Abstract
Purpose
A significant variability of the second harmonic (2ndH) phase of steady-state pattern electroretinogram (SS-PERG) in intrasession retest has been recently described in glaucoma patients (GP), which has not been found in healthy subjects. To evaluate the reliability of phase variability in retest (a procedure called RE-PERG or REPERG) in the presence of cataract, which is known to affect standard PERG, we tested this procedure in GP, normal controls (NC), and cataract patients (CP).
Methods
The procedure was performed on 50 GP, 35 NC, and 27 CP. All subjects were examined with RE-PERG and SS-PERG and also with spectral domain optical coherence tomography and standard automated perimetry. Standard deviation of phase and amplitude value of 2ndH were correlated by means of one-way analysis of variance and Pearson correlation, with the mean deviation and pattern standard deviation assessed by standard automated perimetry and retinal nerve fiber layer and the ganglion cell complex thickness assessed by spectral domain optical coherence tomography. Receiver operating characteristics were calculated in cohort populations with and without cataract.
Results
Standard deviation of phase of 2ndH was significantly higher in GP with respect to NC (P<0.001) and CP (P<0.001), and it correlated with retinal nerve fiber layer (r=−0.5, P<0.001) and ganglion cell complex (r=−0.6, P<0.001) defects in GP. Receiver operating characteristic evaluation showed higher specificity of RE-PERG (86.4%; area under the curve 0.93) with respect to SS-PERG (54.5%; area under the curve 0.68) in CP.
Conclusion
RE-PERG may improve the specificity of SS-PERG in clinical practice in the discrimination of GP.
Introduction
Glaucoma is a progressive optic neuropathy characterized by the apoptosis of retinal ganglion cells (RGCs), which becomes clinically evident anatomically as typical alterations of the optic nerve head (ONH) and retinal nerve fiber layer (RNFL) and functionally as visual field defects.
Standard automated perimetry (SAP) is the main tool for the detection of functional impairment of the visual field. However, it has been reported that at least 25%–40% of RGCs must to be lost before any visual field damage occurs.Citation1,Citation2
In addition, it has been reported that 60% of ocular hypertensive patients who become glaucomatous show ONH and RNFL damage before the occurrence of visual field damage.Citation3
For the anatomical analysis, spectral domain optical coherence tomography (OCT) can provide the objective measures of ONH and RNFL.Citation4–Citation6
Because most of the RGCs are located in the macula, the evaluation of this area and, in particular, of the ganglion cell complex (GCC) has been proposed as a diagnostic tool for the early diagnosis of glaucoma, in addition to the evaluation of ONH and RNFL.Citation7–Citation11
It is well known that in the diagnosis of glaucoma, a variable percentage of RGCs must die before any evidence of damage can be found by using the tools actually available (ie, among the most used, OCT for anatomical impairment and standard achromatic perimetry for functional impairment), regardless of the specific tool that, case by case, is able to detect the damage before the other one.Citation3 On the other hand, it is reasonable to think that, in the natural history of glaucoma, there should be an early stage in which the RGCs are damaged, but still alive. The aim of all the electrophysiologic studies performed on glaucoma patients (GP) was to find an examination able to recognize this early stage of the disease.
