Abstract
Background
Rhegmatogenous retinal detachment (RRD) is the most common form of retinal detachment and an ophthalmic emergency. Here, we compared outcomes of primary RRD eyes operated with conventional scleral buckling (SB) with cryoretinopexy to those operated with standard pars plana vitrectomy (PPV).
Methods
This is an institutional, retrospective, register-based, observational, comparative study. Based on the surgical procedure, 319 eyes of 319 patients were divided into two groups: SB plus cryotherapy (n=50) and PPV (n=269). Changes in intraocular pressure (IOP) and best-corrected visual acuity (BCVA) were recorded at 30 days and reoperation rates within 180 days postoperatively.
Results
Eyes operated with PPV had less reoperations within the first 180 days as compared with SB eyes (P=0.001, log-rank test); however, changes in IOP were more prominent (mean ± standard deviation: +8.1±8.8 vs. +4.4±7.0 mmHg, respectively; P=0.006). Changes in BCVA did not differ between the surgical procedures.
Conclusion
PPV was associated with higher primary anatomic success rates and lower risk of reoperation but significant IOP elevation when compared to SB. These factors should be case-specifically considered when choosing treatment modality for primary RRD.
Introduction
RRD is a potentially sight-threatening vitreoretinal disease and ophthalmic emergency.Citation1 PVR, first reported in 1934 by Gonin, is the most frequent and severe complication of RRD and a major cause of failure of RRD surgery leading to a need of reoperation, with an incidence of 4%–34% in prospective studies.Citation2–Citation4
SB and PPV are the two most commonly used surgical techniques for primary repair of uncomplicated RRD, with anatomic success rates in the range of 85%–91%.Citation5–Citation7 SB was introduced in 1957, and PPV in 1976.Citation8,Citation9 These techniques can also be used combined.Citation10,Citation11 During the last two decades, plenty of clinical studies have been published related to clinical risk factors, ocular characteristics, and features of RRD, as well as methods of reattachment surgery and development of instrumentation. Currently, SB is considered as a reference technique for phakic primary RRD, and PPV as the first choice for primary pseudophakic RRD as well as for posterior and giant retinal tears, and for cases with media opacities and complex retinal pathology.
Here, we investigated these two main surgical RRD techniques, SB and PPV, and compared the early postoperative outcomes and probability of reoperations within 180 days postoperatively.
Materials and methods
Study design
This was an institutional, retrospective, register-based, observational study. Patients were admitted for primary operation for management of RRD in the unit of vitreoretinal surgery, in a tertiary governmental hospital, Helsinki University Hospital, Helsinki, Finland. Our register-based study was conducted according to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Helsinki University Central Hospital and Hjelt Institute, Faculty of Medicine, University of Helsinki. Due to the retrospective nature of the study, patient written informed consent was deemed not required by the Institutional Review Board of Helsinki University Hospital.
Patients and surgical approach
In our study, a total of 319 eyes of 319 patients underwent primary vitreoretinal surgery for RRD between January 1 and December 31 of 2013. Inclusion criteria were, (1) a history of primary RRD, (2) having undergone SB or microincision transconjuctival PPV as the primary procedure, and (3) availability for follow-up for at least 180 days postoperatively. Exclusion criterion was a history of combined phacovitrectomy or the presence of traumatic RRD (n=21) or juvenile RRD (age ≤18 years). No postoperative endophthalmitis was observed in our study. Eyes which have undergone SB plus PPV were not included in the analysis.
Basic patient-related and ophthalmic data collected included age, gender, body mass index, ASA physical status classification, and systemic diseases as well as operation time, extent of RD, status of the lens (crystalline/IOL), presence of VH, presence of PVR (grade C), AL, IOP (mmHg), and BCVA at baseline.
All patients in the SB group underwent conjunctival peritomy, isolation and looping of rectus muscles, external marking of holes/breaks via diathermy, treatment of retinal holes/breaks with cryoretinopexy (ca. −80°C) via indirect ophthalmoscopy, and suturing of segmental circumferential silicone buckles (foam sponge sutured to the sclera) to support retinal holes/breaks with or without external drainage of subretinal fluid. Anterior chamber paracentesis was performed when needed. Intraocular filtered air injection was performed if considered necessary. After conjunctival closure, paraocular antibiotic (gensumycin) and corticosteroid injections (kenalog) were performed. During buckling procedure, the eye fundus was viewed through the indirect ophthalmoscope.
