Abstract
Over the last decade several novel surgical treatment options and devices for glaucoma have been developed. All these developments aim to cause as little trauma as possible to the eye, to safely, effectively, and sustainably reduce intraocular pressure (IOP), to produce reproducible results, and to be easy to adopt. The term “micro-invasive glaucoma surgery (MIGS)” was used for summarizing all these procedures. Currently MIGS is gaining more and more interest and popularity. The possible reduction of the number of glaucoma medications, the ab interno approach without damaging the conjunctival tissue, and the probably safer procedures compared to incisional surgical methods may explain the increased interest in MIGS. The use of glaucoma drainage implants for lowering IOP in difficult-to-treat patients has been established for a long time, however, a variety of new glaucoma micro-stents are being manufactured by using various materials and are available to increase aqueous outflow via different pathways. This review summarizes published results of randomized clinical studies and extensive case report series on these devices, including Schlemm’s canal stents (iStent®, iStent® inject, Hydrus), suprachoroidal stents (CyPass®, iStent® Supra), and subconjunctival stents (XEN). The article summarizes the findings of published material on efficacy and safety for each of these approaches.
Introduction
Glaucoma is one of the leading causes of blindness worldwide.Citation1 Several studies show that elevated intraocular pressure (IOP) is an important risk factor for glaucoma and the progression of the disease.Citation2,Citation3 It could also be demonstrated that lowering of elevated IOP can reduce the risk for progression.Citation4,Citation5 Today it is well accepted to initiate the treatment of patients with glaucoma and ocular hypertension with a medical therapy. If target pressure levels are not achieved and/or the disease is progressing despite using drug combinations, the next step on the therapeutic stepladder is to lower the IOP surgically.Citation6 Despite having a proven record of efficacy and safety, the use of glaucoma medications may cause systemic and local side effects such as ocular surface disease and ocular allergy.Citation7,Citation8 Approximately 50% of glaucoma patients require multiple medical treatments with different drugs.Citation9 Local and systemic side effects, the use of different medications, and the overall complexity of the treatment-scheme may negatively impact adherence and persistance.Citation10–Citation13 Over the last several years new devices for micro-invasive glaucoma surgery (MIGS) were developed and have gained more and more interest. According to Saheb and Ahmed, the term MIGS refers to a group of surgical procedures which share five preferable qualities: an ab interno approach through a clear corneal incision which spares the conjunctiva of incision, a minimally traumatic procedure to the target tissue, an IOP lowering efficacy that justifies the approach, a high safety profile avoiding serious complications compared to other glaucoma surgeries, and a rapid recovery with minimal impact on the patient’s quality of life.Citation14 However, there is currently no single common and widely accepted definition of MIGS. In a workshop of the American Glaucoma Society and the US Food and Drug Administration (FDA) held in February 2014, the term “minimally invasive glaucoma surgery” was characterized by the implantation of a surgical device intended to lower IOP via an outflow mechanism with either an ab interno or ab externo approach, associated with very little or no scleral dissection. In this approach, to define MIGS procedures involving a significant scleral dissection was not classified under the term MIGS.Citation15 All devices do not require a scleral incision and are placed ab interno by using a clear corneal incision. Thus they are frequently used in combination with phacoemulsification and intraocular lens implantation (PE/IOL). MIGS is intended to achieve lower IOP in patients with glaucoma with shorter surgical time, and ideally to achieve a medication sparing effect. All devices work by increasing the outflow of aqueous humor from the anterior chamber, either by directly accessing Schlemm’s canalCitation16–Citation18 or shunting aqueous humor to the suprachoridalCitation19 or subconjunctival space.Citation20 Complications such as hypotony, hyphema, infections of the bleb, revisions of the bleb, and endophthalmitis may occur in up to 35% of patients treated with conventional glaucoma surgery, ie, trabeculectomy (TE).Citation21 MIGS may avoid those complications and therefore provide a valuable treatment option in glaucoma patients, however, the incidence and types of adverse events and possible complications may differ between the different types of MIGS procedures. The technical characteristics of different micro-implants which are available and under development are summarized in .
