Abstract
Background:
Hepatorenal syndrome type 1 (HRS-1) is an uncommon, rapidly progressing, potentially fatal renal failure if left untreated. Terlipressin is a vasopressin analog that is first-line therapy in combination with albumin for treatment of HRS-1 in countries outside of North America. In two previous Phase III clinical trials, terlipressin showed favorable effects on improvement in renal function compared with placebo; however, neither study showed a significant between-group difference in the primary end point. CONFIRM (NCT02770716) is an ongoing clinical trial designed to address operational challenges observed in the previous studies by using a novel, more clinically relevant primary end point than the previous studies.
Methods:
This Phase III, randomized, double-blind, multicenter study is expected to enroll about 300 patients at approximately 70 sites in the US and Canada. Patients with cirrhosis and ascites demonstrating renal impairment via rapidly progressive worsening of serum creatinine (SCr) level (≥199 μmol/L [≥2.25 mg/dL] with a trajectory of SCr doubling over 2 weeks) are randomized 2:1 to receive either terlipressin 1 mg every 6 hrs as an IV bolus injection or placebo. The design of the study is generally similar to previous terlipressin prospective studies in the setting of HRS-1. A key feature differentiating this study from the previous ones centers around a novel, multi-component efficacy variable that extends beyond the traditional outcome of improvement in renal function to include durability of treatment-related effects on renal replacement therapy (RRT) requirements and survival. To meet criteria for the primary efficacy end point in CONFIRM, patients must show not only HRS reversal confirmed by two SCr values ≤1.5 mg/dL, but also survive, without RRT, for at least 10 days after achieving it.
Conclusion:
Data from this pivotal study will demonstrate whether terlipressin treatment is effective as measured by a new, clinically meaningful, multi-component primary efficacy end point.
Introduction
Hepatorenal syndrome type 1 (HRS-1) is an uncommon, serious, potentially reversible, rapidly progressing renal failure that mainly occurs in patients with advanced liver cirrhosis and circulatory dysfunction;Citation1–Citation4 it can be rapidly fatal without treatment.Citation1,Citation5 Currently, there is no approved treatment for HRS-1 in North America. Terlipressin, a synthetic vasopressin analog that acts as a splanchnic vasoconstrictor primarily via vascular vasopressin-1 receptors,Citation6 is recommended as first-line therapy where it is available.Citation7 Although it is approved for the treatment of HRS-1 in many countries in Europe, Asia, and Latin America, it has not yet received approval for use in the United States or Canada. Given that terlipressin is not yet available in North America, the American Association for the Study of Liver Diseases recommends the use of albumin infusion plus administration of vasoactive drugs such as octreotide and midodrine for patients with HRS-1; norepinephrine can also be considered for patients in an intensive care unit setting.Citation8
While there have been many single-center prospective studies reported to date,Citation9–Citation11 relatively few prospective, randomized, controlled, multicenter studies assessing the safety or efficacy of terlipressin in the setting of HRS-1 have been conducted.Citation9–Citation11 The two largest previous studies were the OT-0401 study (n=112; NCT00089570)Citation12 and the “Randomized, placEbo-controlled, double-blind study to confirm the reVERSal of hepatorenal syndromE type 1 with terlipressin” (REVERSE; n=196; NCT01143246).Citation13 The CONFIRM Study (A Multi-Center, Randomized, Placebo Controlled, Double-Blind Study to Confirm Efficacy and Safety of Terlipressin in Subjects With Hepatorenal Syndrome Type 1), currently being conducted in North America, is the third and largest-ever prospective multicenter trial to assess the safety and efficacy of terlipressin in patients with HRS-1. The protocol for CONFIRM was based on results and observations from the previous studies; it was developed, in part, to satisfy regulatory requirements for approval in North America. It was also designed to mitigate the clinical trial operational challenges encountered in the previous prospective studies that may have influenced the results of those trials. A key feature of the CONFIRM study design is the inclusion of a novel, multi-component primary efficacy end point that incorporates not only improvement in renal function (ie, HRS reversal), but also the durability of the improvement, renal replacement therapy (RRT) requirements, and survival. This report describes the end point methodology for CONFIRM, and the rationale for the multi-component primary efficacy measure chosen for the study.