Pattern electroretinogram (PERG) has been shown to be able to analyze the electric activity of RGCsCitation12,Citation13 and, for this reason, to be suitable for the diagnosis of early glaucoma.Citation14,Citation15
PERG has been shown to be abnormal even before the occurrence of visual field defects, as measured by SAP, and of RNFL loss, as assessed by OCT.Citation14
Cross-sectional studies have shown that the PERG is frequently altered in glaucoma suspects and in patients with early glaucoma more than in normal controls (NC).Citation16–Citation20
A specific model of PERG for glaucoma screening (steady-state pattern electroretinogram [SS-PERG]), which is fast and user-friendly, has been developed for the evaluation RGCs dysfunction.Citation21
The PERG is recorded in response to a noninvasive stimulus based on contrast variation of low (transient response) or high (steady-state response) temporal frequency.Citation21 A steady-state stimulus (fast) shows better glaucomatous dysfunction than a transient stimulus (slow), since RGCs are submitted to a greater metabolic stress.Citation22 The steady-state stimulus determines a sinusoidal response that is analyzed by the Fourier transform.Citation23,Citation24 In this way, the second harmonic, that is, the harmonic that has a frequency twice that of the stimulus, can be isolated. Amplitude and phase of this harmonic show typical alterations in glaucoma. In particular, the amplitude is reduced in patients with glaucoma and ocular hypertension (OHT) compared to healthy subjects, while the phase remains constant or at the most tends to delay with age and with the disease.Citation25 SS-PERG has been reported to have high test–retest repeatability, providing, also, a good signal-to-noise ratio.Citation26 In particular, the phase variability was very limited in the retest within and between trials.Citation17,Citation27
Porciatti focused his attention on the phase delay, which tends to increase as the disease progresses. In general, he assumed that the PERG amplitude should be related to the number of surviving RGCs, while the phase should express diminished activity of the existing neurons (synaptic dysfunction). Therefore, he hypothesized that a reduced input to RGCs may be due to a synaptic dysfunction.Citation28 Dendritic modifications precede neuronal apoptosis and can result in reduced responsiveness of RGCs with lower ability to follow stimuli of increased temporal frequency. A phase delay without amplitude reduction could arise from synaptic and transport delays.Citation27,Citation29
The biologic variability of a measurement is not only a physiologic behavior inherent in the instrumental bias, as in diagnostic imaging,Citation30 but also in the different adaptation of the bioelectrical response to an external visual stimulus.Citation22,Citation31
Nevertheless, the amplitude can also be reduced in the presence of nonspecific causes (optic media opacities and others), while phase is a more specific indicator of disease,Citation28 showing, in addition, low variability in the retest within and between trials.Citation17,Citation27
The main problem in the use of electrophysiologic diagnosis in glaucoma is its variability, due to the fact that often, the patients present associated conditions (ie, cataract, diabetic retinopathy, low myopia) that can influence the measurements.Citation32,Citation33 In clinical trials, these kinds of patients are excluded, but in the ordinary practice, they are very common. Probably, this is the reason why PERG is not usually performed worldwide, apart from the specialized laboratories, and is not included in the standard diagnosis tools for glaucoma in any scientific society guidelines.Citation34,Citation35
In a previous study,Citation36 we showed that the individual-intrinsic within-trial variability of the PERG signal in test–retest of the same eye (ie, five consecutive stimulations without pause, a procedure that we called RE-PERG or REPERG) of early GP was greater than the physiologic one present in healthy individuals; in addition, it was also correlated with markers of disease severity such as retinal thickness and visual field indices. The aim of this study was to verify that such variations are not influenced by optic media opacities and, therefore, that the RE-PERG can be more reliable than the standard SS-PERG in the discrimination of GP.
Materials and methods
From January to July 2015, 112 consecutive patients were enrolled in the study. All patients were recruited at the Glaucoma Center of the Brindisi Social Health District, Mesagne, Italy, and at the Department of Ophthalmology and Otolaryngology of the University of Bari, Italy.
The patients were divided into three groups: 50 glaucoma patients (GP), 62 age-matched patients further divided into 35 normal controls (NC), and 27 control cases with no glaucoma, but with various degrees of cataract (cataract patients [CP]).
The criteria for classification in the GP group, in accordance with the European Glaucoma Society (EGS) guidelines (http://www.eugs.org/eng/EGS_guidelines4.asp), were: appearance of optic disc and peripapillary nerve fiber layer glaucoma damage (increased ratio cup/disc, asymmetry ratio of cup/disc, notch or narrowing of the neuroretinal rim, disc hemorrhage, thinning of the peripapillary nerve fiber layer) or visual field suspicious for glaucomatous damage in the absence of clinical signs of other optic neuropathies (default sort, nasal step, paracentral scotoma, altitudinal defect) with a constant elevated intraocular pressure before therapy. The severity of glaucoma was evaluated functionally by SAP and anatomically by the measurement of RNFL and GCC thickness with spectral domain OCT. The NC group included 35 age-matched healthy subjects with no evidence of having any other ocular or general disease or undergoing any ocular or general therapy able to determine the influence on the visual function.