Table 1 Implants used during micro-invasive glaucoma surgery procedures – overview and status
Procedures targeting the trabecular outflow
Currently, three devices (iStent, iStent inject [Glaukos Inc., Laguna Hills, CA, USA], and Hydrus [Ivantis Inc., Irvine, CA, USA]) target the juxtacanalicular part of the trabecular meshwork, which is believed to represent the greatest resistance to aqueous humor outflow in patients with open-angle glaucoma (OAG).Citation22,Citation23 These micro-invasive procedures allow for more direct access to aqueous humor from the anterior chamber into Schlemm’s canal. One limitation of all of these procedures is that the postoperative IOP cannot fall below the episcleral venous pressure (EVP), which is difficult to evaluate but is reported in different studies in a range of 7.6 to 9.1 mmHg.Citation24–Citation26 It was also demonstrated that EVP may be elevated in some glaucoma patients.Citation27
iStent® and iStent® inject
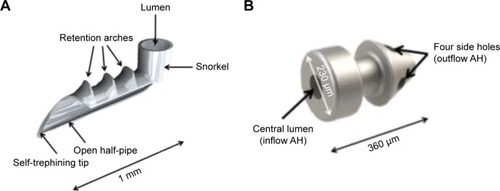
iStent is the first generation trabecular bypass device that is manufactured by Glaukos Inc. This device connects the anterior chamber with Schlemm’s canal. The iStent has CE-mark and was approved in 2012 by the FDA. In Europe, iStent is approved as a stand-alone device or for use in combined cataract/MIGS procedures. The product has a size of 1×0.3 mm, is made from heparin-coated, non-magnetic titanium, and is provided pre-loaded in an inserter ().
Surgical procedure with iStent
The iStent is delivered in an inserter which consists of a 26-gauge disposable instrument which contains the iStent on the tip. The stent is often implanted in a combined procedure with a cataract surgery. The device is implanted through the same temporal clear corneal incision used for PE/IOL. The leading edge of iStent is inserted through the trabecular meshwork into Schlemm’s canal at the nasal position where the tip of the stent is pointing inferiorly. By pushing a button on the inserter, the device is released. There is a right- and left-eye model which are distinguished by the direction of the foot (). Usually, postoperative anti-inflammatory and anti-infective topical medications are applied for approximately 4 weeks.
Figure 1 iStent® and iStent® inject.
Abbreviation: AH, aqueous humour.
Surgical procedure with iStent inject
iStent inject is a much smaller second generation model (). With a length of only 360 μm and a diameter of 230 μm, a single iStent inject stent is currently the smallest medical implant approved for use in the human body during surgical procedures. The iStent inject stents are delivered in an injector system which injects the stents automatically into Schlemm’s canal through a stainless steel insertion tube. The injector is released by the surgeon by pressing a button. The G2-M-IS injector system contains two stents, allowing the insertion of both stents from one injector during the same surgical procedure. Usually two iStent inject stents are implanted nasally into the trabecular meshwork and Schlemm’s canal with a distance of 30 to 60°. The operating microscope is tilted 35°, while the head of the patient is turned 35° counter-clockwise to ensure an optimal view into the chamber angle. The procedure includes a clear corneal incision using topical anesthesia. Usually topical anti-inflammatory and anti-infective medication is applied for 4 weeks postoperatively.
Efficacy of iStent
Efficacy and safety of iStent were evaluated in several clinical studies with different settings. The results of these studies are summarized in and and and .
Figure 2 Development of mean IOP after the implantation of iStent® during combined procedures (implantation with phacoemulsification and IOL implantation).
Abbreviations: IOP, intraocular pressure; IOL, intraocular lens; BM, medicated baseline IOP; BU, unmedicated baseline IOP; U, unmedicated; M, medicated.
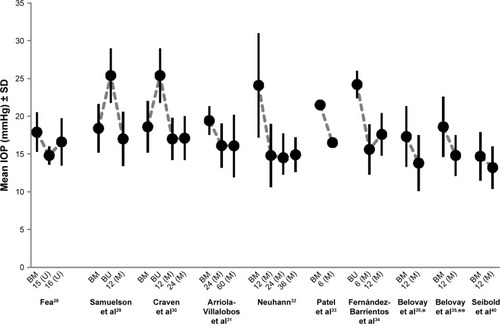
Figure 3 Development of mean IOP after the implantation of iStent® during stand alone procedures.
Abbreviations: IOP, intraocular pressure; BM, medicated baseline IOP; BU, unmedicated baseline IOP; U, unmedicated; M, medicated.