All patients in the PPV group underwent a standard valved transconjunctival (20, 23, or 25 gauge) vitrectomy (Constellation Vision System; Alcon Laboratories, Inc., Fort Worth, TX, USA) with noncontact viewing system (EIBOS; Möller-Wedel, a Haag-Streit Company, Wedel, Germany). Surgical technique consisted of core vitrectomy (three-dimensional vitrectomy mode) with cutting rate up to 5,000 per minute and suction up to 500 mmHg. Perfluorocarbon liquids were used intraoperatively to reattach the retina. Chromodissection with a combination of brilliant blue and trypan blue (0.15%) (MembraneBlue-dual®; DORC, Zuidland, the Netherlands) was performed for epiretinal PVR membrane and ILM staining. Dissection techniques were used to remove preretinal (PVR) membranes lying on the ILM, and ILM peeling was performed if considered necessary. Shaving and removal of peripheral vitreous was done under nurse assistance or using an extra chandelier according to surgeons’ preference. Peripheral vitreous was occasionally visualized using triamcinolone acetonide if needed. Drainage of the subretinal fluid was achieved through a preexisting original retinal break or via a posterior drainage retinotomy in eyes with anterior retinal break(s) with or without perfluorocarbon assistance. In PPV cases, retinal periphery was examined, and the original hole(s)/break(s)/tear(s) was treated with endolaser photocoagulation. Fluid–air exchange was performed and assisted with flute needle suction. Different intraocular tamponade agents were used in the PPV eyes. The choice of tamponade gas, sulfur hexafluoride (20%), perfluoroethane (19%), or perfluoropropane (13.6%), was individualized based on the location and characteristics of RD and expected patient compliance. Silicone oil tamponade was used in eleven eyes. Removal of silicone oil was not counted as revitrectomy. After gas exchange, sclerotomy massage was performed to promote self-sealing. If sclerotomy leakage was suspected, a suture was placed.
Of the operated 319 patients, 50 (15.7%) eyes underwent SB plus cryotherapy, and 269 (84.3%) underwent PPV as initial surgical procedure. The operations were performed mostly under retrobulbar local anesthesia (302 eyes; 95%), and only a minority of the patients received general anesthesia (17 eyes; 5%).
Indication and timing of reoperation was checked from patient archives up to 180 days postoperatively. Postoperatively, operated patients received dexamethasone (1 mg/mL)/chloramphenicol (2 mg/mL) eye drops four times a day for 4 weeks.
VA testing and measurement of IOP
BCVA was measured preoperatively, 1 day postoperatively, and at 30 days postoperatively on a standard Snellen chart at an observation distance of 5 m. No other intermediate-phase controls were routinely performed. When the largest optotype could not be recognized correctly, the classification was very low VA on a semiquantitative scale, such as CF, HM, LP, or NLP. For statistical purposes, the Snellen values were transformed to the equivalent logMAR units. The very low VA measurements were converted as follows: CF 1.9 logMAR, HM 2.3 logMAR, LP 2.7 logMAR, and NLP 3.0 logMAR.Citation12
IOP was measured with Goldmann applanation tonometry.
Statistical analysis
Data are given as mean ± standard deviation and range (min–max) except absolute number and proportion for nominal scale, and were analyzed with SPSS 15.0 (IBM Corp., Armonk, NY, USA) and R 2012 (R Foundation for Statistical Computing, Vienna, Austria). For two-group comparisons, qualitative data were analyzed by two-factor χ2 test, ordinal measurement scale variables by Mann–Whitney U-test, and continuous variables by Student’s t-test. Log-rank test was used to estimate the hazard functions of the groups at observed event time. P≤0.05 was considered statistically significant.
Results
Baseline patient-related and ophthalmic characteristics of SB and PPV groups
A total of 319 eyes were included in our study: 50 eyes in the SB group and 269 eyes in the PPV group. Baseline characteristics of study groups are given in .
Table 1 Baseline variables
There were discrepancies in some patient and ocular baseline characteristics. The age of presentation varied from 20 to 74 years with a mean age of 54.3 years in the SB group as compared with the age of presentation varying from 35 to 101 years with a mean age of 61.4 years in the PPV group (P=0.001, ). The RD patients in the SB group were healthier as defined by ASA physical status classification system compared with RD patients in the PPV group (P=0.002). Noteworthy, the gender distribution differed among the two main study groups, with more males being operated by SB than by PPV (P=0.009). Of note, there were altogether 120 male patients and 199 female patients included in our study. There was no difference in the presence of systemic diseases such as hypertonia, diabetes mellitus, or coronary artery disease among the study groups.
