Abstract
Purpose
To evaluate the efficacy and safety of the biosimilar infliximab in adult patients with inflammatory arthritis switched from reference product in our center.
Patients and methods
In April 2014, patients attending our rheumatology service for infliximab infusions were switched from reference product to the biosimilar infliximab following consent and hospital approval.
Results
Around 34 patients with inflammatory arthritis were switched from reference product to biosimilar infliximab in 2014: 50% female, mean age 55 years (standard deviation=12.9), mean disease duration 14.79 years (9.7), median duration on infliximab 57 months, and two-thirds on oral disease-modifying antirheumatic drugs. There was no difference in efficacy or safety in the first 6 months of therapy. By the end of 2015, the mean follow-up on biosimilar infliximab was 15.8 (standard deviation=6.3) months. Our results showed no significant difference in Health Assessment Questionnaire score, patient global assessment of disease activity, number of disease flares, or the medication dose between the originator and the biosimilar infliximab. However, reported pain and C-reactive protein values were significantly higher during the longer follow-up period (p=0.043, 0.001 respectively). There was no significant difference in the number of adverse events or infusion reactions during follow-up periods. Only five (14.7%) patients discontinued the biosimilar infliximab.
Conclusion
Our patients experienced similar efficacy and safety for managing their arthritis with the biosimilar infliximab as the reference product infliximab, but at a much lower cost.
Introduction
Inflammatory rheumatic diseases are a heterogeneous group of autoimmune conditions, the treatment for which is centered on immunosuppression, which until two decades ago was mainly based on steroids and other disease modifying antirheumatic drugs (DMARDs). The development of targeted biological therapies, genetically engineered proteins derived from human genes specially designed to inhibit specific components of the immune system that play pivotal roles in fueling inflammation,Citation1 had been one of the greatest innovation in rheumatology, and their efficacy was shown by their having achieved better outcomes with longer remissions, less disabilities, and higher quality of life. However, these biological drugs are expensive. The global sales for the licensed original anti-tumor necrosis factor (anti-TNF) drugs (infliximab, adalimumab, etanercept, certolizumab, and golimumab) in 2014 was estimated at over 34 billion US dollars,Citation2 which represents a major cost barrier for a universal health access.
Biosimilar medicine is a biological compound that is highly similar to the reference (original) biological medicine that has already been authorized for use for at least 10 years, without clinically meaningful differences in safety, purity, or potency.Citation3 In other words, it is a cheaper copy of an already authorized biological medicine. The real advantage of biosimilars is the potential costs saving.Citation4 The biosimilar infliximab CT-P13 (under trade names Remsima and Inflectra) was first licensed by the European Medicine Agency in September 2013 for marketing in Europe following demonstration of safety and efficacy in randomized controlled trials.Citation5
In Ireland, according to a recent budget impact analysis, introducing biosimilar infliximab CT-P13 for rheumatoid arthritis alone was estimated to bring about over €5 million of cost savings over 5-year period.Citation6 Another study projected savings of €351 million, €98 million, and €26 million in Germany, UK, and France, respectively, if the biosimilar infliximab was introduced at 30% less price compared to the originator.Citation7 Moreover, subsequent studies in other European countries confirmed the similar finding of substantial cost savings.Citation8,Citation9
The rheumatology department at Unit 1, Merlin Park University Hospital, Galway, is the first unit in Ireland to take the lead on introducing biosimilar infliximab for use in patients with inflammatory rheumatic disease as a switch from the original infliximab. The aim of this study is to show our experience on the biosimilar use in a real-life clinical setting outside clinical trials environment. In the long run, this may pave the road toward switching all patients with rheumatic diseases in Ireland to the biosimilar alternative, thereby improving access to biologic therapies at 30%–50% lower costs.
Patients and methods
In April 2014, 34 consecutive patients who were receiving the reference infliximab (Remicade™, Janssen Biotech, Horsham, PA, USA) for different inflammatory rheumatic diseases consented to switch to the biosimilar infliximab CT-P13 (Inflectra™, Lake Forest, IL, USA) in order to demonstrate safety and efficacy of switching to the biosimilar in routine clinical practice. The Institutional Review Board for Galway University Hospital reviewed and approved the study. All patients gave consent to allow a retrospective review of their data to be used anonymously for scientific studies. Doses, patient-reported outcomes (pain score, patient global assessment [PGA], and Health Assessment Questionnaire [HAQ]), routine hepatic and renal functions tests, full blood counts, and inflammatory markers in addition to self-reported side effects or adverse events were all reported for each infusion visit. Other adverse events were traced and extracted retrospectively from the participant’s medical notes and their rheumatology specialist’s electronic record. Data on safety and efficacy for their last 6 months of use on the reference infliximab (Remicade™) was compared to the data collected prospectively for the use of the biosimilar infliximab CT-P13 (Inflectra™) over the follow-up period until the end of 2015.
