since then, there have been numerous studies reporting the association between OSA and neurocognitive and behavioral morbidity, and several, albeit not all, studies have shown improvements in some of these functions after treatment.Citation32,Citation96–Citation107 Impairments in executive functioning have also been reported: the Tucson Children’s Assessment of Sleep Apnea (TuCASA) study identified a negative correlation between AHI and immediate recall, Full Scale IQ, Performance IQ, and math achievement, while nocturnal hypoxemia adversely affected nonverbal skills.Citation108 Children with OSA have also been shown to take longer and need more learning opportunities to learn a pictorial-based, short-term and long-term declarative memory test.Citation109 From the behavioral standpoint, OSA and habitual snoring have been associated with hyperactivity, attention deficits, concentration difficulties, and impulsivity. Therefore, it should come as no surprise that such children are frequently misdiagnosed as having attention deficit hyperactivity disorder.Citation110–Citation112 Recent 5-year follow up results from the TuCASA study revealed that youth with untreated OSA exhibited hyperactivity, had attention problems and aggressive behaviors, lower social competencies, poorer communication, and/or diminished adaptive skills.Citation113
Notwithstanding such considerations, it is important to emphasize that not all children with OSA exhibit cognitive or behavioral deficits. It is therefore plausible that susceptibility modifiers, both genetic and environmental, may play a role in phenotypic expression.Citation43 Accordingly, several genetic factors have been thus far been identified to account for discrepancies in the cognitive functional performance of children with OSA. For example, differences in systemic inflammatory responses, as reported by plasma CRP levels, will differentiate children with OSA of similar severity who exhibit or do not exhibit cognitive deficits.Citation114 Similarly, genetic variants in a gene critically involved in the formation of free radicals have been shown to account for discrepancies in cognitive outcomes in pediatric OSA.Citation115 Additional factors, such as IGF-1 and apolipoprotein E allelic variants, have also been identified as either protective or detrimental to neurocognitive function.Citation116,Citation117
Khadra et al further hypothesized that OSA causes changes in regional cerebral blood flow during sleep, thus favoring the occurrence of neurocognitive impairments.Citation118 However, these investigators found that the situation was more complex than expected, with both arousal indices and mean arterial pressure being strongly associated with OSA severity. On the one hand, increasing arousal indices were associated with decreasing regional cerebral oxygenation and on the other hand, increasing mean arterial BP was associated with increasing regional brain oxygenation. In other words, OSA can both augment and decrease regional cerebral blood flow. A model based on the interplay between factors such as age, mean arterial pressure, oxygen saturation, REM sleep, gender, arousal index and NREM sleep has been proposed to predict the effect of sleep disordered breathing on regional cerebral oxygenation. The findings linking vascular function and cognitive outcomes were further confirmed in several additional studies, such that the presence of endothelial dysfunction in OSA may serve as a surrogate reporter of altered cognitive functioning.Citation119,Citation120
As mentioned above, improvements in learning and behavior occur following treatment of OSA.Citation107 However, some neurocognitive consequences may only be partially reversible if left too late,Citation121 highlighting the importance of early diagnosis and prompt effective treatment.
Excessive daytime sleepiness
In contrast to adults, EDS does not tend to be as prominent a symptom in pediatric OSA. Nonetheless, children with OSA have been shown to have higher Epworth Sleepiness Scale (ESS) scores compared with controls (8.1 ± 4.9 versus 5.3 ± 3.9) (P < 0.001).Citation122 Objective measurements of EDS using the Multiple Sleep Latency Test have shown that children with OSA have severity-dependent shortening of their sleep latencies but that EDS is relatively infrequent and tends to manifest among more severe and/or obese patients. Interestingly, the magnitude of sleep latency reduction has been shown to be associated with measures of systemic inflammation, such as plasma TNF-α level, the latter being modulated by polymorphisms in the TNF-α gene.Citation37,Citation123
Healthcare utilization
Interestingly, children with OSA have been reported to have increased health care utilization compared with their peers, predominantly for respiratory infections.Citation124,Citation125 From their first year of life to the time of diagnosis, children with OSA had 40% more hospital visits, 20% more repeated visits, and higher prescriptions of medication.Citation124 Although one might argue that children who have recurrent upper respiratory tract infections may be at higher risk of OSA or that children who have more contact with healthcare professionals are more likely to be screened for OSA, another prospective study by the same group showed that after treatment of OSA by AT, health care utilization was significantly reduced to the extent that total annual health care costs were reduced by a third.Citation126 These findings have since been further confirmed in another population-based cohort.Citation127
Nocturnal enuresis
A higher prevalence of nocturnal enuresis has been reported in children with OSA.Citation128–Citation130
It has been postulated that increased enuresis may be due to the dampening effects of OSA on arousal responses, to changes in bladder pressure, or potentially associated with secretion of the hormones involved in fluid regulation. A recent systematic review of the literature identified 14 studies investigating OSA and enuresis, in which a third of the total 3,550 children with OSA had a diagnosis of enuresis.Citation129 In the seven studies with follow up data, post-AT improvements in enuresis occurred. However, the data were derived from studies with weak design and skewed cohorts, and randomized controlled trials are still needed to establish a more definitive cause-effect relationship between OSA and enuresis in children.
In children with sickle cell disease, a prospective, multicenter cohort study of 221 children demonstrated that enuresis was significantly associated with an AHI of >2/hr TST, after adjusting for age and gender.Citation131 The authors recommended that children with sickle cell anemia who present with enuresis should be evaluated by a pulmonologist for sleep disordered breathing.