The operation time was significantly shorter in the SB than in the PPV group (P=0.005, ). As regards the extent of RRD (the total area of detached quadrants), there were statistical differences between the SB and PPV groups (P=0.001). While 19.3% of patients in the PPV group had a detachment larger than two quadrants, only 2% in the SB group presented with this. More accurately, 30 out of 50 eyes (60%) operated with SB had retinal detachment less than one quadrant (3 clock hours), 19 eyes (38%) had less than two quadrants, and in only one eye (2%), three quadrants were detached. No patient in the SB group had a total RRD (four quadrants). On the contrary, only 81 eyes out of 269 (30%) operated with PPV had retinal detachment less than one quadrant, 136 eyes (51%) had less than two quadrants, and 44 eyes (16%) had less than three quadrants. Eight eyes (3%) had a total RRD. All in all, more complicated RRD cases were operated in our PPV group.
Preoperatively, IOP was lower (P=0.042), and occurrence of VH was more common (P=0.001) in eyes operated with PPV than by SB (). No difference was observed regarding the presence of PVR, or the AL of the study eyes between the study groups. Pseudophakic RRD eyes (n=127) were all treated with PPV except for one with SB (P<0.001).
Outcomes in SB and PPV groups
At 30 days postoperatively, IOP was higher in RRD eyes operated with PPV compared to SB (P=0.034, ), with the concomitant IOP pressure change after surgery being higher in the PPV group (P=0.006, ). The IOP change was related to type of endotamponade (). Also, BCVA remained worse in RRD eyes operated with PPV compared to SB (P=0.013). However, the BCVA gain was comparable between the groups (P=nonsignificant).
Table 2 Postoperative variables
Table 3 Postoperative outcome
Table 4 Intraocular pressure change (mmHg) according to endotamponade
Reason for reoperation was retinal redetachment
Noteworthy, primary anatomical surgical success rate differed between the study groups (Kaplan–Meier analysis). We found that the eyes operated with primary SB were reoperated more commonly within the first 180 postoperative days compared with the RRD eyes that underwent primary PPV (P=0.001, ). No gender or age predilection for reoperations was noticed between SB or PPV groups (data not shown).
Figure 1 180-Day anatomic success rates of the study eyes.
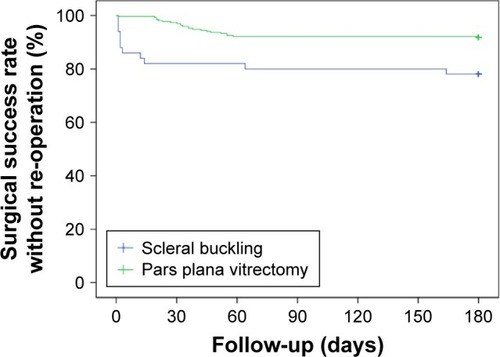
Discussion
In our study, primary RRD eyes operated with three-port PPV had less reoperations due to retinal redetachment within 180 days postoperatively as compared with conventional SB procedure. The first 6 weeks postoperatively are considered as the most important period during which most complications occur, while most of the retinal redetachments seem to occur within six months.Citation13 Only one-sixth of our study eyes were treated with SB, demonstrating per se the current tendency of vitreoretinal surgeons to treat RRDs with PPV.Citation14 The fact that primary SB procedures more often had a need for reoperation is of importance. Noteworthy, previous reports have confirmed that single SB and PPV procedures have similar anatomical and functional outcomes in uncomplicated RRD cases.Citation2,Citation5,Citation6,Citation15 Interestingly, more female than male patients underwent primary RRD surgery in our study hospital, but the reason for this gender discrepancy was not clear.