The CP group included 27 age-matched subjects with no evidence of having any other ocular or general disease or undergoing ocular or general therapy able to determine the influence on the visual function, apart from cataract.
Each participant underwent a comprehensive ophthalmic evaluation, including review of medical history, best-corrected visual acuity testing, intraoccular pressure (IOP) measurement by Goldmann applanation tonometry, ultrasound pachymetry (Pachmate GH55; DGH Technology, Inc., Exton, PA, USA), slit-lamp biomicroscopy, gonioscopy, and dilated fundus examination with a 78 lens. All participants had best-corrected visual acuity ≥20/40 (Snellen acuity), spherical refraction within ±5.0 D, and cylinder correction within ±2.0 diopters, and NC patients had transparent ocular media (nuclear color/opalescence, cortical, or posterior subcapsular lens opacity <1) according to the system of lens opacity Classification System III and open iridocorneal angles on gonioscopy. CP had cataract up to nuclear color N2. Patients with coexisting retinal diseases, diabetes, Parkinson’s disease, or nonglaucomatous optic neuropathies able to determine nonspecific PERG abnormalityCitation37,Citation38 were excluded.
Only one eye of each patient who met the criteria mentioned above was randomly included in the study.
Spectral domain optical coherence tomography
Peripapillary RNFL thickness was assessed by a Zeiss Cirrus HD OCT-500 (software version 7.0.1.290; Carl Zeiss Meditec, Dublin, CA, USA). The protocol Optic Disc Cube 200×200 was used to perform a circular scan 3.46 mm in diameter and was automatically targeted around the optic disc to provide the RNFL thickness of the four quadrants and each of the 12-hour clock positions. The protocol Macular Cube 512×128 was used to obtain measurements of macular thickness.
All images were obtained by the same experienced technician with a signal larger/resistance at 7/10. Three scans of the optic disc and the macular region were consecutively acquired and analyzed for each eye. The measurements of RNFL and GCC were averaged using the data of each of the three scans.
Standard automated perimetry
The visual field was assessed by Humphrey Field Analyzer, model 745i II (Carl Zeiss Meditec AG, Jena, Germany) using the 24-2 Swedish Interactive Threshold Algorithms (SITA) standard strategy. Near addition was added to the subject’s refractive correction. If fixation losses were >20% and false-positive or false-negative results were higher than 15%, the test was repeated. At least two reliable SAPs were performed to minimize the effect of learning.Citation39 Typical glaucomatous defects considered were those with a pattern standard deviation (PSD) significantly higher than the 5% level and/or a glaucoma hemifield test outside the normal limits.
Pattern electroretinogram
Both the SS-PERG and the RE-PERG were recorded with a commercial instrument (RETIMAX Advanced ver. 4.3; CSO, Florence, Italy).
The RE-PERGs were recorded using a method similar to the PERG for Glaucoma (PERGLA) paradigm,Citation21 with some minor changes made by our laboratories. We used a stimulus of horizontal bars with a spatial frequency of 1.7 cycles/degree, which was found in previous studies as the most sensitive in detecting RGCs dysfunction in early glaucoma,Citation40,Citation41 modulated in counterphase at 15 reversals/second and electronically generated on a high-resolution ionized gas electrically charged plasma display (contrast: 90%; luminance: 80 cd/m2; field size: 24° [width] ×24° [height]).
The subjects had undilated pupils of size between 3 and 4 mm, with an appropriate correction for the working distance (57 cm). The signals were recorded from a skin electrode 9 mm Ag/AgCl placed on the lower eyelid. A similar electrode placed on the lid of the unstimulated eye was used as a reference, as described in other studies.Citation36 In all cases, the impedance was below 5 k. The responses were amplified (gain of 100,000), filtered (bandwidth: 130 Hz), and sampled with a resolution of 12 bits. The analysis time was equal to the time period of the stimulus (133 ms).