As mentioned previously in the context of nocturnal cardiac strain, changes in BNP have been demonstrated in children with OSA. As BNP increases sodium and water excretion and also influences hormones in the renin–angiotensin pathway and vasopressin, a population-based cohort study aimed to examine the relationship between BNP levels and enuresis in habitually snoring children. The presence of habitual snoring was associated with an increased prevalence of enuresis, and morning BNP levels were found to be elevated in children with OSA. Furthermore, children with nocturnal enuresis have been shown to have higher BNP levels compared with those without, at any degree of sleep disordered breathing severity, providing support to the hypothesis that sleep fragmentation and increased release of BNP secondary to OSA may contribute to the higher prevalence of enuresis in habitually snoring children.Citation132
Diagnosis
One of the major difficulties with the diagnostic process of pediatric OSA is the fact that history and clinical examination perform poorly. In a recent reassessment of this issue by the American Academy of Pediatrics (AAP), a positive predictive value of 65% for history and 46% for clinical examination were quoted,Citation4 essentially representing a success rate equivalent to that of tossing a coin! Unsurprisingly, the AAP recommendations are that children with symptoms of OSA should be referred for further investigation.Citation4
In this context, the current gold standard for the diagnosis of OSA, as recommended by the AAP, is a nocturnal, in-lab polysomnography (PSG) study. A typical montage would include several electroencephalography (EEG) channels, chin and anterior tibial electromyography (EMG), bilateral electrooculography, pulse oximeter and pulse waveform, nasal pressure transducer, oronasal airflow thermistor, end-tidal capnography, chest and abdominal respiratory inductance plethysmography, body position sensor, microphone, and real-time synchronized video monitoring. Rechtschaffen and Kales devised the initial sleep staging system in 1968,Citation133 which was in use for almost 40 years. The American Academy of Sleep Medicine (AASM) modified and updated the staging criteria in 2007 and further revised it in 2012, with sleep laboratories now scoring according to these newer AASM guidelines.Citation134 This approach provides an objective, quantitative evaluation of disturbances in respiratory parameters and sleep patterns, thus allowing patients to be stratified into disease severities and thereby enabling clinicians to tailor clinical management accordingly. However, stratification into severity categories has thus far been performed empirically, without any critical evidence to support one classification approach versus another. There is as of yet, no international consensus regarding the AHI cutoff values for therapy initiation. The current accepted practice has consisted of the use of an arbitrary cutoff for AHI corresponding to >3 standard deviations beyond the mean of the normative AHI in healthy children. Most clinicians would agree that a child with an AHI >5/hour TST requires treatment and that a child with an AHI <1/hour TST does not have significant OSA. However, the interpretation of the AHI values in between these two cutoffs are fraught with great discord. Some algorithms have recently been proposed and recognize the importance of treating the patient and not just the values obtained from the sleep study.Citation135,Citation136 In addition to polysomnographically derived measures, these algorithms take into account factors such as the severity of symptoms, risk factors, and the presence of any OSA-related morbidity and may therefore provide a more coherent, clinically applicable approach to the diagnosis and prioritization of treatment, when OSA is diagnosed in children.
Treatment
AT is the primary treatment for pediatric OSA for children with adenotonsillar hypertrophy.Citation137 The recently published Childhood Adenotonsillectomy Trial (CHAT), which constitutes the first-ever randomized controlled trial (RCT) for OSA, showed that compared with watchful waiting, the surgical treatment of OSA improved symptoms, behavior, and quality of life, even though improvements in the trial’s primary endpoint, ie, neuropsychological measures of attention and executive function, did not occur.Citation32 However, it is important to note that this study did not include children aged <5 years or children with moderate/severe OSA, as it would have been difficult to ethically justify withholding standard treatment from children with significant oxygen desaturation. Furthermore, similar to the findings reported in previous studies,Citation28 (discussed below), the presence of particular risk categories, such as African American ethnicity and obesity, was associated with a reduced probability for normalization of the sleep study result after surgery.Citation137
Indeed, a large multicenter, retrospective study showed that although the majority of children had marked AHI improvements following AT, residual OSA was still frequent and clinically significant in a relatively large subset.Citation28 Residual OSA was particularly prevalent in obese children, children who had severe OSA before surgery (AHI > 20/hour TST), older children (those aged >7 years), and children with asthma.Citation28 Similar findings have been reported in other studies involving vastly smaller cohorts. In addition, several other risk factors for residual OSA have been identified and include high Mallampati score, African-American ethnicity, craniofacial anomalies, chromosomal defects, and neuromuscular disease (including trisomy 21, achondroplasia, Prader–Willi syndrome, and Pierre Robin syndrome).Citation30,Citation138 Clinicians should be aware that (1) specific protocols may be needed to direct clinicians to automatically pursue post-surgical sleep studies in a subset of children at high risk for residual OSA; (2) the recurrence of OSA symptoms post-AT warrants reevaluation, particularly in children with the aforementioned risk factors. The role of other surgical procedures, such as tonsillotomy (in lieu of tonsillectomy) alone or in combination with adenoidectomy has not been established, even though advocates for these approaches claim similar outcomes with reduced postoperative pain.Citation139,Citation140
In children who manifest residual OSA after AT or in those who present minimally enlarged upper airway or lymphadenoid tissues or who opt not to undergo surgery, positive airway pressure, in the form of CPAP or BiPAP (bilevel positive airway pressure), has been recommended.Citation137 Although positive airway pressure can undoubtedly be a highly effective treatment,Citation141,Citation142 adherence can be particularly challenging in children, particularly those with behavioral problems or developmental delays. Behavioral modification techniques can improve adherence, but are time and labor intensive, often require admission to the inpatient service and therefore add substantial costs to their implementation, and are impossible to implement without a priori engagement with the child and family.Citation143 Indeed, family and demographic factors have been shown to play a large role in CPAP adherence.Citation144
A possible and recently proposed alternative to mask-based positive airway pressure is high flow nasal cannula (HFNC) oxygen therapy. Although there has been only one study so far looking at 12 patients on HFNC,Citation145 the reduction in AHI was comparable with that of CPAP, leading the authors to postulate that HFNC may be a viable and possibly more child-friendly alternative.