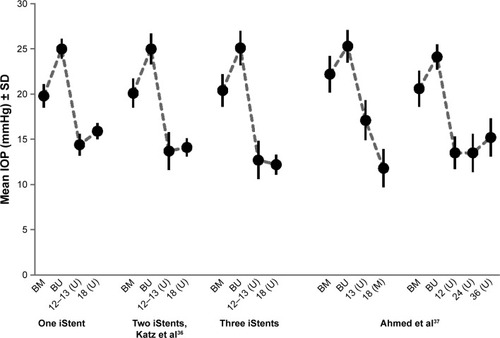
Table 2 iStent® and iStent® inject – overview of published study results
Table 3 iStent® and iStent® inject: proportion of patients achieving target IOP levels of ≤18 mmHg or ≤21 mmHg and/or an IOP reduction of ≥20% versus baseline IOP levels
Efficacy of iStent in combined procedures
In most of the studies iStent was implanted during a combined procedure with cataract surgery including PE/IOL. In a randomized prospective study conducted by Fea, efficacy and safety of iStent combined with cataract surgery were compared with a stand-alone cataract surgery (PE/IOL).Citation28 Mean medically treated baseline IOP (no wash-out phase) in the iStent study arm was 17.3±2.6 mmHg compared to 17.3±3.0 mmHg in the PE/IOL only study arm. At final visit at month 15, mean IOP in the iStent arm was 14.8±1.2 mmHg and 15.7±1.1 mmHg in the PE/IOL arm, respectively (P=0.031). At month 16 after the wash-out of any study medications, mean IOPs increased to 16.6±3.1 mmHg in the iStent arm and 19.2±3.5 mmHg in the PE/IOL arm (P=0.042). The mean number of medications used at baseline was 2.0±0.9 in the iStent arm and 1.9±0.7 in the PE/IOL arm compared to 0.4±0.7 in the iStent and 1.3±1.0 in the PE/IOL arm, respectively, at month 15 (P=0.007).
In another prospective controlled and randomized study with 233 eyes with mild to moderate primary open-angle glaucoma (POAG), exfoliative and pigmentary glaucoma, and IOP values of ≤24 mmHg treated with one to three medications at baseline, were randomized to receive either a stand-alone PE/IOL procedure (n=111) or a combined PE/IOL procedure (n=122) with the implantation of an iStent.Citation29 Mean baseline IOPs were 18.7±3.3 mmHg (on medication) and 25.2±3.5 mmHg (after wash-out) in the iStent study arm, and 18.0±3.0 mmHg (on medication) and 25.5±3.7 mmHg (after wash-out) in the PE/IOL study arm. Reduction of mean IOP at month 12 in comparison to mean baseline IOP after wash-out was similar in both arms, with a decrease of mean IOP of 8.4±3.6 mmHg in the iStent arm and 8.5±4.3 mmHg in the PE/IOL arm, respectively. A significant difference at month 12 was seen in the number of medications used. Fifteen percent of the patients in the iStent arm received medications versus 35% in the PE/IOL arm (P=0.001). Furthermore, 72% of patients in the iStent arm achieved IOP values of ≤21 mmHg and an IOP reduction versus baseline of ≥20% compared to 50% of patients in the PE/IOL arm (P<0.001). The safety profile of both arms was comparable.
In a prospective, randomized, controlled registration trial published by Craven et al efficacy and safety results of iStent were reported for a study period of 24 months.Citation30 Two hundred and forty patients with mild to moderate glaucoma (POAG, exfoliative glaucoma [PEX], and pigmentary glaucoma) were randomized to PE/IOL surgery alone (n=123) or to the combined procedure with iStent (n=117). Mean baseline IOPs were 18.6±3.6 (treated) and 25.4±3.6 mmHg (after wash-out) in the iStent arm, and 17.9±3.0 (treated) and 25.4±3.6 (after wash-out) in the PE/IOL arm, respectively. After 24 months mean IOPs decreased to 17.1±2.9 in the iStent arm and 17.8±3.3 mmHg in the PE/IOL arm. Interestingly, 71 patients (61%) in the iStent arm achieved IOP values, without medication, of ≤21 mmHg versus only 61 patients (53%) in the PE/IOL arm (P=0.0036).
In a non-randomized interventional case-series published by Arriola-Villalobos et al data from 19 glaucoma patients who received one iStent during a combined cataract procedure were reported for a follow-up period of 5 years (mean: 53.7±9.3 months).Citation31 Mean IOP (without wash-out of medications) decreased significantly from 19.3±1.9 mmHg at baseline to 16.3±4.2 mmHg at final visit (−16.3%; P=0.002). The mean number of pressure-lowering medications decreased from 1.3±0.5 at baseline to 0.8±0.9 at final visit (P=0.046).