Patient population
Patients receiving the standard care were recruited at Western Ireland’s largest Rheumatology specialist service unit at Merlin Park University Hospital in Galway city. There was no randomization, and patients were enrolled without prespecified criteria. Any patient receiving the reference infliximab (Remicade™) was considered eligible for switching to the biosimilar infliximab. All patients were in remission at the time of switching. These were the same actual patients who were receiving their routine outpatient rheumatology specialist service for a number of years and attending our center based on their geographical location. Patients had their demographics, clinical, laboratory, and self-reported data collected as part of their routine administrative and clinical encounter for every time they attend our center to receive their care. The clinical data is saved in a purpose-built electronic record used for rheumatology specialist service with access limited to the specialist staff. Patients routinely received their care during their biologics infusion by the rheumatology clinical specialist nurses and other trained nursing staff in the unit. The unit is also covered by an attending consultant rheumatologist and specialist trainees. Patients who reported any illnesses, pain, flares, symptoms deterioration, or adverse events are reviewed and assessed by the appropriate medical staff to further guide their care. Patients who are otherwise in remission continued to receive their biosimilar infliximab (Inflectra™) unless it was recommended to hold or discontinue therapy.
Design
This was a prospective observational study on a cohort of 34 patients who were switched from the reference infliximab to the biosimilar infliximab in a real-life clinical setting. The primary outcome was to compare the safety (adverse events, side effects, morbidity, and mortality) and efficacy (disease activity) indicators of the last 6 months use of the reference infliximab to the following period of use on the biosimilar. The 6-month duration was used as a cut-off index for retrospective examination on the reference infliximab use for the reason of readily available data. The main hypothesis was that the biosimilar infliximab should demonstrate similar safety and efficacy profile to the reference product. The patients who used to attend to receive the reference infliximab (Remicade™) were followed up the same way and received the similar standard of care after switching to the biosimilar infliximab (Inflectra™). Doses were prescribed as per manufacturer according to the indication (for rheumatoid arthritis the loading dose =3 mg/kg at 0, 2, and 6 weeks, then every 8 weeks for maintenance, for psoriatic arthritis the loading dose =5 mg/kg at 0, 2, and 6 weeks then every 8 weeks for maintenance, for ankylosing spondylitis the loading dose =5 mg/kg at 0, 2, and 6 weeks then every 6 weeks for maintenance). The clinical and laboratory outcomes reported were the minimum standards of care for all the patients receiving any other biologic therapy in our center. The mean (standard deviation) duration on biosimilar infliximab (Inflectra™) was 15.8 (6.3) months. Reasons for discontinuation, switching to other agents, adverse effects, malignancy, or mortality were all reported irrespective of their possible causal relationship to the biosimilar infliximab (Inflectra™). All patients who started biosimilar infliximab were included in the final data analysis.
Clinical data
Three patient self-reported outcomes were used to assess disease activity: HAQ on scale 0–3, PGA on scale 0–100, and pain level on scale 0–100. Tender joints count, swollen joints count, or disease activity score (DAS) were reported by medical staff during flares, symptoms deterioration, or adverse events but not routinely for the sake of this study.
Laboratory data
Patients’ bloods for biochemistry, hematology, inflammatory markers, or immunological tests get routinely collected at each visit. These include liver functions tests, urea and electrolytes, complete blood count, serum protein electrophoresis, C-reactive protein (CRP), and erythrocyte sedimentation rate. Any additional blood tests were also collected if indicated under the physician’s discretion.
Statistical analysis
All variables of interest were numerical in nature. SPSS version 22 (StataCorp LP, College Station, TX, USA) was adopted for data analysis. Descriptive statistics were used to report the differences in the outcome variables before and after use of the biosimilar infliximab (Inflectra™). The central tendency for the normally distributed data was expressed in mean (standard deviation), while nonnormally distributed continuous data was expressed in median (range). χ2 test was used to compare categorical variables. As all the data was collected before and after switching for the same patients, all tests were assumed to be paired (related). Continuous variables that are normally distributed were reported using parametric statistics (paired Student’s t-test) while nonparametric alternative (Wilcoxon signed-rank test) was used for nonnormally distributed data. Approximately 95% confidence intervals were reported for the mean differences before and after switching. p values below 0.05 were considered significant.