Since less invasive therapies are more desirable, there has been considerable interest in anti-inflammatory agents, particularly leukotriene-receptor antagonists (such as montelukast) and intranasal steroids.Citation146 Tonsils from children with OSA have been shown to express increased levels of leukotriene receptors 1 and 2 compared with tonsils from children with recurrent tonsillitis.Citation147 Furthermore, the application of leukotriene antagonists in an in vitro cell culture system of tonsillar tissues from children with OSA elicited dose-dependent reductions in cell proliferation and reductions in the secretion of the cytokines TNF-α, IL-6, and IL-12.Citation148 In an open-label intervention study where children with mild OSA received 16 weeks of montelukast, significant reductions in adenoidal size and respiratory-related sleep disturbances occurred.Citation149 These findings have since been reproduced in a recent double-blind, randomized, placebo-controlled trial.Citation150
Similarly, the use of in vitro steroids resulted in decreased proliferative rates of mixed tonsillar tissues, and in increased apoptosis and reduction in the secretion of the proinflammatory cytokines IL-6, IL-8, and TNF-α.Citation151 A randomized crossover trial of 6 weeks treatment with intranasal budesonide for mild OSA showed reductions in the severity of OSA as well as in the size of adenoidal tissues. Importantly, discontinuation of therapy for 8 weeks did not promote the occurrence of rebound symptoms.Citation152 Intranasal fluticasone has also shown similar results.Citation153 Use of both montelukast and nasal budesonide for 12 weeks in children who had residual mild residual OSA after ATCitation154 led to significant improvements in the AHI, nadir oxygen saturation, and in the respiratory arousal index, whereas no significant changes occurred over this time period in the control subjects. Anti-inflammatory therapy appears to be gaining wider acceptance in the treatment of mild OSA, such that studies examining the desirable optimal duration of treatment, longer term outcomes, combinatorial approaches, criteria for patient selection for optimal outcomes, etc, are critically needed.
In selected patient populations, some orthodontic procedures, such as rapid maxillary expansion, have been proposed as efficacious.Citation155–Citation157 Procedures such as tongue-base suspension and uvulopalatopharyngoplasty have also been studied in children with cerebral palsy and OSA.Citation158 Recently, the use of more comprehensive assessments and interventions, including myofascial reeducation has been advocated in a series of uncontrolled studies.Citation159–Citation162
In complex or persistent cases of OSA, sleep endoscopy is a technique that enables the exact level of obstruction in the child to be identified, thus facilitating site-specific surgical therapy.Citation163,Citation164 Further work to delineate the children who would benefit most from these procedures is needed, but some recent work in obese children and in children with trisomy 21 showed that lingual tonsils may contribute to residual disease and that lingual tonsillectomy may be effective in those cases.Citation165–Citation171
Future developments
Although sleep studies provide an objective measure of sleep disturbance, it is now evident that measures derived from sleep studies are not predictive of OSA-associated morbidities, and therefore, their cost–efficiency in the management of habitually snoring children is less than perfect, particularly considering the high cost, intensity of labor, and family burden that such studies impose. It is possible that home-based studies or more restricted multichannel studies may provide a more economical and accessible option in the future, once the appropriate validation studies are conducted. Alternatively, the identification and implementation of diagnostic biomarker approaches may be possible and merits further exploration.Citation172
Another important issue in the context of the evaluation of habitually snoring children is the wide spectrum of phenotypic variance that exists in OSA. As with many other diseases, factors such as individual genetic susceptibility and environmental exposures/lifestyle play major contributing roles to the variance in phenotype. In other words, the optimal threshold for diagnosis and treatment is likely different in different children. Simply stated, not every child fulfilling current polysomnographic criteria for OSA manifests end-organ morbidity,Citation173 and conversely, some children with primary snoring display neurocognitive or cardiovascular sequelae and/or signs of systemic inflammation, despite a normal sleep study.Citation90,Citation110
In the future, algorithms that incorporate measures derived from the sleep study, from blood or urine tests, and from clinical elements obtained during the history and physical examination may provide improved approaches to determining which children require treatment, which children may benefit most from a specific treatment, or which children are at risk for residual disease and require incremental therapies. There has been exciting research into urinary biomarkers as a potential measure. Preliminary data has shown an association between pediatric OSA and significant nocturnal alterations in urinary neurotransmitters.Citation174 It is likely that the episodic hypoxemia and arousals that characterize sleep in OSA patients enhances sympathetic activity, therefore leading to increased levels of urinary epinephrine and norepinephrine. Overnight changes in the levels of three other neurotransmitters, notably increases in gamma-aminobutyric acid (GABA), decreases in taurine, and decreases in β-phenylethylamine (PEA) have appeared to differentiate children with OSA with neurocognitive deficits from those without.Citation174
Conclusion
In summary, the recent decades since the initial description of pediatric OSA in 1976Citation45 have witnessed extensive and meaningful progress in research on the pathophysiology, morbidity, and treatment of pediatric OSA. However, there are still many fundamental questions to be answered. If the age of personalized medicine is to become a reality, a greater understanding of the mechanisms underlying the pathogenesis of the disease will be required to help develop novel biomarkers and individualized therapies.
Acknowledgments
LKG and DG are supported by National Institutes of Health grants HL-65270, HL-086662, and HL-107160. HLT was supported by the Scadding Morriston Davies Fellowship.
Disclosure
The authors report no conflicts of interest in this work.