In a prospective, non-randomized consecutive case series of 62 eyes with POAG, PEX, secondary glaucoma, post-traumatic glaucoma, and ocular hypertension which received iStent during a combined procedure with PE/IOL, Neuhann reported a decrease of mean IOP from 24.1±6.9 mmHg on a mean of 1.8±0.9 medications at baseline to 14.9±2.3 mmHg at month 36. Medications were completely stopped in 74% of eyes at month 36.Citation32
In a prospective case series conducted in the UK, iStent was implanted in 44 eyes with OAG either during a combined PE/IOL (n=40) or a stand-alone procedure (n=4).Citation33 Mean IOP at baseline was 21.5 mmHg and the mean number of medications used 2.3. At month 6 both, mean IOP and mean number of medications used, decreased significantly to 16.5 mmHg (P<0.001) and 0.5 (P<0.001), respectively.
The effects of an implantation of multiple (two or three) iStents were evaluated in studies published by Fernández-Barrientos et al, Belovay et al, Katz et al, Ahmed et al and Donnenfeld et al.Citation34–Citation38 This approach was further followed during the development of iStent inject which is delivered with two micro-stents in one injector.
In the study of Fernández-Barrientos et al the changes of aqueous humor dynamics were evaluated in patients with glaucoma or ocular hypertension undergoing a PE/IOL surgery.Citation34 Patients of one study arm received two iStents together with a PE/IOL procedure; patients of the second study arm received a PE/IOL procedure only. In addition, IOP and the number of medications taken before and 12 months after the surgery were analyzed. Mean baseline IOPs were 24.2±1.8 mmHg in the two-stent arm and 23.6±1.5 mmHg in the PE/IOL only arm. At final visit, mean IOP decreased by 6.6 mmHg in the two-stent arm to 17.6±2.8 mmHg, and by 3.8 mmHg to 19.8±2.3 mmHg (P=0.04) in the PE/IOL arm, respectively. Aqueous outflow facility increased by 275% in the two-stent arm and by 46% in the PE/IOL arm at month 12 compared to baseline (P<0.02). At baseline, mean number of medications used was comparable in both arms with 1.1±0.5 in the two-stent arm and 1.2±0.7 in the PE/IOL arm. No patient in the two-stent arm needed any medication at month 12, compared to a mean number of medications of 0.7±1.8 in the PE/IOL arm (P=0.007).
In a study published by Belovay et al two or three iStents were implanted in each of 53 individual eyes during a PE/IOL surgery.Citation35 Twenty-eight eyes received two iStents, 25 eyes received three iStents. Mean baseline IOPs were 17.3±4.0 mmHg and 18.6±4.0 mmHg in the two- and three-stent treatment arms, respectively. At month 12, mean IOP decreased to 13.8 mmHg and 14.8 mmHg in the two- and three-stent arm, respectively. The difference was found not to be statistically significant. A significant difference was found in the mean number of medications used at final visit, which was reduced from 2.8 and 2.6 at baseline to 1.0 and 0.4 at final visit for the two- and three-iStent arms, respectively (P<0.001). Overall, a 20% reduction of IOP with a reduction of medication of 64% was achieved in the two-iStent arm and a 20% reduction of IOP with a reduction of medications of 85% was achieved in the three-iStent arm.
In a retrospective consecutive case series conducted by Ferguson et al efficacy and safety of the iStent were evaluated in 350 eyes. The implantation of the iStent was performed during a combined procedure with a cataract extraction and phacoemulsification in patients with OAG and cataract.Citation39 Mean medicated preoperative IOP was 19.13±6.34 mmHg. At 2 years after the procedure, mean IOP was significantly lower (15.17±3.53 mmHg; P<0.0001) and the mean number of ocular hypotensive medications decreased from 1.19±1.0 at baseline to 0.61±0.96 at month 24 (P<0.0001). An IOP level of ≤18 mmHg at month 24 was achieved by 52% of the patients without medication.
Seibold et al evaluated the treatment outcomes of iStent (IOP, use of medication) in a retrospective case series of 46 eyes of 45 patients after combined phacoemulsification and the implantation of the trabecular micro-bypass (iStent) in patients with controlled glaucoma.Citation40 Treatment success (defined as a 20% or more reduction in IOP or the discontinuation of at least one medication) was achieved in 76.1% of the patients at month 12 and 41% of patients were medication-free at month 12. Overall mean IOP decreased from 14.7±3.2 mmHg preoperatively to 13.2±2.8 mmHg at month 12 (P=0.01).
Efficacy of iStent in stand-alone procedures
Efficacy and safety of iStent implanted in stand-alone procedures without cataract surgery were evaluated in three clinical studies.