An average of 650 events for SS-PERGs and 5 consecutive blocks of 130 events for RE-PERG was calculated, with automatic rejection of artifacts. The data were then exported to a text file. The amplitude (μV) and phase (πrad) of the second harmonic were then analyzed with the Fourier transform ().
Figure 1 Example of five consecutive steady-state PERGs.
Abbreviation: PERGs, pattern electroretinograms.
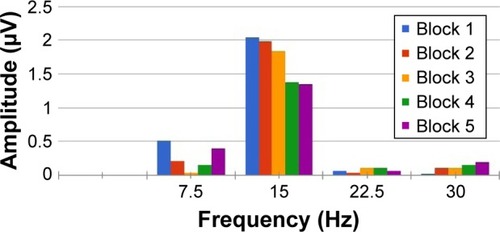
The repeatability of the amplitude and phase of the second harmonic was calculated as the standard deviation of amplitude (SD Ampl) and phase (SD Phase, ). The noise level obtained by recording a response to an occluded stimulus was ≤0.087±0.03 μV in both normal subjects and patients.
Figure 2 Example of phase trend of five consecutive PERGs.
Abbreviation: PERG, pattern electroretinogram.
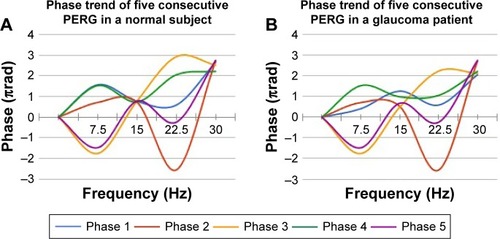
As described previously,Citation31 to avoid the inherent ambiguity for phase values around zero, which is typically associated with spurious variability, it is necessary to subtract multiples of 2π from the recorded value of the modulo (2 less than the recorded value).Citation25 So, phase values are consequently enclosed between 1 and 3 πrad without discontinuities. PERG signal was considered reliable only when the second harmonic was clearly visible in the spectrum of the frequencies.
Statistical analysis was performed using commercial software (MedCalc® 16.8.1.0). A P-value of ≤0.05 was considered statistically significant.
This study follows the tenets of the Declaration of Helsinki for human studies. The study was approved by both the ethical committees of the Brindisi Social Health District and the University of Bari. For this study, written informed consent was obtained from all subjects after the nature of the test and the possible risks were explained in detail.
Results
General demographics of the patients are summarized in ; demographics of GP, NC, and CP are summarized in –, respectively.
Table 1 Demographic and relevant ocular characteristics of the study participants
Table 2 Clinical characteristics of glaucoma patients
Table 3 Clinical characteristics of normal controls
Table 4 Clinical characteristics of cataract patients
We observed a significant reduction of PERG amplitude in GP compared to controls (PERG: 1.36 vs 1.68 μV, P<0.001).
GP showed significantly different mean deviation (MD), PSD, RNFL, and GCC values from the control group (−2.5 vs 0.3, P<0.001; 2.66 vs 1.23 dB, P<0.001; 75.84 vs 92.03 μm, P<0.001; and 67.26 vs 80.14 μm, P<0.001, respectively).
As predicted, the reduction in PERG amplitude positively correlated to MD, RNFL, and GCC and negatively correlated to PSD (MD 0.57, P<0.0001; RNFL 0.35, P=0.0002; GCC 0.31, P=0.0008; and PSD −0.29, P=0.0021; ).