References
- Kheirandish-GozalLGozalDSleep Disordered Breathing in Children A Comprehensive Clinical Guide to Evaluation and TreatmentNew York, NYSpringer Science2012
- BixlerEOVgontzasANLinHMSleep disordered breathing in children in a general population sample: prevalence and risk factorsSleep200932673173619544748
- LiAMSoHKAuCTEpidemiology of obstructive sleep apnoea syndrome in Chinese children: a two-phase community studyThorax2010651199199720965935
- MarcusCLBrooksLJDraperKAAmerican Academy of PediatricsDiagnosis and management of childhood obstructive sleep apnea syndromePediatrics20121303e714e75522926176
- RosenCLLarkinEKKirchnerHLPrevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurityJ Pediatr2003142438338912712055
- TaumanRGozalDObstructive sleep apnea syndrome in childrenExpert Rev Respir Med20115342544021702663
- ArensRMcDonoughJMCostarinoATMagnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndromeAm J Respir Crit Care Med2001164469870311520739
- NandalikeKShiftehKSinSAdenotonsillectomy in obese children with obstructive sleep apnea syndrome: magnetic resonance imaging findings and considerationsSleep201336684184723729927
- DayyatEKheirandish-GozalLSans CapdevilaOMaarafeyaMMGozalDObstructive sleep apnea in children: relative contributions of body mass index and adenotonsillar hypertrophyChest2009136113714419225059
- ArensRSinSNandalikeKUpper airway structure and body fat composition in obese children with obstructive sleep apnea syndromeAm J Respir Crit Care Med2011183678278720935105
- ParikhSRSadoughiBSinSWillenSNandalikeKArensRDeep cervical lymph node hypertrophy: A new paradigm in the understanding of pediatric obstructive sleep apneaLaryngoscope201312382043204923666635
- ArensRSinSWillenSRhino-sinus involvement in children with obstructive sleep apnea syndromePediatr Pulmonol2010451099399820648667
- GozalDPediatric OSA: a case for “United We Stand” in the way of a breathPediatr Pulmonol201045121151115220812245
- KimJBhattacharjeeRDayyatEIncreased cellular proliferation and inflammatory cytokines in tonsils derived from children with obstructive sleep apneaPediatr Res200966442342819581829
- SerperoLDKheirandish-GozalLDayyatEGoldmanJLKimJGozalDA mixed cell culture model for assessment of proliferation in tonsillar tissues from children with obstructive sleep apnea or recurrent tonsillitisLaryngoscope200911951005101019266584
- GoldbartADKrishnaJLiRCSerperoLDGozalDInflammatory mediators in exhaled breath condensate of children with obstructive sleep apnea syndromeChest2006130114314816840394
- LiAMHungETsangTInduced sputum inflammatory measures correlate with disease severity in children with obstructive sleep apnoeaThorax2007621757916928708
- LiAMWongEKewJHuiSFokTFUse of tonsil size in the evaluation of obstructive sleep apnoeaArch Dis Child200287215615912138072
- MarcusCLMcColleySACarrollJLLoughlinGMSmithPLSchwartzARUpper airway collapsibility in children with obstructive sleep apnea syndromeJ Appl Physiol19947729189248002548
- SchwartzARSmithPLCrossTalk proposal: the human upper airway does behave like a Starling resistor during sleepJ Physiol20135912229223223740879
- GozalDBurnsideMMIncreased upper airway collapsibility in children with obstructive sleep apnea during wakefulnessAm J Respir Crit Care Med2004169216316714525806
- CarreraHLMcDonoughJMGallagherPRUpper airway collapsibility during wakefulness in children with sleep disordered breathing, as determined by the negative expiratory pressure techniqueSleep201134671772421629359
- ThongJFPangKPClinical parameters in obstructive sleep apnea: are there any correlations?J Otolaryngol Head Neck Surg200837689490019128723
- StraussTSinSMarcusCLUpper airway lymphoid tissue size in children with sickle cell diseaseChest201214219410022241762
- HuangJPintoSJAllenJLUpper airway genioglossal activity in children with sickle cell diseaseSleep201134677377821629365
- GozalDKheirandish-GozalLChildhood obesity and sleep: relatives, partners, or both? – a critical perspective on the evidenceAnn NY Acad Sci20121264113514122882312
- RedlineSTishlerPVSchluchterMAylorJClarkKGrahamGRisk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problemsAm J Respir Crit Care Med19991595 Pt 11527153210228121
- BhattacharjeeRKheirandish-GozalLSpruytKAdenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective studyAm J Respir Crit Care Med2010182567668320448096
- CanapariCAHoppinAGKinaneTBThomasBJTorrianiMKatzESRelationship between sleep apnea, fat distribution, and insulin resistance in obese childrenJ Clin Sleep Med20117326827321677896
- TaumanRGulliverTEKrishnaJPersistence of obstructive sleep apnea syndrome in children after adenotonsillectomyJ Pediatr2006149680380817137896
- MitchellRBBossEFPediatric obstructive sleep apnea in obese and normal-weight children: impact of adenotonsillectomy on quality-of-life and behaviourDev Neuropsychol200934565066120183725
- MarcusCLMooreRHRosenCLChildhood Adenotonsillectomy Trial (CHAT)A randomized trial of adenotonsillectomy for childhood sleep apneaN Engl J Med2013368252366237623692173
- SpruytKSans CapdevilaOSerperoLDKheirandish-GozalLGozalDDietary and physical activity patterns in children with obstructive sleep apneaJ Pediatr20101565724730 730. e120138306
- BhattacharjeeRKimJKheirandish-GozalLGozalDObesity and obstructive sleep apnea syndrome in children: a tale of inflammatory cascadesPediatr Pulmonol201146431332320967842
- GozalDSleep, sleep disorders and inflammation in childrenSleep Med200910Suppl 1S12S1619647481
- TaumanRO’BrienLMGozalDHypoxemia and obesity modulate plasma C-reactive protein and interleukin-6 levels in sleep-disordered breathingSleep Breath2007112778417171553