Katz et al evaluated the effect of the implantation of either one, two or three iStents during stand-alone procedures without PE/IOL in 119 patients with OAG.Citation36 Thirty and eight subjects received one, 41 subjects two, and 40 subjects three iStents, respectively. At month 12 an unmedicated IOP of ≤18 mmHg and an IOP reduction of ≥20% without ocular hypotensive medication were achieved by 89.2%, 90.2%, and 92.1% of eyes in the one-, two-, and three-stent subgroups, respectively. At month 18, a medication was required by seven subjects (18.4%) in the one-stent subgroup, four subjects (9.8%) in the two-stent subgroup, and three subjects (7.5%) in the three-stent subgroup, respectively. At month 18, mean unmedicated IOPs were 15.9±0.9 mmHg, 14.1±1.0 mmHg, and 12.2±1.1 mmHg for the one-, two-, and three-stent subgroups, respectively.
The IOP lowering effect of the implantation of two trabecular micro-bypass stents (iStent) and postoperative medical treatment with a prostaglandin-analog (travoprost) in patients with OAG, which was preoperatively uncontrolled with two ocular hypotensive medications, was evaluated in a non-randomized, prospective open-label study by Ahmed et alCitation39 Overall, 39 phakic patients with medicated IOP between 18 mmHg and 30 mmHg and baseline IOP levels between 22 mmHg and 38 mmHg received two iStents and postoperative medical treatment with travoprost. Follow-up visits were scheduled over 18 months and a wash-out of medications was performed postoperatively at month 13. All patients achieved an IOP reduction of ≥20% and target IOP levels of ≤18 mmHg at month 12. At month 18, mean medicated IOP decreased from 22.2±2.0 mmHg at baseline on two medications to 13.0±2.4 mmHg on one medication. The mean unmedicated IOP decreased from 25.3±1.8 mmHg preoperatively to 17.1±2.2 mmHg at month 13.
Donnenfeld et al examined efficacy and safety of the implantation of two iStents in phakic or pseudophakic patients with OAG and IOP levels between 18 mmHg and 30 mmHg on one preoperative ocular hypotensive medication over a period of 36 months.Citation40 The primary efficacy endpoint, which was defined as a reduction of IOP of ≥20% from unmedicated baseline IOP at month 12, was achieved in 36 of 39 eyes (92.3%). At month 36, mean IOP decreased significantly by 9.1±2.7 mmHg (P<0.001) or 37% versus unmedicated baseline IOP, and by 5.5±2.7 mmHg (P<0.001) or 26% versus medicated baseline IOP.
Efficacy of iStent inject
iStent inject is a micro-stent system which uses the smallest of all micro-stents available (). Two of these micro-devices are delivered in one injector, and it offers the convenient option to implant both micro-stents during the same procedure into the trabecular meshwork. In all published clinical studies, iStent inject was used during stand-alone procedures in phakic or pseudophakic patients. The results of these studies are summarized in and .
Figure 4 Development of mean IOP after the implantation of iStent inject® during stand alone procedures.
Abbreviations: IOP, intraocular pressure; POAG, primary open-angle glaucoma; PEX, exfoliative glaucoma; BM, medicated baseline IOP; BU, unmedicated baseline IOP; U, unmedicated; M, medicated.
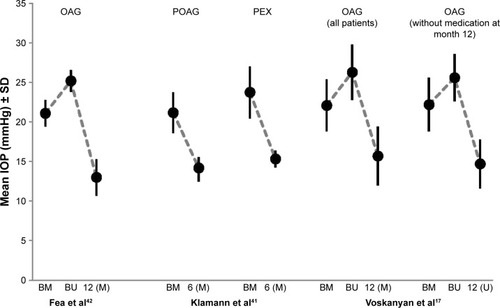
In a study published by Voskanyan et al two iStent inject were implanted in each of 99 individual eyes.Citation17 Mean baseline IOPs were 22.1±1.3 mmHg (with medication) and 26.3±3.5 mmHg (after wash-out). At month 12, IOP decreased by 40.2% versus baseline to 15.7±3.7 mmHg. Of the patients, 24.4% required a medical treatment. IOP ≤18 mmHg with and without medication was achieved by 81% and 66% of the patients, respectively. An IOP decrease of ≥20% and ≥30% without medication versus baseline was achieved by 72% and 61% of the patients, respectively. At month 12, 86.9% of subjects had reduced their number of medications: in 15.2% and 71.7% of subjects the number of medications was reduced by one, and two or more medications, respectively.