Results
Patients’ characteristics
Thirty-four patients were switched from the reference infliximab to the biosimilar infliximab. Approximately 50% were female, mean age of 55 years, and all were Caucasians ( and ). Overall mean disease duration was 14.79 years, and the patients were on the reference infliximab for a median duration of 57 months.
Table 1 Main patient characteristics
Table 2 Patient characteristic’s stratified by gender
Seventeen patients (50%) had rheumatoid arthritis (64% rheumatoid factor positive, 57% anti-CCP positive), nine patients (26.5%) had ankylosing spondylitis (78% human leukocyte antigen B27 positive), four patients (11.8%) had psoriatic arthritis, and three patients (8.8%) had inflammatory bowel disease-related spondyloarthropathy. There was one patient with juvenile inflammatory arthritis. Around 70.6% of patients had erosive disease, and 23.5% had extra-articular disease. Approximately 32.4% were on infliximab monotherapy, while 35.3% were on methotrexate and the rest were on other disease modifying antirheumatic drugs (sulfasalazine =4, leflunomide =2, cyclosporin =2, methotrexate + other DMARD combination =3). Only two patients were on steroids.
Efficacy outcomes
Patient-reported outcomes (CRP, pain, PGA, and HAQ) were compared before and after switching to the biosimilar infliximab (). Mean (standard deviation) PGA for the last 6 months on the reference infliximab was 28.3 (23.2) compared to 35.2 (25.6) following biosimilar switching without significant difference (p=0.111). Median HAQ on reference infliximab also did not differ significantly following switching (0.38 vs 0.69, p=0.111).
Figure 1 Box-plots for the main study outcomes.
Abbreviations: PGA, patient global assessment; HAQ, Health Assessment Questionnaire.
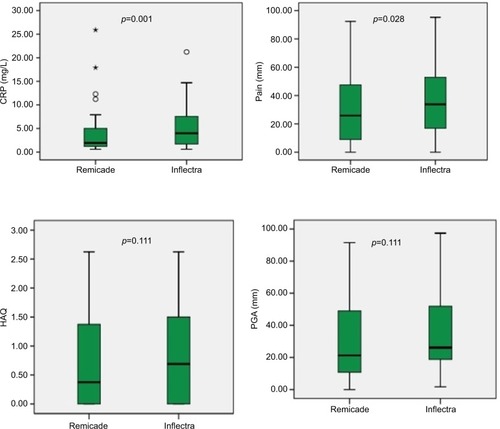
Patients reported significantly higher level of pain on biosimilar infliximab compared to reference infliximab. CRP had increased statistically significantly following switching to the biosimilar infliximab (from 1.95 to 4.0, p=0.001). Doses used for both the reference and the biosimilar infliximab were similar. Flare was defined as an episode of increased symptoms that necessitated a form of additional intervention (eg, analgesia, systemic steroids, or local injection), whether self or physician-assisted. There were total of 14 flare events () during the biosimilar use compared to 10 events during the reference infliximab use (p=0.402, without adjusting for the duration exposure, 6 months vs 15.8 months).
Table 3 Overall main study findings
Safety outcomes
Discontinuation, morbidity, and mortality
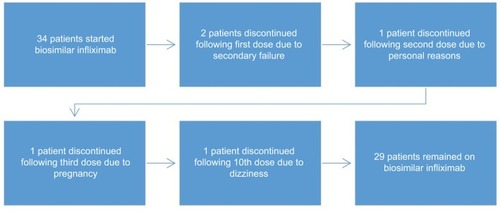
Of the 34 patients () who started the biosimilar infliximab, five patients had discontinued their use: pregnancy =1, secondary failure =2, side effect =1 (dizziness), and one patient had withdrawn consent and was switched back to reference infliximab following second dose for the reason that he was subjectively feeling worse without objective deterioration or sustaining adverse events. One patient experienced dizziness after the 10th dose that necessitated discontinuation and switching to alternative biologic following normal MRI brain and partial resolution of symptoms. The two patients who sustained secondary failure were switched to alternative biologic after only one dose of biosimilar, also indicating failure to the reference infliximab. One elderly female died with pneumonia and heart failure following two doses of the biosimilar. One female developed non-small-cell lung cancer after having received seven doses of the biosimilar and three doses of the reference infliximab.
Infusion-related adverse events
One epileptic patient on antiepileptic drugs had a seizure during the reference infliximab infusion; this was an isolated episode and resulted in a temporary discontinuation, but the patient later resumed his therapy without further events. Also, one patient on the biosimilar infliximab experienced nausea and abdominal pain post infusion; this was also an isolated event and did not recur on subsequent infusions. The infusion-related events for both the reference and biosimilar infliximab took place later during the course of therapy. No patient on the biosimilar experienced allergic or anaphylactic reactions, and the drug was well tolerated.