- GozalDSerperoLDKheirandish-GozalLCapdevilaOSKhalyfaATaumanRSleep measures and morning plasma TNF-alpha levels in children with sleep-disordered breathingSleep201033331932520337189
- TamCSWongMMcBainRBaileySWatersKAInflammatory measures in children with obstructive sleep apnoeaJ Paediatr Child Health200642527728216712558
- GozalDSerperoLDSans CapdevilaOKheirandish-GozalLSystemic inflammation in non-obese children with obstructive sleep apneaSleep Med20089325425917825619
- KhalyfaACapdevilaOSBuazzaMOSerperoLDKheirandish-GozalLGozalDGenome-wide gene expression profiling in children with non-obese obstructive sleep apneaSleep Med2009101758618261956
- KimJBhattacharjeeRKhalyfaADNA methylation in inflammatory genes among children with obstructive sleep apneaAm J Respir Crit Care Med2012185333033822077067
- TanHLGozalDWangYAlterations in circulating T-cell lymphocyte populations in children with obstructive sleep apneaSleep201336691392223729935
- Kheirandish-GozalLGozalDGenotype-phenotype interactions in pediatric obstructive sleep apneaRespir Physiol Neurobiol Epub432013
- GuilleminaultCEldridgeFLSimmonsFBDementWCSleep apnea in eight childrenPediatrics19765812330934781
- SerrattoMHarrisVJCarrIUpper airways obstruction. Presentation with systemic hypertensionArch Dis Child19815621531557469469
- RossRDDanielsSRLoggieJMMeyerRABallardETSleep apnea-associated hypertension and reversible left ventricular hypertrophyJ Pediatr198711122532552956404
- AdegunsoyeARamachandranSEtiopathogenetic mechanisms of pulmonary hypertension in sleep-related breathing disordersPulm Med2012201227359122848814
- NisbetREGravesASKleinhenzDJThe role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in miceAm J Respir Cell Mol Biol200940560160918952568
- AbmanSHIvyDDRecent progress in understanding pediatric pulmonary hypertensionCurr Opin Pediatr201123329830421572384
- MarcusCLGreeneMGCarrollJLBlood pressure in children with obstructive sleep apneaAm J Respir Crit Care Med19981574 Pt 1109811039563725
- KohyamaJOhinataJSHasegawaTBlood pressure in sleep disordered breathingArch Dis Child200388213914212538317
- HorneRSYangJSWalterLMElevated blood pressure during sleep and wake in children with sleep-disordered breathingPediatrics20111281e85e9221708802
- AminRSCarrollJLJeffriesJLTwenty-four-hour ambulatory blood pressure in children with sleep-disordered breathingAm J Respir Crit Care Med2004169895095614764433
- VerdecchiaPSchillaciGBorgioniCAltered circadian blood pressure profile and prognosisBlood Press Monit19972634735210234138
- AminRSomersVKMcConnellKActivity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathingHypertension2008511849118071053
- NisbetLCYiallourouSRWalterLMHorneRSBlood pressure regulation, autonomic control and sleep disordered breathing in childrenSleep Med Rev EpubJuly122013
- LiAMAuCTHoCFokTFWingYKBlood pressure is elevated in children with primary snoringJ Pediatr20091553362368. e119540515
- KwokKLNgDKCheungYFBP and arterial distensibility in children with primary snoringChest200312351561156612740274
- GoldbartADLevitasAGreenberg-DotanSB-type natriuretic peptide and cardiovascular function in young children with obstructive sleep apneaChest2010138352853520558551
- GozalDKheirandish-GozalLSerperoLDSans CapdevilaODayyatEObstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomyCirculation2007116202307231417967978
- Kheirandish-GozalLBhattacharjeeRKimJClairHBGozalDEndothelial progenitor cells and vascular dysfunction in children with obstructive sleep apneaAm J Respir Crit Care Med20101821929720203242
- Kheirandish-GozalLEtzioniTBhattacharjeeRObstructive sleep apnea in children is associated with severity-dependent deterioration in overnight endothelial functionSleep Med201314652653123643649
- DubernBAggounYBouléMFaurouxBBonnetDTounianPArterial alterations in severely obese children with obstructive sleep apnoeaInt J Pediatr Obes20105323023620210676
- BhattacharjeeRKimJAlotaibiWHKheirandish-GozalLCapdevilaOSGozalDEndothelial dysfunction in children without hypertension: potential contributions of obesity and obstructive sleep apneaChest2012141368269122030801
- GozalDKheirandish-GozalLThe obesity epidemic and disordered sleep during childhood and adolescenceAdolesc Med State Art Rev2010213480490 viii21302856
- PearsonTAMensahGAAlexanderRWCenters for Disease Control and PreventionAmerican Heart AssociationMarkers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart AssociationCirculation2003107349951112551878
- PasceriVWillersonJTYehETDirect proinflammatory effect of C-reactive protein on human endothelial cellsCirculation2000102182165216811056086
- PasceriVChengJSWillersonJTYehETChangJModulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugsCirculation2001103212531253411382718
- Kheirandish-GozalLCapdevilaOSTaumanRGozalDPlasma C-reactive protein in nonobese children with obstructive sleep apnea before and after adenotonsillectomyJ Clin Sleep Med20062330130417410279
- GozalDKheirandish-GozalLBhattacharjeeRKimJC-reactive protein and obstructive sleep apnea syndrome in childrenFront Biosci (Elite Ed)201242410242222652648
- IngramDGMatthewsCKEffect of adenotonsillectomy on c-reactive protein levels in children with obstructive sleep apnea: a meta-analysisSleep Med201314217217623317933
- GozalDKaditisAGKhalyfaAAssociation between variants in CRP and IL-6 genes and susceptibility to OSA in children: A candidate-gene association study in European American and South-East European populationsIn press2013
- O’BrienLMSerperoLDTaumanRGozalDPlasma adhesion molecules in children with sleep-disordered breathingChest2006129494795316608943
- KimJBhattacharjeeRSnowABCapdevilaOSKheirandish-GozalLGozalDMyeloid-related protein 8/14 levels in children with obstructive sleep apnoeaEur Respir J201035484385019608587