Overview Of Study Methodology And Primary End Point
CONFIRM (NCT02770716) is a Phase III study investigating the effects of intravenous (IV) terlipressin for the treatment of HRS-1. It is expected to enroll a total of 300 patients at approximately 70 sites in the United States and Canada. The protocol was approved by the institutional review board and/or independent ethics committee at each study site, and the study is being conducted in accordance with the International Council for Harmonisation Good Clinical Practice guidelines,Citation14 the Declaration of Helsinki,Citation15 and all applicable regulatory guidelines. Written informed consent will be obtained from each patient or a legally authorized representative prior to enrollment in the study.
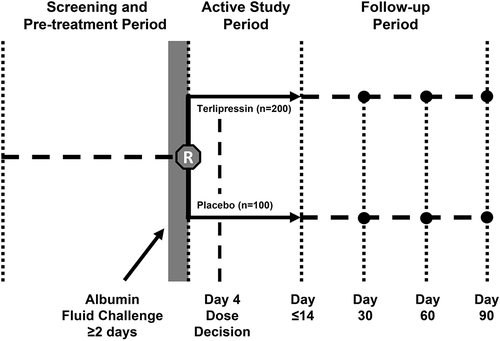
The parallel-group study design of CONFIRM is substantially similar to that of REVERSE ().Citation16 CONFIRM patients are required to be at least 18 years of age, have a diagnosis of cirrhosis and ascites, and demonstrate rapidly progressive worsening of renal function to a serum creatinine (SCr) level of ≥199 μmol/L (≥2.25 mg/dL), with a trajectory for SCr to double over 2 weeks, as determined using the same nomogram as REVERSE.Citation16 Exclusion criteria for CONFIRM are consistent with the previously reported studies,Citation12,Citation16 with a few exceptions. Importantly, unlike in the previous studies, patients with one or more events of large-volume paracentesis of ≥4 L within 2 days of randomization, and those with transjugular intrahepatic portosystemic shunt within 30 days of randomization are excluded from CONFIRM; patients with microhematuria in the setting of urinary catheterization and those receiving octreotide and midodrine up to the time of enrollment are eligible for CONFIRM. The study population in CONFIRM is considerably larger than in either of the previous studies, with a larger proportion of patients being randomized to terlipressin (2:1 ratio for terlipressin versus placebo) than in the prior studies (1:1 ratio for terlipressin versus placebo). Based on a 2:1 randomization ratio of terlipressin to placebo, and an interim analysis conducted after 50% of the patients completed study Day 14 or discharge, the planned sample size of 300 patients will provide 90% power to detect a statistically significant difference in verified HRS reversal between the groups, as determined by two consecutive SCr values ≤133 μmol/L (≤1.5 mg/dL) at least 2 hrs apart while on treatment (ie, ≤24 hrs after the final dose of study drug) by Day 14 or discharge. Also, unlike in the previous studies, patients who experience reversible peripheral or skin ischemia in CONFIRM may continue to study drug after appropriate dose adjustment at the investigator’s discretion. An overview of key differences between CONFIRM and the previous prospective studies of terlipressin in patients with HRS-1 is shown in .
