Abstract
Introduction
Substantial evidence suggests that drospirenone-containing oral contraceptives may cause a higher risk of venous thrombotic events than earlier-generation oral contraceptives.
Methods
To gain insight into recent real-world implications, we conducted an analysis using the US Food and Drug Administration’s Adverse Event Reporting System.
Results
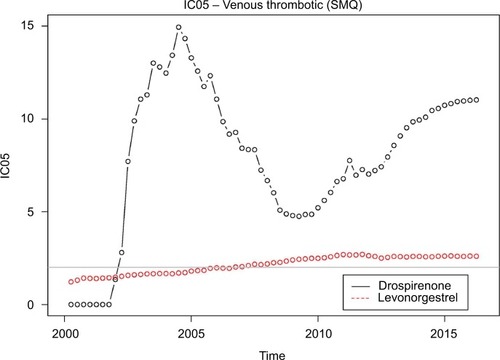
Venous thrombotic events continue to be reported at a much higher rate with drospirenone-containing oral contraceptives than the general background. The disproportionality has been rising since 2010. The same behavior is not seen with levonorgestrel-containing oral contraceptives.
Conclusion
Our results are consistent with decreased physician and patient awareness of risks associated with drospirenone-containing oral contraceptives.
Introduction
Oral contraceptives are among the most widely used drugsCitation1 and questions about oral contraceptive safety have occupied regulators, physicians, and patients for decades. Venous thrombotic risks associated with oral contraceptives have attracted particular scrutiny. A substantial body of evidence linked the first generation of oral contraceptive pills (OCPs) to significant risk of venous thromboembolism (VTE). The estrogen-like compounds in these OCPs appeared to be the cause. Subsequently, drug developers decreased the VTE risk of OCPs by lowering the delivered estrogen content by adding progestins such as levonorgestrel.Citation2 More recently, OCPs with the progestin drospirenone (Yaz/Yasmin) have enjoyed considerable commercial success, in part by emphasizing potential benefits such as reduced acne.Citation3,Citation4
Evidence began to emerge in the 2000s, however, that drospirenone-containing OCPs may cause higher risk of VTE than earlier generation OCPs. Recent systematic reviews show elevated risk,Citation5,Citation6 but others emphasize the modest absolute risk: “Regardless of whether the thrombotic risk of drospirenone OCs compared to levonorgestrel OCs is increased by 1.5-fold or threefold, the absolute risk is still low.”Citation7
The US Food and Drug Administration’s Adverse Event Reporting System (FAERS) database contains voluntarily submitted adverse event reports. The data can be downloaded from the FDA’s website (http://bit.ly/2naXeJU). To shed light on real-world experience with drospirenone OCPs and using standard analytical techniques, we considered reporting of VTE associated with drospirenone OCPs as compared with the rest of the drugs in FAERS and also considered reporting of VTE associated with levonorgestrel OCPs as compared with the rest of the drugs in the database.
Methods
For each year since the introduction of drospirenone-containing OCPs, we compared the cumulative reporting of VTE events in the FAERS database associated with these OCPs with the general background using a standard measure of disproportionality. We conducted a similar analysis for levonorgestrel-containing OCPs. Because this study involved analysis of publicly available deidentified data, no Institutional Board Approval was required.
Identification of VTE events
Our analysis uses the Standardized MedDRA Query (SMQ) “Embolic and Thrombotic Events, Venous.” provides the included MedDRA preferred terms. SMQs are groupings of MedDRA preferred terms that relate to a defined medical condition or area of interest. The included terms may relate to signs, symptoms, diagnoses, syndromes, physical findings, laboratory, and other physiologic test data, etc, related to the medical condition or area of interest. SMQs were developed to facilitate retrieval of MedDRA-coded data as a first step in investigating drug safety issues in pharmacovigilance and clinical development. Below, we refer to the “Embolic and Thrombotic Events, Venous” SMQ endpoint as “Venous Thrombotic (SMQ).”
Table 1 MedDRA terms included in the SMQ Embolic and Thrombotic Events, Venous
Inclusion criteria for oral contraceptives
The medical literature concerning epidemiological studies of oral contraceptives frequently groups oral contraceptives according to progestin (eg, Dinger et alCitation8 or Jick et alCitation9). In our analysis, we considered two progrestins: drospirenone and levonorgestrel. We included products that were listed on the investigational new drug application for each of these progestins and that have oral contraception as an indication. lists the specific products that we included.
Table 2 Specific drugs included
Disproportionality metric
We used the lower bound of a 90% interval for the “information component (IC)” statistic.Citation10 This statistic is commonly referred to as IC05. Because it is the lower end of the interval, IC05 is always closer to one than the IC and is thus more conservative than IC. The IC05 calculations stratify by age, sex, and year of report.
In practice, disproportionality metrics are often used with “signal thresholds” that dictate whether or not a given drug–outcome pair generates a signal. For example, Szarfman et alCitation11 proposed using a threshold of 2 for the “EB05” measure, quantitatively very similar to the IC05.Citation11 We indicate this threshold in .
Figure 1 IC05 values for drospirenone-containing OCPs and levonorgestrel-containing OCPs.