Infection risk and other adverse events
All patients in our center get pre anti-TNF TB screening with Quantiferon™ (Cellestis, Carnegie, Australia) and CXR. No TB reactivation reported on the biosimilar infliximab in our patients. Serious adverse event was defined as an event that necessitated a visit to the general practitioner, to the hospital emergency room, or to our center and resulted in temporary or permanent discontinuation (). Two patients on the reference infliximab sustained serious adverse events (lower limb cellulitis =1, pleuritic chest pain =1) compared to three patients on the biosimilar who experienced similar events (atypical chest pain =2, asthma exacerbation =1). Five patients sustained respiratory tract infections on the reference infliximab compared to eight on biosimilar. Six patients on the biosimilar infliximab experienced gastroenteritis that necessitated temporary discontinuation (two patients with inflammatory bowel disease-related arthritis had three episodes). Two patients had urinary tract infections on the reference infliximab compared to one on biosimilar. One patient encountered ear infection (treated topically) on the reference infliximab, and one patient had an eye infection (treated topically) during biosimilar use. None of these infections demanded hospital admission, except for one occasion of leg cellulitis during use of reference infliximab.
Table 4 Main safety outcomes
Discussion
This is the first switching experience of its kind in Ireland and Western Europe. There are very few reports on switching to biosimilar infliximab CT-P13 on adult patients with inflammatory rheumatic diseases. The largest two clinical trials so far, PLANETRACitation10 and PLANETAS,Citation11 had published their data only in April 2016 as an extension study to their original randomized controlled trials, and both trials showed that switching to biosimilar infliximab was of comparable safety and efficacy profile to the reference product (switching was carried out between weeks 54 and 102 of the study). Other than clinical trials, there is only one published report (in late 2015 from Finland) on switching to biosimilar CT-P13 in a real-life clinical setting,Citation12 which was a prospective study of 39 patients who were switched to biosimilar infliximab for a median duration of 11 months; this study also demonstrated similar safety and efficacy outcomes in comparison to reference infliximab, however with a higher discontinuation rate at 28% – over half of them was for subjective reasons. Our study so far showed the longest longitudinal data available on biosimilar CT-P13 in patients with rheumatic diseases in a routine clinical practice outside randomized controlled trial setting, with mean follow-up duration of 15.8 months by end of 2015. The discontinuation rate of 14.7% was comparable to the other reports.Citation10–Citation12 Few recent reportsCitation13–Citation16 on experience with biosimilar infliximab have emerged at the late EULAR 2016 meeting in London, and they also confirmed the similar findings of comparable safety and efficacy profile to their reference product. Preliminary 1-year results from a larger study from Norway, the NOR-SWITCH study, have been presented at the American College of Rheumatology 2016 meeting. Around 199 of 500 patients having arthritis were being treated with rheumatoid-approved drugs. At 12 months, their safety and efficacy data were in line with expectations.Citation17
With the exception to pain and CRP, all other outcome indicators were comparable before and after switching. The CRP had increased statistically significantly following switching; however, it had still remained within the normal range (1.95 vs 4.0, our lab normal CRP cut off reference is <5). The pain increase was also statistically significant but of small size (from 28 to 38).
There were no serious infections or TB reactivation encountered during biosimilar use. Five patients experienced a total of six gastroenteritis events, two of them known to have inflammatory bowel disease-related arthropathy. The patient who died was a frail 80-year-old female who was on the reference infliximab for 58 months and had had hip surgery for fracture within last 12 months. The patient who developed non-small-cell lung cancer was a 67-year-old female smoker who declined earlier investigations when suspicion was raised and the biosimilar was discontinued.
This is one of the very few available reports on switching to biosimilar infliximab for rheumatic diseases in a real-life clinical setting. The main strength was the fact that it was carried out in the same environment as the routine outpatient clinical care. The follow-up duration is one of the longest available, but less than 2 years. Our study has important limitations, including a small sample size, being from a single center, and being observational rather than a randomized, blinded clinical trial. The lack of data on menopausal status of the women included in our study and lack of serum immunogenicity markers before and after switch are among other limitations.
Conclusion
Our patients who were switched from the reference to the biosimilar infliximab experienced comparable efficacy and safety profile over the follow-up period (15.8 months) without major safety issues in keeping with the other previous reports.Citation10–Citation12 Larger switching studies with longer follow-up duration are ongoing, and needed, to support these findings. Major cost savings are anticipated (30%–50%) with switching to biosimilar infliximab.