Table 1 Overview Of Key Differences Between The Study Designs Of OT-0401, REVERSE, And CONFIRM
Figure 1 CONFIRM study design. Copyright © 2012. Dove Medical Press. Adapted from Boyer TD, Medicis JJ, Pappas SC, Potenziano J, Jamil K. A randomized, placebo-controlled, double-blind study to confirm the reversal of hepatorenal syndrome type 1 with terlipressin: the REVERSE trial design. Open Access J Clin Trials. 2012;4:39–49.Citation16
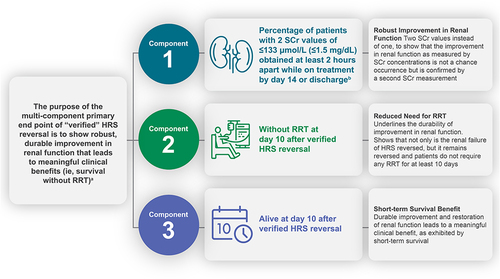
The key feature differentiating CONFIRM from previous studies is the novel, multi-component primary end point—referred to as “verified HRS reversal”—chosen for CONFIRM. Patients must achieve all three specific components in order to meet the primary end point. This multi-component end point extends beyond the traditional study end point of HRS reversal, based on SCr alone, to include additional components of RRT and survival for confirmation of clinically meaningful benefits associated with terlipressin treatment. RRT is defined as any procedure to replace non-endocrine kidney function, including intermittent hemodialysis, ultrafiltration, continuous hemofiltration and hemodialysis, peritoneal dialysis, and other dialysis and filtration techniques. CONFIRM is the first prospective, controlled study to include these additional components as part of a primary measure of efficacy. Specifically, the primary efficacy measure for this study is defined as the percentage of patients with verified HRS reversal, as determined by two consecutive SCr values ≤133 μmol/L at least 2 hrs apart while on treatment; once verified reversal is achieved, patients must also remain alive without RRT for at least 10 days in order to meet criteria for the primary study end point (). The first component of this end point, HRS reversal, allows for a second (confirmatory) SCr value to be obtained as soon as 2 hrs after the first value, unlike the previous studies, which required up to 48 hrs for the second confirmatory SCr value. This shorter, more clinically feasible 2 hrs window is long enough to indicate that the first laboratory value is not spurious and is indeed reflective of treatment-related improvement in renal function, while minimizing the risk of dropouts due to patients being discharged from the hospital before the second SCr value can be obtained. So, the first component of the end point shows robust improvement in renal function as indicated by reversal to ≤133 μmol/L (≤1.5 mg/dL), and the second component (avoidance of RRT for at least 10 days) emphasizes the durability of this improvement in renal function. RRT is an invasive and costly procedure and carries its own risks; thus, avoiding this procedure for at least 10 days after achieving the second confirmatory SCr is a clinically meaningful measure of efficacy. The third component is survival for at least 10 days beyond the date of the second confirmatory value. Even though HRS-1 is one of the many complications of cirrhosis, and these patients often have other comorbidities that impact survival (eg, esophageal variceal hemorrhage, hepatic encephalopathy), a requirement that a study patient must survive for at least 10 days as part of the primary end point underscores the effect of the treatment on this key clinical outcome. Altogether, these three components of the primary end point result in a clinically meaningful, robust end point () by requiring that the treatment-related improvement in renal function be durable enough to have a significant impact on patient morbidity and mortality.
Figure 2 Overview of the multi-component primary end point in CONFIRM. aVerified reversal is assessed as percentage of patients achieving this end point on terlipressin vs placebo; open-label albumin is administered in both treatment groups. bTreatment period is up to Day 14 or discharge, whichever comes first.