- KimJBhattacharjeeRKheirandish-GozalLSpruytKGozalDCirculating microparticles in children with sleep disordered breathingChest2011140240841721273295
- BhushanBKhalyfaASpruytKFatty-acid binding protein 4 gene polymorphisms and plasma levels in children with obstructive sleep apneaSleep Med201112766667121664182
- PunjabiNMSorkinJDKatzelLIGoldbergAPSchwartzARSmithPLSleep-disordered breathing and insulin resistance in middle-aged and overweight menAm J Respir Crit Care Med2002165567768211874813
- LamJCMakJCIpMSObesity, obstructive sleep apnoea and metabolic syndromeRespirology201217222323621992649
- AssoumouHGGaspozJMSforzaEObstructive sleep apnea and the metabolic syndrome in an elderly healthy population: the SYNAPSE cohortSleep Breath201216389590221927990
- BasogluOKSaracFSaracSUluerHYilmazCMetabolic syndrome, insulin resistance, fibrinogen, homocysteine, leptin, and C-reactive protein in obese patients with obstructive sleep apnea syndromeAnn Thorac Med20116312012521760842
- PillaiAWarrenGGunathilakeWIdrisIEffects of sleep apnea severity on glycemic control in patients with type 2 diabetes prior to continuous positive airway pressure treatmentDiabetes Technol Ther201113994594921714680
- BhushanBMisraAGuleriaRObstructive sleep apnea is independently associated with the metabolic syndrome in obese Asian Indians in northern IndiaMetab Syndr Relat Disord20108543143520715932
- AhmedQAMetabolic complications of obstructive sleep apnea syndromeAm J Med Sci20083351606418195586
- RedlineSStorfer-IsserARosenCLAssociation between metabolic syndrome and sleep-disordered breathing in adolescentsAm J Respir Crit Care Med2007176440140817541017
- KorenDLevitt KatzLEBrarPCGallagherPRBerkowitzRIBrooksLJSleep architecture and glucose and insulin homeostasis in obese adolescentsDiabetes Care201134112442244721933909
- LesserDJBhatiaRTranWHSleep fragmentation and intermittent hypoxemia are associated with decreased insulin sensitivity in obese adolescent Latino malesPediatr Res201272329329822669298
- TaumanRO’BrienLMIvanenkoAGozalDObesity rather than severity of sleep-disordered breathing as the major determinant of insulin resistance and altered lipidemia in snoring childrenPediatrics20051161e66e7315995020
- KaditisAGAlexopoulosEIDamaniEObstructive sleep-disordered breathing and fasting insulin levels in nonobese childrenPediatr Pulmonol200540651552316193477
- GozalDCapdevilaOSKheirandish-GozalLMetabolic alterations and systemic inflammation in obstructive sleep apnea among non-obese and obese prepubertal childrenAm J Respir Crit Care Med2008177101142114918276939
- KhalyfaAGharibSAKimJPeripheral blood leukocyte gene expression patterns and metabolic parameters in habitually snoring and non-snoring children with normal polysomnographic findingsSleep201134215316021286499
- ZongJLiuYHuangYSerum lipids alterations in adenoid hypertrophy or adenotonsillar hypertrophy children with sleep disordered breathingInt J Pediatr Otorhinolaryngol201377571772023434201
- Kheirandish-GozalLSans CapdevilaOKheirandishEGozalDElevated serum aminotransferase levels in children at risk for obstructive sleep apneaChest20081331929918187742
- VerhulstSLJacobsSAertsLSleep-disordered breathing: a new risk factor of suspected fatty liver disease in overweight children and adolescents?Sleep Breath200913220721019002513
- VerhulstSLRoomanRVan GaalLDe BackerWDesagerKIs sleep-disordered breathing an additional risk factor for the metabolic syndrome in obese children and adolescents?Int J Obes (Lond)200933181318779826
- GozalDSleep-disordered breathing and school performance in childrenPediatrics19981023 Pt 16166209738185
- ChervinRDRuzickaDLHobanTFEsophageal pressures, polysomnography, and neurobehavioral outcomes of adenotonsillectomy in childrenChest2012142110111022302302
- GiordaniBHodgesEKGuireKEChanges in neuropsychological and behavioral functioning in children with and without obstructive sleep apnea following TonsillectomyJ Int Neuropsychol Soc201218221222222272653
- LandauYEBar-YishayOGreenberg-DotanSGoldbartADTarasiukATalAImpaired behavioral and neurocognitive function in preschool children with obstructive sleep apneaPediatr Pulmonol201247218018821905262
- BourkeRAndersonVYangJSCognitive and academic functions are impaired in children with all severities of sleep-disordered breathingSleep Med201112548949621493135
- GaretzSLBehavior, cognition, and quality of life after adenotonsillectomy for pediatric sleep-disordered breathing: summary of the literatureOtolaryngol Head Neck Surg2008138Suppl 1S19S2618164375
- WeiJLBondJMayoMSSmithHJReeseMWeatherlyRAImproved behavior and sleep after adenotonsillectomy in children with sleep-disordered breathing: long-term follow-upArch Otolaryngol Head Neck Surg2009135764264619620583
- WeiJLMayoMSSmithHJReeseMWeatherlyRAImproved behaviour and sleep after adenotonsillectomy in children with sleep-disordered breathingArch Otolaryngol Head Neck Surg20071331097497917938319
- AbmanSJobeAChernickVNHLBI working group reportStrategic plan for pediatric respiratory diseases research: an NHLBI working group reportPediatr Pulmonol200944121319086051
- GozalDKheirandish-GozalLNeurocognitive and behavioral morbidity in children with sleep disordersCurr Opin Pulm Med200713650550917901756
- ChervinRDRuzickaDLGiordaniBJSleep-disordered breathing, behaviour, and cognition in children before and after adenotonsillectomyPediatrics20061174e769e77816585288
- Montgomery-DownsHECrabtreeVMGozalDCognition, sleep and respiration in at-risk children treated for obstructive sleep apnoeaEur Respir J200525233634215684300
- FriedmanBCHendeles-AmitaiAKozminskyEAdenotonsillectomy improves neurocognitive function in children with obstructive sleep apnea syndromeSleep2003268999100514746381