Another study on the implantation of two iStent inject in each eye during stand-alone procedures without PE/IOL was reported by Klamann et al.Citation41 In this study, 17 patients with POAG, 15 patients with PEX, and three patients with pigmentary glaucoma were included. In the subgroup of patients with POAG, mean IOP decreased significantly from 21.2±2.6 mmHg to 14.2±1.3 mmHg after 6 months and the mean number of medications used decreased from 2.2±0.9 at baseline to 0.9±0.6 at month 6. In the patient subgroup with PEX, mean IOP was significantly reduced from 23.8±3.2 mmHg at baseline to 15.3±1.1 mmHg at month 6, and the mean number of medications was reduced from 2.3±1.2 at baseline to 1.0±0.3 at month 6. In patients with POAG and PEX, mean number of medications was reduced by −1.3 and −1.29 at month 6.
Finally, iStent inject was evaluated in comparison with medical therapy using a fixed combination of a prostaglandin and timolol, by Fea et alCitation42 Ninety and four and 98 eyes were treated with the implantation of two iStent inject or a fixed combination of latanoprost and timolol or travoprost and timolol, respectively. Mean baseline IOPs in the iStent inject and medication study arms were 26.3±2.5 mmHg and 24.8±2.7 mmHg, respectively. Decrease of IOP was comparable in both treatment arms. At month 12, mean IOP decreased to 13.0±2.3 mmHg in the iStent arm and to 13.2±2.0 mmHg in the medication arm. Four patients in the iStent arm were taking medications at month 12.
Safety profile and adverse events of iStent and iStent inject
Blood reflux from Schlemm’s canal into the anterior chamber is a common process that occurs intraoperatively. This reflux may be seen as a positive and normal sign which occurs when iStent and iStent inject are well positioned in the trabecular meshwork. The most common adverse events in all studies were minor, and include temporary obstructions of the iStent, which were resolved in most cases by Neodym-YAG laser treatment, and malpositioned micro-stents. No postoperative hypotony, loss of endothelial cells, and signs of inflammation were reported in any of the studies. As for all trabecular procedures, caution should be applied in patients with elevated EVP, ie, in patients with lower baseline IOPs and in patients with obesity or metabolic syndrome.
Hydrus® micro-stent
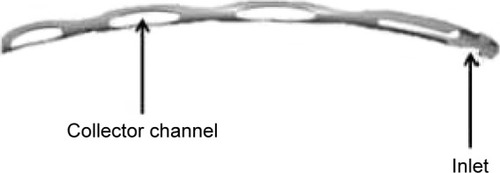
The Hydrus micro-stent is a so-called “intracanalicular scaffold”; an 8 mm long slightly shaped device () which is implanted inside Schlemm’s canal. The micro-stent is implanted ab interno with a pre-loaded injector through a clear corneal incision into Schlemm’s canal. After the implantation, the Hydrus micro-stent dilates Schlemm’s canal in the complete nasal quadrant, allowing aqueous humor to bypass the trabecular meshwork through multiple collector channels ().
Surgical procedure with the Hydrus micro-stent
The Hydrus micro-stent is implanted in the nasal hemisphere or the inferior temporal quadrant through a 1–1.5 mm clear corneal incision. The beveled tip of the injector is used to perforate the trabecular meshwork, and to position the micro-stent in Schlemm’s canal by rotating the advancement mechanism. Usually acetylcholine is recommended to establish a miosis, and a viscoelastic substance is used to stabilize the anterior chamber. At the end of the procedure the viscoelastic substance is removed and the anterior chamber inflated with balanced salt solution. A topical antibiotic and a topical corticosteroid are recommended during the first postoperative period.
Pfeiffer et al reported results of a controlled, prospective, randomized, single-masked 2-year clinical study comparing the efficacy and safety of a combined procedure with the Hydrus micro-stent and cataract surgery using PE/IOL and cataract surgery alone.Citation18
Efficacy of Hydrus
In this study, prior glaucoma medications were discontinued and washed-out before baseline IOP measurements were conducted. Mean baseline IOPs were 26.3±4.4 mmHg in the Hydrus/PE/IOL study arm and 26.6±4.2 mmHg in the PE/IOL study arm. At the 24-month follow-up visit, mean IOPs were 16.9±3.3 mmHg in the Hydrus/PE/IOL study arm and 19.2±4.7 mmHg in the PE/IOL study arm. At 24 months, a 20% reduction from baseline IOP was achieved by 80% of patients treated with Hydrus/PE/IOL as compared to 46% of patients treated with PE/IOL only. The percentage of unmedicated patients at month 24 was significantly higher in the Hydrus/PE/IOL study arm (72.9%) compared to the PE/IOL study arm (37.8%).