The IC and IC05 are used by the World Health Organization for safety assessment in its program for International Drug Monitoring.Citation10,Citation12
Results
A signal of disproportionate reporting for drospirenone-containing OCPs appeared in 2002, well before any epidemiological studies raised any concerns, and persists to this day. It is unclear why the IC05 declined in the 2005–2010 period (albeit remaining well above the standard signaling threshold of 2), but it has increased steadily since then and is currently above 11. The IC05 for drospirenone-containing OCPs is approximately five times higher than that for levonorgestrel-containing OCPs.
shows the IC05 values for each quarter from the first quarter of 2000 through the first quarter of 2016.
Discussion
Spontaneous report databases remain a mainstay of modern pharmacoepidemiology. Lester et alCitation13 provide a recent demonstration of the importance of spontaneous report analyses in characterizing drug safety issues. The article considered all drug label changes in 2010 and reported that “Spontaneous reports were the most common evidence source from which drug safety issues were identified that resulted in safety-related label changes in 2010 when analyzed both by unique safety issue and drug (52% and 55% of all evidence sources, respectively).” Moore et alCitation14 conducted a similar exercise, in their case looking at 2009 label changes involving major regulatory safety actions. The authors state that spontaneous reports “formed the basis of 77 of 135 new regulatory actions (57%) and 19 of 25 new boxed warnings (76%).” Hennessy and StromCitation15 state: “Spontaneous reporting systems remain to this day a crucial means of uncovering important postapproval drug safety information.”
Spontaneous report data have some inherent, well-documented limitations relying as they do on voluntary reporting. Underreporting is a particular concern that has been well documented and, furthermore, the data provide limited temporal information to inform analyses.Citation16 Some authors refer to the possibility of “stimulated” or publicity-triggered reporting to FAERS. A recent comprehensive review of FDA safety alerts suggests modest evidence for significant reporting changes associated with the issuance of alerts.Citation17 Some earlier studies drew similar conclusions.Citation18,Citation19 Disproportionality analyses provide limited opportunity for adjustment for potential confounding and this possibility cannot be ruled out.
Conclusion
Our analysis suggests that drospirenone-containing OCPs may result in many more VTEs than levonorgestrel-containing OCPs and that the gap is widening in recent years.
Disclosure
Dr Madigan testified for plaintiffs in litigation related to Yaz in 2011. The authors report no other conflicts of interest in this work.
References
- IMS Institute for Healthcare InformaticsMedicine use and spending shifts: a review of the use of medicines in the United States in 2014Parsippany, NJIMS Institute for Healthcare Informatics2015
- ManzoliLDe VitoCMarzuilloCBocciaAVillariPOral contraceptives and venous thromboembolismDrug Saf201235319120522283630
- The Food and Drug AdministrationYaz: Warning LetterSilver Spring, MDThe Food and Drug Administration2008 Available from: http://bit.ly/2FjVSbPAccessed March 08, 2018
- GeampanaAPregnancy is more dangerous than the pill: a critical analysis of professional responses to the Yaz/Yasmin controversySocial Sci Med2016166916
- WuCQGrandiSMFilionKBAbenhaimHAJosephLEisenbergMJDrospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic reviewBJOG2013120780181123530659
- de BastosMStegemanBHRosendaalFRCombined oral contraceptives: venous thrombosisCochrane Database Syst Rev20143CD010813
- JickSSDrospirenone-containing oral contraceptives may increase the risk of venous thromboembolismEvid Based Nurs20141737124170817
- DingerJCHeinemannLAJKuhl-HabichDThe safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance study on Oral Contraceptives based on 142,475 women-years of observationContraception200775534435417434015
- JickHJickSSMyersMWVasilakisCGurewichVRisk of idiopathic cardiovascular death and nonfatal venous thromboembolism in women using oral contraceptives with differing progestagen componentsLancet200534615891593
- NorenGNHopstadiusJBateAShrinkage observed-to-expected ratios for robust and transparent large-scale pattern discoveryStat Methods Med Res2013221576921705438
- SzarfmanAMachadoSGO’NeillRTUse of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports databaseDrug Saf200225638139212071774
- HopstadiusJNorénGNBateAEdwardsIRImpact of stratification on adverse drug reaction surveillanceDrug Saf200831111035104818840023
- LesterJNeyarapallyGALipowskiEGrahamCFHallMDal PanGEvaluation of FDA safety – related drug label changes in 2010Pharmacoepidemiol Drug Saf201322330230523280652
- MooreTJSinghSFurbergCDThe FDA and new safety warningsArch Int Med20121721788022232155
- HennessySStromBLImproving postapproval drug safety surveillance: getting better information soonerAnn Rev Pharmacol Toxicol201555758725292435
- HazellLShakirSAUnder-reporting of adverse drug reactions: a systematic reviewDrug Saf20062938539616689555
- HoffmanKBDemakasARDimbilMTatonettiNPErdmanCBStimulated reporting: the impact of US Food and Drug Administration-issued alerts on the Adverse Event Reporting System (FAERS)Drug Saf2014371197198025255848
- van HunselFvan PuijenbroekEde Jong-van den BergLvan GrootheestKMedia attention and the influence on the reporting odds ratio in disproportionality analysis: an example of patient reporting of statinsPharmacoepidemiol Drug Saf2010191263219953500
- MooreTJCohenMRFurbergCDSerious adverse drug events reported to the Food and Drug Administration, 1998–2005Arch Int Med2007167161752175917846394