Acknowledgments
Statistical software was provided by the National University of Ireland, Galway (NUIG).
Disclosure
Prof. John J Carey has received speaker fees from Hospira UK, Hospira Canada, Pfizer Canada, Pfizer Brazil. He has received education and research grants from Centocor, USA, MSD. Ireland and travel support from MSD Ireland. This work was presented as a poster abstract at the EULAR 2016 (European League Against Rheumatism) meeting in London, UK (DOI: 10.1136/annrheumdis-2016-eular.2924). The authors report no other conflicts of interest in this work.
References
- WebMed [homepage on the Internet]Biologics for Rheumatoid Arthritis Treatment – Enbrel, Humira, Remicade, and More Available from: http://www.webmd.com/rheumatoid-arthritis/guide/biologics
- ResearchandMarketsCompetitor Analysis: TNF Antibodies 2015 – Biosimilars and Biosuperiors of Humira, Remicade and Enbrel Available from: http://www.researchandmarkets.com/research/xr7tsg/competitor
- European Medicines AgencyQ&A: Similar biological products – Biosimilar medicines Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/document_listing/document_listing_000318.jsp
- LapadulaGFerraccioliGFBiosimilars in rheumatology: pharmacological and pharmacoeconomic issuesClin Exp Rheumatol2012304 Suppl 73S102S10623078880
- BajiPPéntekMCzirjákLEfficacy and safety of infliximab-biosimilar compared to other biological drugs in rheumatoid arthritis: a mixed treatment comparisonEur J Health Econ201415Suppl 1S53S6424832836
- McCarthyGEbel BitounCGuyHIntroduction of an Infliximab Biosimilar (CT-P13): a five-year budget impact analysis for the treatment of rheumatoid arthritis in IrelandValue Health2013167A558
- WhitehouseJWalshKPapandrikopoulouAHoadRThe cost saving potential of utilizing biosimilar medicines in biologic naive severe rheumatoid arthritis patientsValue Health2013167A573
- BrodszkyVBajiPBaloghOPéntekMBudget impact analysis of biosimilar infliximab (CT-P13) for the treatment of rheumatoid arthritis in six Central and Eastern European countriesEur J Health Econ201415S16571
- JhaAUptonADunlopWCAkehurstRThe budget impact of biosimilar infliximab (Remsima®) for the treatment of autoimmune diseases in five European countriesAdv Ther201532874275626343027
- YooDHProdanovicNJaworskiJEfficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension studyAnn Rheum Dis201776235536327130908
- ParkWYooDHMirandaPEfficacy and safety of switching from reference infliximab to CT-P13 compared with maintenance of CT-P13 in ankylosing spondylitis: 102-week data from the PLANETAS extension studyAnn Rheum Dis201776234635427117698
- NikiphorouEKautiainenHHannonenPClinical effectiveness of CT-P13 (Infliximab biosimilar) used as a switch from Remicade (infliximab) in patients with established rheumatic disease. Report of clinical experience based on prospective observational dataExpert Opin Biol Ther201515121677168326549204
- GlintborgBKringelbachTSørensenIJNon-medical switch from originator to biosimilar infliximab in patients with inflammatory arthritis – impact on s-infliximab and antidrug-antibodies. Results from the Danish Rheumatologic Biobank and Danbio RegistryPresented at: 17th Annual European Congress of RheumatologyJune 8–11, 2016London, UK eular abstract archive
- RubioERuizALόpezJProspective study of 78 patients treated with infliximab biosimilar Remsima®Ann Rheum Dis2016751006
- BatticciottoAParisiSLi GobbiGFSafety and efficacy of switching from innovator to biosimilar infliximab in patients affected by spondyloarthritis. A 6-month observational studyAnn Rheum Dis201675Suppl 2180626582822
- SheppardMHadaviSHayesFKentJDasguptaBPreliminary data on the introduction of the infliximab biosimilaR (CT-P13) to a real world cohort of rheumatology patientsAnn Rheum Dis2016751011
- GollGLOlsenIGJorgensenKKBiosimilar infliximab (CT-P13) is not inferior to originator infliximab: results from a 52-week randomized switch trial in NorwayPresented at: ACR 2016 Annual Meeting2016 Abstract 19L. Available from: http://acrabstracts.org/abstract/biosimilar-infliximab-ct-p13-is-not-inferior-to-originator-infliximab-results-from-a-52-week-randomized-switch-trial-in-norway/