The reasons for inferiority of SB procedure in our study could be related to various patient-, eye-, and/or surgical technique-related characteristics. Preoperative risk factors contributing to RRD reoperation are related to longer duration of retinal detachment, presence of PVR, extent of RD, presence of VH, structure of the eyeball (longer AL, myopia), systemic diseases, and medication of the patient.Citation16 Generally, PVR formation is considered more pronounced in younger patientsCitation2 which, however, was not confirmed in our study population. In patients with RRD of more than 1-week duration, tissue remodeling phases such as mesenchymal transformation of RPE cells toward fibroblast- or macrophage-like cell morphology are activated, and the risk for PVR is therefore considered higher.Citation3,Citation17 Preoperatively, a minority of our primary RRD eyes were diagnosed with characteristics of advanced PVR formation (star folds or epiretinal membranes) by biomicroscopy, which was 4% in the SB group compared with 11% in the PPV group, paralleling previous studies.Citation18 Since rate of preexisting PVR did not significantly differ between our SB and PPV study groups, preoperatively observed PVR per se should therefore not be related to the increased reoperation rate observed in our SB group.
Also, intravitreal hemorrhage has been considered as a high-risk factor related to reoperation rate.Citation19 In our study, VH was diagnosed significantly more often in eyes operated with PPV than in SB eyes, which would not explain the fact that prognosis as regards the reoperation rate was worse in SB eyes as compared to PPV eyes. Therefore, VH does not seem to affect the reoperation rate in our study. Neither is the reoperation rate directly associated with involved detached retinal quadrants. Interestingly, in our study, pseudophakic RRD eyes operated with PPV had more extensive detachments to start with (ie, total area of involved retinal quadrants was larger), but these eyes had better postoperative outcome and were reoperated less often than SB eyes. Previously, it has been postulated that the intact lens provides a physical barrier for transmission of inflammatory cytokines from the anterior chamber to the vitreous cavity reducing thus the risk of redetachment.Citation20 Neither can the reoperations be related to duration of surgical procedure, as the eyes operated with PPV in our study had longer duration of surgery but better postoperative prognosis.
Based on our study results, we think that cryotherapy used in SB eyes could cause more changes in retinal tissue microarchitecture than laser in PPV eyes. Cryotherapy could increase local vitreous-related biochemical factors such as various growth factors, extracellular matrix proteolytic factors, and other factors contributing thus to breakdown of blood–retinal barrier and PVR development in eyes.Citation21,Citation22 During microincisional valved PPV procedure, more complex removal of PVR-causing RPE cells and clumps as well as removal of the irregular and rolled edges of the retinal break(s) can be accomplished. More complete removal of these PVR-causing cells (RPE cells, glial cells, fibroblasts, myofibroblasts, erythrocytes), the cytokines (interleukin-1, interleukin-6, interleukin-8, interleukin-10, interferon gamma, angiopoietin-2, transforming growth factor-β1), and matrix metalloproteinases 2 and 9 diminishes PVR processCitation17,Citation22,Citation23 and decreases the reoperation rate in vitrectomized eyes compared with SB-operated eyes.
Additional use of cryotherapy per se could also be related to higher risk of reoperations in SB eyesCitation24–Citation26 by inducing more inflammation and contraction in the SB-treated eyes than endolaser treatment in the PPV eyes. Moreover, loss of vitreous during external drainage of subretinal fluid and incarceration of vitreous to the external drainage sclerotomy can cause additional traction and lead to secondary hole/break formation in SB eyes. Perioperatively undetected retinal holes may also play a role in higher redetachment rate in SB-operated eyes. Detection of holes might, however, be improved if extra chandelier light is used during the SB procedure.Citation27 Lower reoperation rate in PPV eyes can also be associated with transconjunctival vitrectomies that are known to reduce the surgical trauma and postoperative inflammation.Citation28,Citation29
The major limitation of our study was its retrospective and register-based design with limited postoperative follow-up. Therefore, intraoperative complications arising from surgery (such as type and number of iatrogenic breaks, subretinal hemorrhage, passage of liquid perfluorocarbon to the subretinal space, perforation of sclera/choroid) were not available for analysis. Another major limitation was that the operations were not done by the same vitreoretinal surgeon. Notably, our department is the highest-volume ophthalmic vitreoretinal department in Finland, and all the acute-onset emergency patients with RRD in the whole of Finland during the weekends can be operated in our hospital. Therefore, we consider that the criteria for choosing the type of SB or PPV operation were generally well established among experienced VR surgeons and in compliance with the recommended guidelines,Citation2 diminishing the surgeon-related confounding effects. In our center, we prefer to perform the SB surgery for young, phakic patients with attached hyaloid, clear media, and proper identification of retinal hole(s)/break(s) and/or tears. However, when the RRD case is more complicated (pseudophakic RRD, VH, multiple breaks, posterior and/or large breaks), we tend to perform primary PPV. Another limitation of our study is the heterogeneous nature of the study population with no age-or gender-matching between our study groups that cannot be overcome in real-world clinical studies.