- KaemingkKLPasvogelAEGoodwinJLLearning in children and sleep disordered breathing: findings of the Tucson Children’s Assessment of Sleep Apnea (tuCASA) prospective cohort studyJ Int Neuropsychol Soc2003971016102614738283
- Kheirandish-GozalLDe JongMRSpruytKChamuleauSAGozalDObstructive sleep apnoea is associated with impaired pictorial memory task acquisition and retention in childrenEur Respir J201036116416920075057
- O’BrienLMMervisCBHolbrookCRNeurobehavioral implications of habitual snoring in childrenPediatrics20041141444915231906
- BarnesMEGozalDMolfeseDLAttention in children with obstructive sleep apnoea: an event-related potentials studySleep Med201213436837722425681
- BarnesMEHussEAGarrodKNImpairments in attention in occasionally snoring children: an event-related potential studyDev Neuropsychol200934562964920183724
- PerfectMMArchboldKGoodwinJLLevine-DonnersteinDQuanSFRisk of behavioral and adaptive functioning difficulties in youth with previous and current sleep disordered breathingSleep2013364517B525B23543901
- GozalDCrabtreeVMSans CapdevilaOWitcherLAKheirandish-GozalLC-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged childrenAm J Respir Crit Care Med2007176218819317400731
- GozalDKhalyfaACapdevilaOSKheirandish-GozalLKhalyfaAAKimJCognitive function in prepubertal children with obstructive sleep apnea: a modifying role for NADPH oxidase p22 subunit gene polymorphisms?Antioxid Redox Signal201216217117721902598
- GozalDSans CapdevilaOMcLaughlin CrabtreeVSerperoLDWitcherLAKheirandish-GozalLPlasma IGF-1 levels and cognitive dysfunction in children with obstructive sleep apneaSleep Med200910216717318314384
- GozalDCapdevilaOSKheirandish-GozalLCrabtreeVMAPOE epsilon 4 allele, cognitive dysfunction, and obstructive sleep apnea in childrenNeurology200769324324917636061
- KhadraMAMcConnellKVanDykeRDeterminants of regional cerebral oxygenation in children with sleep-disordered breathingAm J Respir Crit Care Med2008178887087518658114
- HoganAMHillCMHarrisonDKirkhamFJCerebral blood flow velocity and cognition in children before and after adenotonsillectomyPediatrics20081221758218595989
- GozalDKheirandish-GozalLBhattacharjeeRSpruytKNeurocognitive and endothelial dysfunction in children with obstructive sleep apneaPediatrics20101265e1161e116720956420
- GozalDPopeDWSnoring during early childhood and academic performance at ages thirteen to fourteen yearsPediatrics200110761394139911389263
- MelendresMCLutzJMRubinEDMarcusCLDaytime sleepiness and hyperactivity in children with suspected sleep-disordered breathingPediatrics2004114376877515342852
- KhalyfaASerperoLDKheirandish-GozalLCapdevilaOSGozalDTNF-α gene polymorphisms and excessive daytime sleepiness in pediatric obstructive sleep apneaJ Pediatr20111581778220846669
- TarasiukAGreenberg-DotanSSimon-TuvalTElevated morbidity and health care use in children with obstructive sleep apnea syndromeAm J Respir Crit Care Med20071751556117038661
- ReuveniHSimonTTalAElhayanyATarasiukAHealth care services utilization in children with obstructive sleep apnea syndromePediatrics20021101 Pt 1687212093948
- TarasiukASimonTTalAReuveniHAdenotonsillectomy in children with obstructive sleep apnea syndrome reduces health care utilizationPediatrics2004113235135614754948
- TsouYALinCCLaiCHDoes Adenotonsillectomy really reduced clinic visits for pediatric upper respiratory tract infections? A national database study in TaiwanInt J Pediatr Otorhinolaryngol201377567768123394793
- WeiderDJHauriPJNocturnal enuresis in children with upper airway obstructionInt J Pediatr Otorhinolaryngol1985921731824030239
- JeyakumarARahmanSIArmbrechtESMitchellRThe association between sleep-disordered breathing and enuresis in childrenLaryngoscope201212281873187722549900
- AlexopoulosEIKostadimaEPagonariIZintzarasEGourgoulianisKKaditisAGAssociation between primary nocturnal enuresis and habitual snoring in childrenUrology200668240640916904463
- LehmannGCBellTRKirkhamFJEnuresis associated with sleep disordered breathing in children with sickle cell anemiaJ Urol2012188Suppl 4S1572S1576
- Sans CapdevilaOCrabtreeVMKheirandish-GozalLGozalDIncreased morning brain natriuretic peptide levels in children with nocturnal enuresis and sleep-disordered breathing: a community-based studyPediatrics20081215e1208e121418450864
- RechtschaffenAKalesAA manual of standardized terminology, techniques and scoring system for sleep stages of human subjectsLos AngelesBrain Information Service/Brain Research Institute, University of California1968
- BerryRBBrooksRGamaldoCEHardingSMMarcusCLVaughnBVAmerican Academy of Sleep MedicineThe AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications2nd edDarien, ILAmerican Academy of Sleep Medicine2012
- KaditisAKheirandish-GozalLGozalDAlgorithm for the diagnosis and treatment of pediatric OSA: a proposal of two pediatric sleep centersSleep Med201213321722722300748
- GozalDKheirandish-GozalLNew approaches to the diagnosis of sleep-disordered breathing in childrenSleep Med201011770871320621557
- MarcusCLBrooksLJDraperKAAmerican Academy of PediatricsDiagnosis and management of childhood obstructive sleep apnea syndromePediatrics2012130357658422926173
- GuilleminaultCHuangYSGlamannCLiKChanAAdenotonsillectomy and obstructive sleep apnea in children: a prospective surveyOtolaryngol Head Neck Surg2007136216917517275534
- CantarellaGViglioneSFortiSMinettiAPignataroLComparing postoperative quality of life in children after microdebrider intracapsular tonsillotomy and tonsillectomyAuris Nasus Larynx201239440741022118950
- EricssonELundeborgIHultcrantzEChild behaviour and quality of life before and after tonsillotomy versus tonsillectomyInt J Pediatr Otorhinolaryngol20097391254126219539380
