Abstract
Purpose
This study evaluated visual function and anatomic and vision-related quality-of-life outcomes in recalcitrant neovascular age-related macular degeneration (AMD) subjects switched to aflibercept (Eylea®) from ranibizumab (Lucentis®).
Methods
In a single-center study conducted in Barrie, ON, 40 patients with persistent fluid despite previous ranibizumab treatment were switched to aflibercept with 3 consecutive monthly doses. Main outcome measure was mean change from baseline to week 12 in Pelli–Robson contrast sensitivity (CS). Secondary outcomes were mean change in best corrected visual acuity (BCVA), central retinal thickness (CRT), and National Eye Institute 25-Item Visual Function Questionnaire (NEI VFQ-25) score. A two-sided paired t-test was used in the statistical data analysis to compare the means of continuous variables.
Results
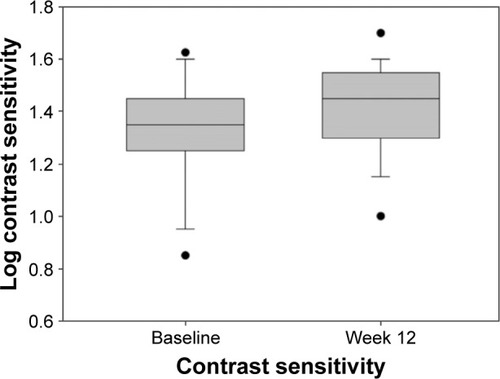
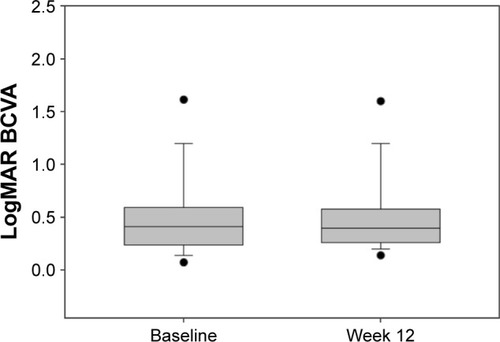
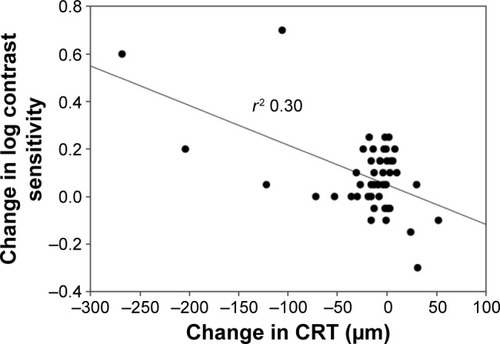
Forty-nine eyes (baseline visual acuity [VA] >6/120) were evaluated. Ranibizumab injections (mean ± standard deviation [SD] 28.2±22.1 [range 3–86]) were administered prior to treatment switch. Mean CS improved from 1.32 at baseline to 1.40 log units at week 12. VA was stable throughout. Mean CRT decreased from 354 µm at baseline to 332 µm at week 12 (−22 µm, P=0.004). Twenty-six (65%) patients experienced an overall improvement in NEI VFQ-25 score. Interestingly, a correlation was observed between improvement in log CS and CRT change (P=0.000046).
Conclusion
Contrast sensitivity improved statistically and significantly, and CRT decreased from baseline to week 12 after a switch to aflibercept from ranibizumab. Analysis of CS as an independent outcome end point in neovascular AMD treatment switch studies may provide a more complete understanding of visual response.