Table 5 CC and SL-P between MD PSD, RNFL, GCC, PERG amplitude, PERG phase, SD phase in 50 glaucoma patients
To better understand the influence of comorbidities on the electrophysiologic diagnosis of glaucoma, we evaluated SS-PERG amplitude and RE-PERG phase variability (SD phase), respectively. In particular, we assigned a score of 1 or 0 to the pathologic or normal outcome of SS-PERG and RE-PERG. We considered a low amplitude in SS-PERG (<1.5 μm) and a high phase variability of PERG signal in RE-PERG (>0.15 SD) as pathologic.Citation36,Citation41
summarizes the SS-amplitude and the RE-PERG phase SD for each patient of the study. The scatter diagram shows that a significant reduction in SS-PERG amplitude (<1.5 μV) can be observed both in GP and CP with a consistent overlap among the groups; it is not the same for phase variability of PERG signal in RE-PERG >0.15 SD, which better discriminates GP from CP.
Figure 3 Scatter diagram shows distribution of phase standard deviation (SD) and PERG amplitude for each patient of the study.
Abbreviations: PERG, pattern electroretinogram; SS-PERG, steady-state pattern electroretinogram.
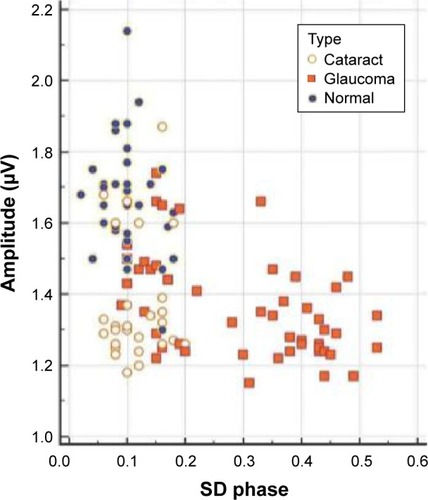
In the GP group, SS-PERG showed a specificity of 82.1% (95% confidence interval [CI]:a 66.5–92.5). In the CP group, SS-PERG showed abnormal results in 24 cases (85%). In both GP and CP groups, considered as a whole, total specificity of SS-PERG dropped to 54.5% (95% CI: 41.8%–66.9%) due to false-positive higher incidence. In the GP group, RE-PERG showed a specificity of 84.6% (95% CI:a 69.5%–91.1%; ). In the CP group, RE-PERG showed abnormal results in two cases (7%). In both GP and CP groups, considered as a whole, RE-PERG total specificity increased from 84.6% to 86.4% (75.7–93.6, ).
Table 6 ROCs without (A) and with (B) cataract patients
Discussion
It was found that variations in the phase are little affected by lens opacities and deterioration of optics that instead cause a nonspecific reduction of PERG amplitude.Citation25 Other causes of nonspecific reduction of the PERG amplitude are myopia and diabetic retinopathy.Citation32,Citation33 Because of the variability of PERG amplitude due to nonspecific causes, the implication for glaucoma is that a worsening of the quality of the visual stimulus, as in CP, may display nonspecific PERG amplitude reductions due to stimulus deterioration, but not PERG phase delays, which remain related only to the disease.
Starting from the evidences provided by Porciatti about the little influence of the optic media on the phase delay, we decided to study not its absolute value, but its retest variability. In a previous study,Citation29 we found that this parameter was significantly different among healthy subjects and GP. In particular, we found that coefficient of variability of the phase was significantly increased in early GP (8.97%±2.52%) and glaucoma suspects (7.30%±2.51%) compared to healthy subjects (3.54%±1.13%; P<0.0001); in addition, it was correlated with PSD (P=0.0009), GCC (P=0.028), and RNFL (P=0.0078) exclusively in early GP. The great advantage of using this parameter was that, for analyzing the intrapatient, intratest variability, it did not need a normative database ().
We suppose that the SD phase, not based on the absolute value of the phase, is not influenced by optic media opacities. Therefore, the aim of this study was to verify the specificity of this new paradigm (called RE-PERG) in the presence of cataract.
Our results show that SS-PERG and RE-PERG have the same specificity in GP; but by mixing GP and CP, the specificity of SS-PERG drops to 54.5%, whereas the specificity of RE-PERG remains high (86.4%).