During our study, the economic aspects did not affect the decision-making of the surgical process. The VR surgeons were able to choose whatever instrumentation was considered the best choice for the individual patient. Therefore, our results might not be generalized to other kinds of health care systems around the world, where the patient might pay for the treatment by himself/herself or where hospital-related resources are more limited.
The strength of our study is that we analyzed all consecutive primary RRD eyes operated in our tertiary institution within 1 year. Our RRD study eyes represented mostly cases with acute onset, with inflammation and cell proliferation phases being activated in less than 1-week duration.Citation30 Including more complicated RRD eyes with longer duration might surely have affected our study results. In addition to improved surgical technology, improving postsurgical prognosis of RRD eyes also demands deeper understanding of the biochemical signaling mechanisms associated with intraocular fibrosis-related tissue repair mechanisms.Citation31 In the future, search for potential biomarkers, that is, differentially expressed proteins linked to RRD inflammatory pathways, might provide us with knowledge that might help in monitoring and predicting the development of the RRD in eyes with or without preoperative PVR disease.Citation28,Citation29 Prospective randomized studies are needed to answer these open questions more accurately.
Conclusion
RRD eyes operated with primary PPV had higher anatomic success rates within 180 days as compared with SB eyes, despite the fact that PPV eyes had more complicated RRD to start with (VH, extent of RD, IOL) as compared with less complicated RRD in SB eyes. In our study, the patients in the SB group were younger, healthier, had smaller RRD area, less often had intravitreal hemorrhage, and had better initial preoperative BCVA. Despite these facts, they experienced more retinal redetachments postoperatively. Benefits and disadvantages between SB and PPV treatment modalities should be case-specifically considered in primary RRD.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Abbreviations
| AL | = | axial length |
| ASA | = | American Society of Anesthesiologists |
| BCVA | = | best-corrected visual acuity |
| CF | = | counting fingers |
| HM | = | hand motion |
| ILM | = | internal limiting membrane |
| IOL | = | intraocular lens |
| IOP | = | intraocular pressure |
| logMAR | = | logarithm of minimum angle of resolution |
| LP | = | light perception |
| NLP | = | no light perception |
| PPV | = | pars plana vitrectomy |
| PVR | = | proliferative vitreoretinopathy |
| RD | = | retinal detachment |
| RPE | = | retinal pigment epithelial |
| RRD | = | rhegmatogenous retinal detachment |
| SB | = | scleral buckling |
| VA | = | visual acuity |
| VH | = | vitreous hemorrhage |
Acknowledgments
The authors thank Mrs Sanna Piiponniemi for excellent technical assistance.
Disclosure
The authors report no conflicts of interests in this work.
References
- FeltgenNWalterPRhegmatogenous retinal detachment – an ophthalmologic emergencyDtsch Arztebl Int20141111–21222 quiz 2224565273
- HeimannHBartz-SchmidtKUBornfeldNWeissCHilgersRDFoersterMHScleral Buckling versus Primary Vitrectomy in Rhegmatogenous Retinal Detachment Study GroupScleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment: a prospective randomized multicenter clinical studyOphthalmology2007114122142215418054633
- PastorJCProliferative vitreoretinopathy: an overviewSurv Ophthalmol19984313189716190
- KwonOWSongJHRohMIRetinal detachment and proliferative vitreoretinopathyDev Ophthalmol20165515416226501375
- SunQSunTXuYPrimary vitrectomy versus scleral buckling for the treatment of rhegmatogenous retinal detachment: a meta-analysis of randomized controlled clinical trialsCurr Eye Res201237649249922577767
- SoniCHainsworthDPAlmonyASurgical management of rhegmatogenous retinal detachment: a meta-analysis of randomized controlled trialsOphthalmology201312071440144723511114
- NooriJBilonickRAEllerAWScleral buckle surgery for primary retinal detachment without posterior vitreous detachmentRetina201636112066207127172097