Safety profile and adverse events of Hydrus
Forty-seven out of 50 patients in the Hydrus/PE/IOL study arm and 43 patients in the PE/IOL study arm were evaluable at month 24. Significantly more patients in the Hydrus/PE/IOL study arm (12%) developed focal peripheral anterior synechiae, typically present as focal iris tissue adhesion to the device or to the chamber angle and located at or near the inlet of the Hydrus micro-stent. Interestingly the presence of these synechiae seemed not to have any influence on the study outcome. Other adverse events were similarly distributed among the two study arms.
Procedures targeting the suprachoroidal space
CyPass® micro-stent
The CyPass micro-stent (Alcon Inc., Fort Worth, TX, USA) creates an outflow pathway from the anterior chamber to the suprachoroidal space (). The device, with a length of 6.35 mm, is placed through a clear corneal incision after being inserted on a small guidewire with a special tip that separates the iris from the scleral spur (). Openings along the whole length of the micro-stent allow aqueous humor outflow into the suprachoroidal space.
Surgical procedure with the CyPass micro-stent
CyPass is implanted through a 1.5 mm clear corneal incision. Usually acetylcholine is injected into the anterior chamber to achieve a strong miosis. Then, a viscoelastic substance is injected in order to provide a stable anterior chamber. The procedure is controlled using a gonioscopy lens. The micro-device is inserted on a guidewire between the ciliary body and sclera until the suprachoroidal space is reached and the retention features are then released. The viscoelastic substance is removed via irritation and aspiration.
Three studies have been published on efficacy and safety of CyPass in patients with mild to moderate OAG.Citation19,Citation43,Citation44 In a study published by Hoeh et al CyPass was implanted in a combined procedure with cataract extraction and the implantation of an IOL.Citation19 In another study published by García-Feijoo et al CyPass was implanted in patients with phakic and pseudophakic eyes.Citation43 In both studies the procedure was performed without a wash-out period of the medication used. In a third study published most recently by Vold et al the supraciliary micro-stent (CyPass) was implanted in a combined procedure with a cataract extraction and phacoemulsification.Citation44
Efficacy of CyPass
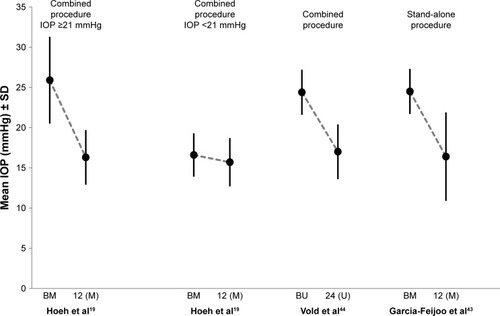
The first study involved 167 eyes of 142 patients. Mean baseline IOP of the patient cohort was 20.2±6.0 mmHg.Citation19 The eyes were analyzed in two subgroups – one with medicated baseline IOP levels of ≥21 mmHg and another with baseline IOP levels of <21 mmHg. Overall, mean IOP decreased from 20.2±6.0 mmHg to 15.9±3.1 mmHg at 12 months. Mean IOP decreased in the subgroup of patients with higher baseline IOP levels from 25.9±5.4 mmHg to 16.3±3.4 mmHg, and in the subgroup of patients with lower IOP baseline levels from 16.6±2.7 mmHg to 15.7±3.0 mmHg (). The number of medications used dropped from 2.1 at baseline to 1.1 at month 12 in the patient subgroup with higher baseline IOPs and from 2.0 at baseline to approximately 0.4 at month 12 in patients with lower IOPs. The second study involved 65 eyes from which 55 eyes were analyzed at the 12-month visit.Citation43 This study was performed as a stand-alone procedure in patients with phakic or pseudophakic eyes. After 12 months, mean IOP was decreased from 24.5±2.8 mmHg to 16.4±5.5 mmHg () while the number of medications was reduced from 2.2 on average at baseline to 1.4 at month 12. The third study was a multicenter, interventional randomized trial.Citation44 In this study, subjects with OAG and cataract with mean diurnal IOP between 21 mmHg and 33 mmHg were randomized to phacoemulsification only (n=131) or supraciliary stenting phacoemulsification and the implantation of a CyPass stent to the supraciliary space (n=374). Mean preoperative IOP in the phacoemulsification and the micro-stent arm were 24.5±3.0 mmHg and 24.4±2.8 mmHg, respectively. The mean numbers of medications in the two arms at baseline were comparable with 1.3±1.0 and 1.4±0.9 medications in the phacoemulsification and the micro-stent arm, respectively. At month 24, IOP was reduced by 7.4 mmHg in the micro-stent arm versus 5.4 mmHg in the phacoemulsification arm. Of the patients in the micro-stent arm, 85% did not require medications at month 24 and the mean number of medications was reduced by 67% (0.2±0.6 medications). Seventy-seven percent of subjects in the micro-stent arm and 60% of subjects in the phacoemulsification arm achieved an unmedicated IOP decrease versus baseline of ≥20%.