Introduction
Age-related macular degeneration (AMD) is a leading cause of severe vision loss in people aged 55 years and older in developed countries.Citation1,Citation2 The neovascular form of AMD, involving choroidal neovascularization (CNV) associated with fluid exudation or bleeding, accounts for the majority of cases of AMD-related severe central vision loss.Citation3 Upregulation of angiogenic factors, including vascular endothelial growth factor (VEGF), has been implicated in the development and progression of AMD.Citation4 The management of neovascular AMD has been transformed over the past decade with the development and advent of intravitreal anti-VEGF treatment, which is significantly more effective in preventing progressive vision loss from neovascular AMD than previous alternative treatment modalities, including laser photocoagulation and photodynamic therapy. Moreover, anti-VEGF therapy has been shown to significantly improve visual acuity (VA) in many patients with neovascular AMD or other retinal diseases.Citation5
Current management of neovascular AMD involves the use of continuing anti-VEGF treatment to control signs of exudation by blocking the growth of abnormal blood vessels to prevent vision loss and potentially improve VA. Intravitreal anti-VEGF agents ranibizumab (Lucentis®; Novartis Pharmaceuticals Canada Inc, Dorval, QC, Canada) and aflibercept (Eylea®; Bayer Inc, Mississauga, ON, Canada) are Health Canada-approved options for the treatment of neovascular AMD. Large multicenter clinical trials have demonstrated that monthly ranibizumab, a recombinant VEGF-specific monoclonal antibody fragment, prevents vision loss in most patients with neovascular AMD, with significant visual gain achieved in some.Citation6,Citation7 The phase 3 randomized VIEW clinical studies demonstrated that intravitreal aflibercept injection every 8 weeks, following 3 initial monthly injections, was noninferior to monthly ranibizumab, which was measured by the primary end point of proportion of subjects who maintained vision (loss of <15 letters compared to baseline) at week 52.Citation8
Intravitreal aflibercept injection is a recombinant fusion protein of key domains from human VEGF receptors 1 and 2 with the constant region (Fc) of human immunoglobulin G. Aflibercept binds to both VEGF-A and proangiogenic placental growth factor. The binding affinity of aflibercept to VEGF is substantially higher than that of ranibizumab,Citation9 with a greater potency in vitro and a longer duration of action allowing potentially for less frequent dosing.Citation8 Research findings in eyes with neovascular AMD demonstrate a mean duration of complete VEGF suppression of 36.4 days for ranibizumab compared with ~70 days for aflibercept injection.Citation10,Citation11 Switching treatment-resistant neovascular AMD patients to aflibercept is an option that may improve visual and anatomic outcomes.Citation12,Citation13
Visual function assessment in determining quality of vision
Assessment of the overall interaction with the external environment through the visual system is measured using various methods, including VA (100% contrast, Early Treatment Diabetic Retinopathy Study [ETDRS], and Snellen charts), contrast sensitivity (CS) testing (100%–0.6% contrast), Humphrey visual field test, and color vision (eg, Isihara charts, Farnsworth dichotomous test). The ability to perform everyday visual tasks, such as reading, recognizing faces, driving, telephone use, and using household appliances, depends on visual components beyond high-contrast visual distance acuity, including CS, near vision, color vision, and sensitivity to glare. The Snellen Eye Chart is primarily used to measure VA and is depicted as a series of high contrast black-on-white letters in different sizes. Relatively small changes in refractive status can be detected by this test, and it is a useful standard for defining vision changes caused by spherical blur. As various types of vision loss are not caused by spherical blur (eg, cataracts, glaucoma, and irregular astigmatism), the Snellen VA test may not be an adequate or appropriate measure in all cases.
CS measures an individual’s ability to detect low contrast images and to perceive differences between light and dark, whereas the Snellen VA chart test measures the level at which an individual can identify fine detail at high contrast using black-on-white letters. Using low contrast images, the CS method can detect subtle vision changes that may be concealed by acuity.Citation14 CS could be considered as an adjunct to standard acuity testing, to better assess visual capability, as well as being a useful measure for evaluating a wide range of ocular conditions (eg, diabetic eye disease, macular degeneration, cataracts, glaucoma, traumatic brain injury, amblyopia, and optic nerve disorders).Citation14 CS is a sensitive and arguably essential outcome end point for a complete assessment of visual function and visual performance, including driving, mobility, reading speed, and general vision-related quality of life.Citation15 Additionally, CS has potential applications when measuring the vision of patients with high visual requirements, including athletes and public service personnel. CS charts that utilize letters are familiar to both the patient and the practitioner, in comparison to other nonletter-based CS charts.Citation16 The Pelli–Robson CS test is considered a quick and reliable method in clinical settings.Citation17,Citation18 Patients scoring below 1.65 log on the Pelli–Robson CS test are considered to have impaired visual function, with a 6-letter change in CS equating to a 15-letter (or 3 lines) change in VA.Citation19
Both VA and CS are associated with the ability to perform daily vision-related activitiesCitation20 and are considered as significant independent parameters in the determination of visual impairment and quality of life. However, in clinical studies, change in VA over time is commonly used as the main primary outcome measure as an effective and reliable indicator of functional deficit or improvement. When measuring VA, single optotypes (usually letters, numbers, or geometric symbols) with high contrast are presented to the patient; however, the environment is not always seen in high contrast as presented on a standard acuity chart. Therefore, using CS determination to measure the ability to recognize low contrast patterns may detect functional impairment not evident when measuring VA only.Citation21
Study results have indicated that VA and CS do not reliably demonstrate the same parallel progression in visual function loss, although they show a moderate correlation in eyes with neovascular AMD.Citation21 Both parameters provide important and supplementary information about visual disability,Citation21 supporting the role of multiple visual end point evaluations in interventional studies.Citation21,Citation22 To better assess the overall visual functioning following anti-VEGF treatment switch, this study was conducted to determine and evaluate change from baseline to week 12 in Pelli–Robson CS as the primary clinical end point in patients with recalcitrant exudative AMD, despite previous ranibizumab treatment who were switched to aflibercept therapy. Other outcome measures recorded and assessed were mean change in best corrected visual acuity (BCVA), mean change in central retinal thickness (CRT), and vision-related quality of life (or visual functioning) assessed using the National Eye Institute 25-Item Visual Function Questionnaire (NEI VFQ-25) single composite score. The latter captures key dimensions of self-reported vision-targeted health status and visual functioning in patients with chronic eye disease.