Based on the outcome of this study, we suppose that increased phase variability in RE-PERG could be the expression of the lower ability to follow stimuli due to RGCs’ preapoptotic synaptic dysfunction in glaucoma.
Further studies are required: first, the procedure should be validated in other laboratories also to confirm our results; second, its reliability should also be verified in other conditions potentially able to bias the results (diabetic retinopathy, low myopia, etc); third, longitudinal studies should be carried out to verify its predictive value in ocular hypertensive patients; finally, it would be also helpful to verify the variations of the SD phase under therapy (both topical hypotensive and neuroprotective).
Acknowledgments
The abstract of this paper was presented at the AISG/SIGLA 2016 Congress held in Catania (Italy) on 7–11 June 2016 and at the 2016 ESCRS Congress held in Athens (Greece) on 26–28 February 2016, as a presentation with interim findings. The abstract was not published anywhere, apart from the official program of the congress. This paper is original and has not been submitted elsewhere.
Disclosure
The authors report no conflicts of interest in this work.
References
- HarwerthRSCarter-DawsonLShenFSmithEL3rdCrawfordMLGanglion cell losses underlying visual field defects from experimental glaucomaInvest Ophthalmol Vis Sci199940102242225010476789
- Kerrigan-BaumrindLAQuigleyHAPeaseMEKerriganDFMitchellRSNumber of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same personsInvest Ophthalmol Vis Sci200041374174810711689
- KassMAHeuerDKHigginbothamEJThe Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucomaArch Ophthalmol2002120670113 discussion 829–830
- WollsteinGIshikawaHWangJBeatonSASchumanJSComparison of three optical coherence tomography scanning areas for detection of glaucomatous damageAm J Ophthalmol20051391394315652826
- WollsteinGSchumanJSPriceLLOptical coherence tomography (OCT) macular and peripapillary retinal nerve fiber layer measurements and automated visual fieldsAm J Ophthalmol2004138221822515289130
- HougaardJLHeijlABengtssonBGlaucoma detection by stratus OCTJ Glaucoma200716330230617438424
- TanOChopraVLuATHDetection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomographyOphthalmology2009116122305231419744726
- RaoHLZangwillLMWeinrebRNSamplePAAlencarLMMedeirosFAComparison of different spectral domain optical coherence tomography scanning areas for glaucoma diagnosisOphthalmology201011791692169920493529
- MoriSHangaiMSakamotoAYoshimuraNSpectral-domain optical coherence tomography measurement of macular volume for diagnosing glaucomaJ Glaucoma201019852853420164794
- KotowskiJWollsteinGFolioLSIshikawaHSchumanJSClinical use of OCT in assessing glaucoma progressionOphthalmic Surg Lasers Imaging201142 SupplS6S1421790113
- CurcioCAAllenKATopography of ganglion cells in human retinaJ Comp Neurol199030015252229487
- MaffeiLFiorentiniLElectroretinographic responses to alternating gratings before and after section of the optic nerveScience198121144859539557466369
- ZrennerEThe physiological basis of the pattern electroretinogramProg Retin Res19909427464
- VenturaLMSorokacNLos SantosNRThe relationship between retinal ganglion cell function and retinal nerve fiber thickness in early glaucomaInvest Ophthalmol Vis Sci20064793904391116936103
- BachMHoffmannMBUpdate on the pattern electroretinogram in glaucomaOptom Vis Sci200885638639518521020
- PfeifferNBachMThe pattern-electroretinogram in glaucoma and ocular hypertension. A cross-sectional and longitudinal studyGer J Ophthalmol19921135401477616
- BowdCTafreshiAVizzeriGZangwillLMSamplePAWeinrebRNRepeatability of pattern electroretinogram measurements using a new paradigm optimized for glaucoma detectionJ Glaucoma200918643744219680050