- MarcusCLWardSLMalloryGBUse of nasal continuous positive airway pressure as treatment of childhood obstructive sleep apneaJ Pediatr1995127188947608817
- MarcusCLRosenGWardSLAdherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apneaPediatrics20061173e442e45116510622
- SawyerAMGooneratneNSMarcusCLOferDRichardsKCWeaverTEA systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventionsSleep Med Rev201115634335621652236
- DiFeoNMeltzerLJBeckSEPredictors of positive airway pressure therapy adherence in children: a prospective studyJ Clin Sleep Med20128327928622701385
- McGinleyBHalbowerASchwartzARSmithPLPatilSPSchneiderHEffect of a high-flow open nasal cannula system on obstructive sleep apnea in childrenPediatrics2009124117918819564298
- Kheirandish-GozalLKimJGoldbartADGozalDNovel pharmacological approaches for treatment of obstructive sleep apnea in childrenExpert Opin Investig Drugs20132217185
- GoldbartADGoldmanJLLiRCBrittianKRTaumanRGozalDDifferential expression of cysteinyl leukotriene receptors 1 and 2 in tonsils of children with obstructive sleep apnea syndrome or recurrent infectionChest20041261131815249436
- DayyatESerperoLDKheirandish-GozalLLeukotriene pathways and in vitro adenotonsillar cell proliferation in children with obstructive sleep apneaChest200913551142114919118273
- GoldbartADGoldmanJLVelingMCGozalDLeukotriene modifier therapy for mild sleep-disordered breathing in childrenAm J Respir Crit Care Med2005172336437015879419
- GoldbartADGreenberg-DotanSTalAMontelukast for children with obstructive sleep apnea: a double-blind, placebo-controlled studyPediatrics20121303e575e58022869829
- Kheirandish-GozalLSerperoLDDayyatECorticosteroids suppress in vitro tonsillar proliferation in children with obstructive sleep apnoeaEur Respir J20093351077108419047310
- Kheirandish-GozalLGozalDIntranasal budesonide treatment for children with mild obstructive sleep apnea syndromePediatrics20081221e149e15518595959
- BrouilletteRTManoukianJJDucharmeFMEfficacy of fluticasone nasal spray for pediatric obstructive sleep apneaJ Pediatr2001138683884411391326
- KheirandishLGoldbartADGozalDIntranasal steroids and oral leukotriene modifier therapy in residual sleep-disordered breathing after tonsillectomy and adenoidectomy in childrenPediatrics20061171e61e6616396849
- PirelliPSaponaraMGuilleminaultCRapid maxillary expansion in children with obstructive sleep apnea syndromeSleep200427476176615283012
- VillaMPMalagolaCPaganiJRapid maxillary expansion in children with obstructive sleep apnea syndrome: 12-month follow-upSleep Med20078212813417239661
- VillaMPMianoSRizzoliAMandibular advancement devices are an alternative and valid treatment for pediatric obstructive sleep apnea syndromeSleep Breath201216497197621948042
- HartzellLDGuilloryRMMunsonPDDunhamAKBowerCMRichterGTTongue base suspension in children with cerebral palsy and obstructive sleep apneaInt J Pediatr Otorhinolaryngol201377453453723357781
- GuilleminaultCHuangYSMonteyrolPJSatoRQuoSLinCHCritical role of myofascial reeducation in pediatric sleep-disordered breathingSleep Med201314651852523522724
- HuangYSGuilleminaultCPediatric obstructive sleep apnea and the critical role of oral-facial growth: evidencesFront Neurol2012318423346072
- RuoffCMGuilleminaultCOrthodontics and sleep-disordered breathingSleep Breath201216227127321559930
- GuilleminaultCMonteyrolPJHuynhNTPirelliPQuoSLiKAdeno-tonsillectomy and rapid maxillary distraction in pre-pubertal children, a pilot studySleep Breath201115217317720848317
- LinACKoltaiPJSleep endoscopy in the evaluation of pediatric obstructive sleep apneaInt J Pediatr2012201257671922518178
- TruongMTWooVGKoltaiPJSleep endoscopy as a diagnostic tool in pediatric obstructive sleep apneaInt J Pediatr Otorhinolaryngol201276572272722421163
- MaturoSCHartnickCJPediatric lingual tonsillectomyAdv Otorhinolaryngol20127310911122472240
- SedaghatARFlax-GoldenbergRBGaylerBWCaponeGTIshmanSLA case-control comparison of lingual tonsillar size in children with and without Down syndromeLaryngoscope201212251165116922374875
- Abdel-AzizMIbrahimNAhmedAEl-HamamsyMAbdel-KhalikMIEl-HoshyHLingual tonsils hypertrophy; a cause of obstructive sleep apnea in children after adenotonsillectomy: operative problems and managementInt J Pediatr Otorhinolaryngol20117591127113121737150
- FrickeBLDonnellyLFShottSRComparison of lingual tonsil size as depicted on MR imaging between children with obstructive sleep apnea despite previous tonsillectomy and adenoidectomy and normal controlsPediatr Radiol200636651852316596369
- GuimaraesCVKalraMDonnellyLFThe frequency of lingual tonsil enlargement in obese childrenAJR Am J Roentgenol2008190497397518356444
- SchaafWEWoottenCTDonnellyLFYingJShottSRFindings on MR sleep studies as biomarkers to predict outcome of genioglossus advancement in the treatment of obstructive sleep apnea in children and young adultsAJR Am J Roentgenol201019451204120920410404
- DonnellyLFShottSRLaRoseCRChiniBAAminRSCauses of persistent obstructive sleep apnea despite previous tonsillectomy and adenoidectomy in children with down syndrome as depicted on static and dynamic cine MRIAJR Am J Roentgenol2004183117518115208134
- GozalDSerum, urine, and breath-related biomarkers in the diagnosis of obstructive sleep apnea in children: is it for real?Curr Opin Pulm Med201218656156722965273
- Kheirandish-GozalLGozalDThe multiple challenges of obstructive sleep apnea in children: diagnosisCurr Opin Pediatr200820665065319005333
- Kheirandish-GozalLMcManusCJKellermannGHSamieiAGozalDUrinary neurotransmitters are selectively altered in children with obstructive sleep apnea and predict cognitive morbidityChest201314361576158323306904