Methods
This single-center study was an institutional review board (IRB)-approved, investigator-masked, prospective, interventional, noncontrolled, single-arm study that included 40 randomly selected neovascular AMD patients with persistent fluid on spectral domain optical coherence tomography (SD-OCT) following at least three intravitreal injections of ranibizumab in the previous 6 months. If both eyes of individual subjects met the study inclusion criteria, they were included in the study population dataset ().
Table 1 Inclusion and exclusion criteria
Subjects were switched to 2 mg aflibercept injection on a dosing protocol that matched the recommended treatment posology for neovascular AMD, involving treatment initiation with three doses administered once a month for the first 3 months (weeks 0, 4, and 8), followed by an injection every 2 months for the first 12 months of treatment. Subjects completed clinical examination at baseline and week 12. Assessments included the Pelli–Robson log CS measurement at 1 m, BCVA converted to logarithm of minimum angle of resolution (logMAR) using standard methods,Citation23 CRT using SD-OCT (Heidelberg Spectralis; Heidelberg Engineering Inc., Vista, CA, USA), and the NEI VFQ-25 questionnaire with composite score.
Statistical data analysis was performed once all patients had completed the week 12 visit, using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). A two-sided paired t-test was used to compare the means of continuous variables. If not otherwise stated, all values are presented as mean ± standard deviation (SD). A P-value <0.05 was considered statistically significant.
The study was conducted in accordance with the principles of the Declaration of Helsinki and in compliance with Good Clinical Practice and applicable regulatory requirements. The study procedure and informed consent document were approved by IRB services (Aurora, ON, Canada) prior to initiating the study. Written informed consent was obtained from all participants before study enrollment.
Results
Characteristics of study population
A total of 49 eyes of 40 patients (27 male and 13 female) with treatment-resistant neovascular AMD were evaluated at baseline and at the week 12 visit following treatment switch to aflibercept from ranibizumab. No patients were lost to follow-up, and there were no treatment discontinuations. There were no ocular or nonocular adverse events in the patient population during the duration of the study. The baseline age was 80.2±6.8 years (mean ± SD, range 68–93 years). The mean number of ranibizumab injections in the previous 6 months before switch to aflibercept was 5.1 (range 3–6). Ranibizumab injections (mean ± SD 28.2±22.1 [range 3–86]) were administered before initiation of aflibercept treatment. The duration of prior ranibizumab therapy was 36.8±24.7 months (mean ± SD, range 6–94), with a treatment start date from 2008 to 2015.
Measures of visual function and anatomic outcomes
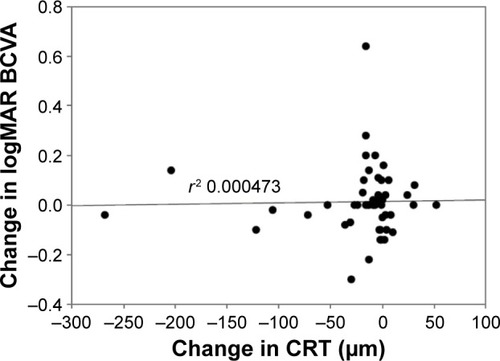
The key outcome results are shown in –. The mean CS increased from 1.32 log units at baseline to 1.40 log units at week 12 (P=0.00079). Mean VA was stable from baseline to week 12: mean logMAR VA was 0.51878 at baseline and 0.53204 at week 12 (median 0.4 and 0.42, respectively). At week 12, there was a statistically significant decrease of 22 µm in mean CRT from 354 µm at baseline to 332 µm at week 12 (P=0.004). There was no demonstrated relationship between change in CRT and VA measurements (P=0.88). There was however an associated relationship between improvement in CS and mean change in CRT (P=0.000046).