- SchepensCLOkamuraIDBrockhurstRJThe scleral buckling procedures. I. Surgical techniques and managementAMA Arch Ophthalmol195758679781113478226
- MachemerRPars plana vitrectomy. IntroductionTrans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol1976813 Pt 1350351
- WongCWYeoIYLohBKScleral buckling versus vitrectomy in the management of macula-off primary rhegmatogenous retinal detachment: a comparison of visual outcomesRetina201535122552255726049617
- HaugstadMMoosmayerSBragadóttirRPrimary rhegmatogenous retinal detachment – surgical methods and anatomical outcomeActa Ophthalmol Epub20161118
- Schulze-BonselKFeltgenNBurauHHansenLBachMVisual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity testInvest Ophthalmol Vis Sci20064731236124016505064
- LeeEEl HousseiniZSteelDHWilliamsonTHAn analysis of the outcomes for patients with failed primary vitrectomy for rhegmatogenous retinal detachmentGraefes Arch Clin Exp Ophthalmol2014252111711171624668386
- El-AmirANKeenanTDAbu-BakraMTannerVYeatesDGoldacreMJTrends in rates of retinal surgery in England from 1968 to 2004: studies of hospital statisticsBr J Ophthalmol200993121585159019671530
- D’AmicoDJClinical practice. Primary retinal detachmentN Engl J Med2008359222346235419038880
- ChaudharyRDretzkeJScottRLoganABlanchRClinical and surgical risk factors in the development of proliferative vitreoretinopathy following retinal detachment surgery: a systematic review protocolSyst Rev20165110727391963
- GarwegJGTappeinerCHalberstadtMPathophysiology of proliferative vitreoretinopathy in retinal detachmentSurv Ophthalmol201358432132923642514
- MitryDSinghJYorstonDThe predisposing pathology and clinical characteristics in the Scottish retinal detachment studyOphthalmology201111871429143421561662
- YeungLYangKJChenTLAssociation between severity of vitreous haemorrhage and visual outcome in primary rhegmatogenous retinal detachmentActa Ophthalmol20088616516917995984
- KonCHAsariaRHOcclestonNLKhawPTAylwardGWRisk factors for proliferative vitreoretinopathy after primary vitrectomy: a prospective studyBr J Ophthalmol200084550651110781515
- MoysidisSNThanosAVavvasDGMechanisms of inflammation in proliferative vitreoretinopathy: from bench to bedsideMediators Inflamm2012201281593723049173
- LoukovaaraSLehtiKRobciucAIncreased intravitreal angio-poietin-2 levels associated with rhegmatogenous retinal detachmentGraefes Arch Clin Exp Ophthalmol2014252688188824218041
- KitaTHataYAritaRRole of TGF-beta in proliferative vitreoretinal diseases and ROCK as a therapeutic targetProc Natl Acad Sci U S A200810545175041750918952846
- LincoffHKreissigIThe mechanism of the cryosurgical adhesion. IV. Electron microscopyMod Probl Ophthalmol197210991065056389
- VeckeneerMvan OverdamKBouwensDRandomized clinical trial of cryotherapy versus laser photocoagulation for retinopexy in conventional retinal detachment surgeryAm J Ophthalmol2001132334334711530046
- van MeursJCFeronEvan RuyvenRMulderPVeckeneerMPostoperative laser coagulation as retinopexy in patients with rhegmatogenous retinal detachment treated with scleral buckling surgery: a prospective clinical studyRetina200222673373912476099
- SeiderMINomidesREHahnPMruthyunjayaPMahmoudTHScleral buckling with chandelier illuminationJ Ophthalmic Vis Res201611330430927621789
- RecchiaFMScottIUBrownGCBrownMMHoACIpMSSmall-gauge pars plana vitrectomy: a report by the American Academy of OphthalmologyOphthalmology201011791851185720816248
- Iwashi-ShimaCSatoTBandoHIkedaTEmiKAnatomic and functional outcomes of 25-gauge vitrectomy for repair of eyes with rhegmatogenous retinal detachment complicated by proliferative vitreoretinopathyClin Ophthalmol201372043204924143072
- PastorJCRojasJPastor-IdoateSDi LauroSGonzalez-BuendiaLDelgado-TiradoSProliferative vitreoretinopathy: a new concept of disease pathogenesis and practical consequencesProg Retin Eye Res20165112515526209346
- LiMLiHJiangPLiuXXuDWangFInvestigating the pathological processes of rhegmatogenous retinal detachment and proliferative vitreoretinopathy with metabolomics analysisMol Biosyst20141051055106224556753