- BowdCTafreshiAZangwillLMMedeirosFASamplePAWeinrebRNPattern electroretinogram association with spectral domain-OCT structural measurements in glaucomaEye201125222423221183943
- BowdCVizzeriCTafreshiAZangwillLMSamplePAWeinrebRNDiagnostic accuracy of pattern electroretinogram optimized for glaucoma detectionOphthalmology2009116343744319167080
- TafreshiARacetteLWeinrebRNPattern electroretinogram and psychophysical tests of visual function for discriminating between healthy and glaucoma eyesAm J Ophthalmol2010149348849520172073
- PorciattiVVenturaLMNormative data for a user-friendly paradigm for pattern electroretinogram recordingOphthalmology2004111116116814711729
- PorciattiVSorokacNBuchserWHabituation of retinal ganglion cell activity in response to steady state pattern visual stimuli in normal subjectsInvest Ophthalmol Vis Sci20054641296130215790894
- CooleyJWTukeyJWAn algorithm for the machine calculation of complex Fourier seriesMath Comput19651990297301
- O’NeillMAFaster than fast FourierByte1998134293300
- VenturaLMPorciattiVIshidaKFeuerWJParrishRK2ndPattern electroretinogram abnormality and glaucomaOphthalmology20051121101915629814
- YangASwansonWHA new pattern electroretinogram paradigm evaluated in terms of user friendliness and agreement with perimetryOphthalmology2007114467167917398319
- FredetteMJAndersonDRPorciattiVFeuerWReproducibility of pattern electroretinogram in glaucoma patients with a range of severity of disease with the new glaucoma paradigmOphthalmology2008115695796317976726
- PorciattiVVenturaLMPhysiologic significance of steady-state pattern electroretinogram losses in glaucoma: clues from simulation of abnormalities in normal subjectsJ Glaucoma200918753554219745668
- FrishmanLJFreemanAWTroyJBSchweitzer-TongDEEnroth-CugellCSpatiotemporal frequency responses of cat retinal ganglion cellsJ Gen Physiol19878945996283585279
- AraieMTest–retest variability in structural parameters measured with glaucoma imaging devicesJpn J Ophthalmol201357112423138681
- PorciattiVVenturaLMAdaptive changes of inner retina function in response to sustained pattern stimulationVision Res200949550551319124035
- VenturaLMGolubevIFeuerWJPorciattiVThe PERG in diabetic glaucoma suspects with no evidence of retinopathyJ Glaucoma201019424324719528818
- OnerAGumusKArdaHKarakucukSMirzaEPattern electroretinographic recordings in eyes with myopiaEye Contact Lens200935523824119672200
- European Glaucoma SocietyTerminology and guideline for glaucoma4th ed Available from: http://www.eugs.org/eng/EGS_guidelines4.aspAccessed January 11, 2017
- PrumBEJrRosenbergLFStevenJPrimary open-angle glaucoma preferred practice pattern® guidelinesOphthalmology2016123141111
- MavilioAScrimieriFErricoDCan variability of pattern ERG signal help to detect retinal ganglion cells dysfunction in glaucomatous eyes?Biomed Res Int2015201557131426167489
- LamBLFeuerWJAbukhalilFPorciattiVHauswirthWWGuyJLeber hereditary optic neuropathy gene therapy clinical trial recruitment: year 1Arch Ophthalmol201012891129113520837795
- Bodis-WollnerIVisual electrophysiology in Parkinson’s disease: PERG, VEP and visual P300Clin Electroencephalogr19972831431479241467
- HeijlALindgrenGOlssonJThe effect of perimetric experience in normal subjectsArch Ophthalmol1989107181862642703
- PorciattiVFalsiniBScaliaGFaddaAFontanesiGThe pattern electroretinogram by skin electrodes: effect of spatial frequency and ageDoc Ophthalmol19887011171223229289
- FalsiniBMarangoniDSalgarelloTStructure–function relationship in ocular hypertension and glaucoma: interindividual and interocular analysis by OCT and pattern ERGGraefes Arch Clin Exp Ophthalmol200824681153116218386035
- DeLongERDeLongDMClarke-PearsonDLComparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approachBiometrics19884438378453203132