Figure 2 Change in log contrast sensitivity versus change in central retinal thickness at week 12.
Vision-related quality of life measured using NEI VFQ-25
Of 40 patients, 26 (65%) experienced an overall improvement in NEI VFQ-25 composite score from baseline to week 12; 8 of 40 (20%) patients experienced no change; and 6 of 40 (15%) experienced an overall decrease in composite score.
Discussion
The efficacy evaluation following treatment switch to aflibercept from ranibizumab incorporated both CS and VA measurement as independent outcome measures at 12 weeks following an initial treatment-loading phase of 3 consecutive monthly aflibercept injections. Overall, the study population received close to the planned 4-weekly aflibercept dosing after switch initiation. Adherence to strict inclusion criteria ensured that the study population included only suboptimal responders with persistent fluid, despite previous near monthly ranibizumab treatment.
It was shown that in this switch population, there was a statistically significant improvement in the primary outcome end point of change in CS from baseline to week 12, with an average CS change of two letters broadly comparable to a five-letter (one line on ETDRS chart) difference in VA in terms of vision function performance. It has been reported, for example, that a 6-letter difference in CS had a similar impact on self-reported difficulty with everyday vision-related tasks as a 15-letter (3 lines on ETDRS chart) VA difference in a population-based sample of individuals aged 65 years and older.Citation19 The evaluation of change in CS may be a clinically meaningful measure of treatment benefit in terms of quality of vision on anti-VEGF therapy. Published literature has shown that CS is an important measure of visual function in patients with subfoveal CNV due to AMD, and the potential benefits of treatment may not be completely characterized by VA measurement alone.Citation24 Obtaining information on the overall quality of vision that includes VA and CS as efficacy outcomes may provide physicians, optometrists, and general ophthalmologists with a better and more complete understanding of their patient’s visual status and help improve patient care.Citation20,Citation25 Pauleikhoff Citation26 noted in a review of natural history and treatment outcomes in neovascular AMD that reducing the risk of further VA and CS loss might allow neovascular AMD patients to maintain better vision-related functional abilities.
This study demonstrated that there is a general and immediate decreasing trend in mean CRT after treatment switch to aflibercept, while no statistically significant change from baseline VA was noted. In this neovascular AMD population of suboptimal responders to ranibizumab, persistence of fluid is associated with impairment in visual function as measured by CS. The fluid reduction seen following a switch to aflibercept is associated with an improved CS and a positive change in NEI VFQ-25 scores.
Numerous studies have evaluated the effectiveness of switching to aflibercept from another anti-VEGF agent in treatment-resistant neovascular AMD patients with persistent or recurrent fluid, despite prior multiple anti-VEGF injections. Wykoff et alCitation27 reported 6-month results from the TURF trial providing evidence that aflibercept treatment may be anatomically valuable in some recalcitrant exudative AMD eyes, while maintaining prior visual gains attained with ranibizumab treatment. A decrease in the proportion of CNV lesions that were graded as active at 12 months after switch from ranibizumab to aflibercept was noted by Barthelmes et al.Citation28 Chang et alCitation29 prospectively assessed the efficacy of aflibercept in a cohort of 40 treatment-resistant neovascular AMD patients; at 12 months after switch, mean BCVA improved from baseline by 4.7 letters (95% CI: 2.1–7.3, P<0.001) and CRT decreased by 97.2 µm (95% CI: 54.4–140.1, P<0.001). A meta-analysis noted a small but statistically significant improvement in BCVA 6 months following treatment switch to aflibercept in patients with treatment-resistant neovascular AMD on another anti-VEGF agent.Citation12
The NEI VFQ-25 questionnaire captures patient-reported visual function and was used to assess vision-related quality of life before and after treatment switch. The fact that two-thirds of patients experienced an overall improvement in NEI VFQ-25 total score at week 12 compared with baseline score is encouraging. However, the study sample is too small to allow evaluation of the statistical relationship between different subgroup responses, although this might usefully be addressed in a future study analysis. In the VIEW studies, involving treatment-naive neovascular AMD patients, total NEI VFQ-25 score was a prespecified secondary efficacy variable and improved scores were observed for both ranibizumab and aflibercept treatment groups, with the greatest improvements observed for mental health and general vision.Citation8,Citation30 Yuzawa et alCitation30 reported results evaluating change in composite NEI VFQ-25 score based on categorical change in VA (worsened, unchanged, and improved) over 52 weeks in the VIEW studies, comparing the approved dosing for each agent. Meaningful improvement in NEI VFQ-25 composite score was attained only in patients who gained five ETDRS letters or more from baseline VA to week 52.