Discussion
The CONFIRM study was designed to address the operational difficulties encountered in the previous placebo-controlled Phase III studies of terlipressin. These operational challenges affected the results of those studies.Citation12,Citation13 The primary efficacy end points in OT-0401 and REVERSE required that patients achieve SCr values ≤133 μmol/L (≤1.5 mg/dL) on two occasions up to 48 hrs apart. The rationale was that the relatively long 48 hrs window for obtaining the confirmatory SCr value of ≤133 μmol/L (≤1.5 mg/dL) after a first value would clearly establish that the study treatment resulted in a durable improvement in renal function, and that the potential confounding associated with spurious results and/or variability among study laboratories would be minimal. However, because of the standard clinical practices at the time those studies were conducted, some study patients were discharged from the hospital soon after the first SCr value ≤133 μmol/L (≤1.5 mg/dL) was achieved and before the second (ie, 48 hrs confirmatory) SCr value was obtained. CONFIRM has a much shorter window to collect the second confirmatory SCr, which should reduce the risk of losing patients before the second SCr value is obtained. Importantly, in addition to the inclusion of the assessment of durability in renal function reflected by improvement in SCr, the CONFIRM multi-component primary efficacy end point, “verified HRS reversal,” incorporates two of the most important, clinically meaningful outcomes in patients with HRS-1. The additional components of the end point go beyond renal function to show that not only is renal failure of HRS reversed with terlipressin treatment, but it remains reversed and results in patient survival for at least 10 days without requiring RRT after treatment is discontinued ().
There are other key differences between the design of CONFIRM and the previous Phase III studies of terlipressin (see ). In REVERSE, study discontinuation was required for patients experiencing any ischemic event.Citation13,Citation16 In CONFIRM, patients who experience cardiac or mesenteric ischemia will be discontinued from the study, but those experiencing a reversible peripheral or skin ischemic adverse event will be allowed to continue. Also, because new diagnostic criteria for HRS eliminate the strict 221 μmol/L (2.5 mg/dL) SCr threshold in lieu of changes over time,Citation17 the inclusion criteria for participation in CONFIRM were modified such that the threshold for the second SCr value is 199 μmol/L (2.25 mg/dL), rather than 221 μmol/L (2.5 mg/dL) as in previous studies.
The potential limitations to the use of SCr as a measure of renal function in this setting should be recognized. Although a 2 hrs window for verification of HRS reversal was deemed sufficient to meet regulatory requirements for approval in North America, it is possible that increases in SCr levels may have been delayed and/or were undetectable within the 2 hrs window used for verification of HRS reversal in the current study. Additionally, accurate SCr levels are dependent on steady-state glomerular filtration rate, and may be nonspecific to the chronic kidney disease process.Citation18 Future approaches should include biomarkers that can confirm HRS reversal.
Conclusion
CONFIRM is the largest prospective, randomized clinical trial ever conducted in patients with HRS-1 as defined by rigorous inclusion and exclusion criteria. The novel, multi-component primary end point of “verified HRS reversal” chosen for this study was developed based on observations from the previous large prospective trials of terlipressin in this setting, along with guidance from regulatory health authorities. This end point includes requirements to satisfy the need for both an easy-to-obtain, commonly used measure of HRS treatment efficacy in everyday clinical practice (durable, clinically meaningful change in SCr) and the more robust evidence to demonstrate treatment-related benefits in clinical outcomes (reduction in RRT and improved patient survival) to support regulatory approvals.
Abbreviations
HRS, hepatorenal syndrome; HRS-1, HRS type 1; IV, intravenous; REVERSE, Randomized, placEbo-controlled, double-blind study to confirm the reVERSal of hepatorenal syndromE type 1 with terlipressin; RRT, renal replacement therapy; SCr, serum creatinine.
Acknowledgments
This study is sponsored by Mallinckrodt Pharmaceuticals. Medical writing and editorial support, which was conducted in accordance with Good Publication Practice (GPP3) and the International Committee of Medical Journal Editors (ICMJE) guidelines, was provided by Michael Morren, RPh, MBA, of Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and sponsored by Mallinckrodt Pharmaceuticals.