When reviewing the results of our study, differences in participant selection, baseline features, and inclusion criteria limit generalizability and direct comparisons between reported anti-VEGF switch studies in neovascular AMD. The sample size of the switch cohort in this study was relatively small, many patients had received chronic long-term ranibizumab treatment, and follow-up of participants was limited at 12 weeks only. Comparative analyses involving a control arm of patients continuing on ranibizumab despite recurrent or persistent fluid might have strengthened the overall analysis. A crossover study design incorporating a reswitch arm from aflibercept back to ranibizumab may be a useful consideration in the development of future anti-VEGF treatment switch studies. It would also be of interest to conduct a larger study to evaluate long-term outcomes, including the potential for greater improvement in vision-related functional benefit with continuing treatment, after a treatment switch to aflibercept from ranibizumab.
Despite limitations, the study results reported herein demonstrate interesting findings with regard to disease progression that may be useful in the design of future switch studies in neovascular AMD. VA and CS should be considered as significant independent parameters for determining degree of visual impairment and treatment response, as they provide predictive information on visual disability and vision-related quality of life. Using the Pelli–Robson chart, CS determination is a standardized technique that is straightforward and reproducible; therefore, we suggest that CS be more widely considered as a valuable additional outcome end point in future clinical studies of neovascular AMD, diabetic macular edema (DME), and retinal vein occlusion.
Switching of anti-VEGF agents in neovascular AMD patients is a viable treatment strategy to improve outcomes among initial nonresponders or for patients exhibiting recalcitrant exudative disease activity on continued anti-VEGF treatment. The 2016 Preferences and Trends Membership Survey by the American Society of Retina Specialists found that more than 80% of respondents considered that persistent or recurrent fluid on OCT best determines inadequate response to an anti-VEGF treatment in neovascular AMD, and more than 75% considered it reasonable to switch due to inadequate response after three to six initial injections.
Conclusion
Other studies have shown that improvements in anatomic parameters and stable or moderately improved VA outcomes may be attained in treatment-resistant neovascular AMD patients switched to intravitreal aflibercept from another anti-VEGF agent. Results of this prospective, interventional investigation reveal a statistically significant improvement in CS and a statistically significant reduction in CRT over 12 weeks after treatment switch to aflibercept from ranibizumab in recalcitrant neovascular AMD patients, with no meaningful change from baseline VA observed. In our study series, a demonstrated correlation was observed between CRT decrease and improvement in Pelli–Robson log CS score at 12 weeks after treatment switch to aflibercept. Vision functioning, assessed using the NEI VFQ-25 score, improved from baseline to week 12 in two-thirds of the anti-VEGF switch population.
The observed positive correlation between improvement in CS and reduction in CRT supports the argument that measurement of CS may provide a more complete understanding of the benefits of anti-VEGF treatment on vision-related functional performance in patients with neovascular AMD. As emphasized earlier, measurement of CS could provide useful early information on visual impairment not identifiable on VA assessment alone, as well as provide another method for monitoring global treatment benefit and individual patient responses. Overall, CS measurement may provide a better understanding of visual performance challenges faced by individuals with vision impairment. An extended study duration involving a larger patient population would allow for further investigation and potential validation of results with regard to improvement in CS and other visual functioning parameters. Further analyses exploring multiple measures of visual function as efficacy end points in treatment studies of DME and other retinal diseases are warranted.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank all the patients who participated in the study, the study team, and Jaya Shumoogam of Inspired Science, London, UK, who provided medical writing and editorial support. The study was in part supported by Bayer Inc.
Disclosure
The authors report no conflicts of interest in this work.
References
- CongdonNO’ColmainBKlaverCCEye Diseases Prevalence Research GroupCauses and prevalence of visual impairment among adults in the United StatesArch Ophthalmol2004122447748515078664
- BunceCWormaldRLeading causes of certification for blindness and partial sight in England & WalesBMC Public Health200665816524463
- BresslerNMBresslerSBFineSLAge-related macular degenerationSurv Ophthalmol19883263754132457955
- FerraraNGerberHPLeCouterJThe biology of VEGF and its receptorsNat Med20039666967612778165
- ModiYSTanchonCEhlersJPComparative safety and tolerability of anti-VEGF therapy in age-related macular degenerationDrug Saf201538327929325700714
- RosenfeldPJBrownDMHeierJSMARINA Study GroupRanibizumab for neovascular age-related macular degenerationN Engl J Med2006355141419143117021318
- BrownDMKaiserPKMichelsMANCHOR Study GroupRanibizumab versus verteporfin for neovascular age-related macular degenerationN Engl J Med2006355141432144417021319
- HeierJSBrownDMChongVVIEW 1 and VIEW 2 Study GroupsIntravitreal aflibercept (VEGF trap-eye) in wet age-related macular degenerationOphthalmology2012119122537254823084240
- PapadopoulosNMartinJRuanQBinding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumabAngiogenesis201215217118522302382
- MuetherPSHermannMMDrögeKKirchhofBFauserSLong-term stability of vascular endothelial growth factor suppression time under ranibizumab treatment in age-related macular degenerationAm J Ophthalmol2013156598999323938122
- FauserSSchwabeckerVMuetherPSSuppression of intraocular vascular endothelial growth factor during aflibercept treatment of age-related macular degenerationAm J Ophthalmol2014158353253624879948
- Seguin-GreensteinSLightmanSTomkins-NetzerOA meta-analysis of studies evaluating visual and anatomical outcomes in patients with treatment resistant neovascular age-related macular degeneration following switching to treatment with afliberceptJ Ophthalmol20162016409585227042342
- BakallBFolkJCBoldtHCAflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumabAm J Ophthalmol201315611522.e123706500
- PelliDGRobsonJGWilkinsAJThe design of a new letter chart for measuring contrast sensitivityClin Vis Sci19882187199
- WoodJMOwensDAStandard measures of visual acuity do not predict drivers’ recognition performance under day or night conditionsOptom Vis Sci200582869870516127335
- ElliottDBSandersonKConkeyAThe reliability of the Pelli-Robson contrast sensitivity chartOphthalmic Physiol Opt199010121242330208
- MäntyjärviMLaitinenTNormal values for the Pelli-Robson contrast sensitivity testJ Cataract Refract Surg200127226126611226793
- ThayaparanKCrosslandMDRubinGSClinical assessment of two new contrast sensitivity chartsBr J Ophthalmol200791674975217166891
- RubinGSBandeen-RocheKHuangGHThe association of multiple visual impairments with self-reported visual disability: SEE projectInvest Ophthalmol Vis Sci2001421647211133849
- FletcherDCSchuchardRAVisual function in patients with choroidal neovascularization resulting from age-related macular degeneration: the importance of looking beyond visual acuityOptom Vis Sci200683317818916534460
- BellmannCUnnebrinkKRubinGSMillerDHolzFGVisual acuity and contrast sensitivity in patients with neovascular age-related macular degeneration. Results from the Radiation Therapy for Age-Related Macular Degeneration (RAD-) StudyGraefes Arch Clin Exp Ophthalmol20032411296897413680248
- CsakyKGRichmanEAFerrisFLReport from the NEI/FDA ophthalmic clinical trial design and endpoints symposiumInvest Ophthalmol Vis Sci200849247948918234989
- HolladayJTProper method for calculating average visual acuityJ Refract Surg19971343883919268940
- OwsleyCContrast sensitivityOphthalmol Clin North Am200316217117712809156
- MonésJRubinGSContrast sensitivity as an outcome measure in patients with subfoveal choroidal neovascularisation due to age-related macular degenerationEye (Lond)200519111142115015467700
- PauleikhoffDNeovascular age-related macular degeneration: natural history and treatment outcomesRetina20052581065108416340538
- WykoffCCBrownDMMaldonadoMECroftDEAflibercept treatment for patients with exudative age-related macular degeneration who were incomplete responders to multiple ranibizumab injections (TURF trial)Br J Ophthalmol201498795195524518078
- BarthelmesDCampainANguyenPFight Retinal Blindness! Project InvestigatorsEffects of switching from ranibizumab to afliber-cept in eyes with exudative age-related macular degenerationBr J Ophthalmol2016100121640164526994110
- ChangAABroadheadGKHongTIntravitreal aflibercept for treatment-resistant neovascular age-related macular degeneration: 12-month safety and efficacy outcomesOphthalmic Res2015552849026637166
- YuzawaMFujitaKWittrup-JensenKUImprovement in vision-related function with intravitreal aflibercept: data from phase 3 studies in wet age-related macular degenerationOphthalmology2015122357157825439429