Author Contributions
Study design: SCP, KJ, AS. Study investigator: FW, AS. Enrolled patients: FW, AS. Collection and assembly of data: FW, SCP, KJ. Data analysis: SCP, KJ. Data interpretation: all authors. Manuscript preparation: SCP, KJ, AS. Manuscript review and revisions: all authors. Final approval of manuscript: all authors. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
K Jamil is an employee of Mallinckrodt Pharmaceuticals. S C Pappas has received payment of personal fees from Conatus, Orphan Therapeutics, LLC, Mallinckrodt, and Serono. F Wong has received grants and payment of personal fees from Mallinckrodt and Sequana Medical and has received payment of personal fees from Ferring. A Sanyal is President of Sanyal Biotechnology; has stock options in Durect, Exhalenz, Genfit, Indalo, Nash Pharmaceuticals, and Tiziana; has received grants from Axcella, Bristol Myers, Echosense, Intercept, Mallinckrodt, Merck, and Salix; has received payment of personal fees from Eli Lilly, Pfizer, Siemens, and Terns; and has received grants and payment of personal fees from Boehringer Ingelheim, Conatus, Gilead, Novartis, and Novo Nordisk. The authors report no other conflicts of interest in this work.
References
- Salerno F, Gerbes A, Gines P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56(9):1310–1318. doi:10.1136/gut.2006.107789
- Arroyo V, Gines P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. Hepatology. 1996;23(1):164–176. doi:10.1002/hep.510230122
- Marrero JA, Hepatorenal syndrome. NORD Guide to Rare Disorders. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:344–345.
- DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007 Dec;(165):1–209.
- Alessandria C, Ozdogan O, Guevara M, et al. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology. 2005;41(6):1282–1289. doi:10.1002/hep.20687
- Jamil K, Pappas SC, Devarakonda KR. In vitro binding and receptor-mediated activity of terlipressin at vasopressin receptors V1 and V2. J Exp Pharmacol. 2018;10:1–7. doi:10.2147/JEP.S146034
- EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–460. doi:10.1016/j.jhep.2018.03.024
- Runyon BA. Practice Guideline Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. Alexandria, VA: American Association for the Study of Liver Disease; 2012.
- Gifford FJ, Morling JR, Fallowfield JA. Systematic review with meta-analysis: vasoactive drugs for the treatment of hepatorenal syndrome type 1. Aliment Pharmacol Ther. 2017;45(5):593–603. doi:10.1111/apt.13912
- Facciorusso A, Chandar AK, Murad MH, et al. Comparative efficacy of pharmacological strategies for management of type 1 hepatorenal syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2017;2(2):94–102. doi:10.1016/S2468-1253(16)30157-1
- Wang H, Liu A, Bo W, Feng X, Hu Y. Terlipressin in the treatment of hepatorenal syndrome: a systematic review and meta-analysis. Medicine (Baltimore). 2018;97(16):e0431. doi:10.1097/MD.0000000000010431
- Sanyal AJ, Boyer T, Garcia-Tsao G, et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134(5):1360–1368. doi:10.1053/j.gastro.2008.02.014
- Boyer TD, Sanyal AJ, Wong F, et al. Terlipressin plus albumin is more effective than albumin alone in improving renal function in patients with cirrhosis and hepatorenal syndrome type 1. Gastroenterology. 2016;150(7):1579–1589. doi:10.1053/j.gastro.2016.02.026
- International Council for Harmonisation Working Group. ICH Harmonised Guideline: Integrated Addendum to ICH E6(R1): Guideline for Good Clinical Practice ICH E6(R2). ICH consensus guideline. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH); November 9, 2016. Washington, DC. Available from: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2__Step_4_2016_1109.pdf. Accessed September 20, 2019.
- World Medical Association General Assembly. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. October, 2013. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed September 20, 2019.
- Boyer TD, Medicis JJ, Pappas SC, Potenziano J, Jamil K. A randomized, placebo-controlled, double-blind study to confirm the reversal of hepatorenal syndrome type 1 with terlipressin: the REVERSE trial design. Open Access J Clin Trials. 2012;4:39–49. doi:10.2147/OAJCT.S31844
- Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62(4):968–974. doi:10.1016/j.jhep.2014.12.029
- Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20(3):672–679. doi:10.1681/ASN.2008070669