Figure 7 Mean IOP ± SD at baseline and 12 months after the implantation of CyPass.
Abbreviations: IOP, intraocular pressure; BM, medicated baseline IOP; BU, unmedicated baseline IOP; M, medicated; U, unmedicated.
Safety profile and adverse events of CyPass
In the study by Hoeh et al the two most frequent complications were early hypotony in 13.8% of patients, which resolved within 1 month and an IOP increase of ≥10.0 mmHg (3.0%).Citation19 An obstruction of the implant was seen in 5.4% of the patients. García-Feijoo et al reported 11% of cases with IOP levels of >30 mmHg which persisted more than 1 month after the procedure. Cataract progression (12.2%) and transient hyphema (6.2%) were other adverse events reported in this study.Citation43 In the study of Vold et al 37% of subjects in the micro-stent arm and 36% of the phacoemulsification arm experienced ocular adverse events through month 24. Most frequently reported adverse events were transient (≤30-day duration) best-corrected visual acuity loss of ≥2 lines lasting up to 24 months (8.8% micro-stent arm, 15.3% phacoemulsification arm), iritis (8.6% micro-stent arm, 3.8% phacoemulsification arm), and corneal edema (3.5% micro-stent arm, 1.5% phacoemulsification arm). Of micro-stent-related adverse events, eight stent obstructions (2.1%), two instances of malpositioning, and two instances of migration/dislocation were observed.
MIGS procedure opening a subconjunctival filtration pathway
Subconjunctival filtration generates a new, non-physiological pathway for the outflow of aqueous humor. This route for outflow increase is already the basis for conventional trabeculectomy and for glaucoma epibulbar shunt surgeries.
XEN gel stent
The XEN gel stent (Allergan plc, Dublin, Ireland) is made of porcine collagen cross-linked with glutaraldehyde. The stent is 6 mm in length and has a lumen diameter of 45 μm. The stent is stiff when dehydrated but becomes soft and flexible when it comes into contact with the aqueous humor. Originally three different lumen diameters of the stent were investigated (45 μm, 63 μm, and 140 μm), however, only the 45 μm type is currently being further examined in ongoing clinical trials (). The outflow volume follows Poiseuille’s law of laminar flow, where the diameter and length of the tube define the amount of outflow. The procedure is limited to eyes without conjunctival scarring.
Surgical procedure with the XEN gel stent
The implantation procedure can be done as stand-alone, or in combination with cataract surgery. The XEN gel stent is placed through a small, self-sealing, clear corneal incision using an inserter. The device is implanted into the subconjunctival space opposite the incision, thus, the procedure does not disrupt the conjunctival and subconjunctival tissue. A fistula is created, resulting in a bleb. Because this bleb is a significant risk factor for scar formation, the use of an antimetabolite such as mitomycin C (MMC) is recommended. Thus, MMC 10 μg is usually injected under the conjunctiva approximately 20 minutes before the procedure.
Only one clinical study with the 45 μm XEN gel stent has been published recently. Effectiveness and safety of phacoemulsification combined with the XEN implantation surgery was evaluated in patients with cataract and OAG in a prospective, 12-month follow-up study involving 30 eyes with at least two medications to control IOP. The combined procedure was performed through two temporal incisions after administering subconjunctival mitomycin.Citation45
Efficacy of XEN gel stent
The mean preoperative IOP was 21.2±3.4 mmHg, with an average of 3.1 drugs. At month 12, IOP decreased by 29.3% to 15.0 mmHg, which is equivalent to a 6.2 mmHg IOP decrease. Unfortunately there was no parallel group in which the effect of phacoemulsification and cataract surgery could be evaluated.
Safety profile and adverse events of XEN gel stent
Overall, adverse events occurred in three eyes. Two eyes did not complete the procedure (280° subconjunctival hemorrhage and XEN extrusion during repositioning). The third case had an encapsulation of the bleb 5 months after the surgery. Currently there is very limited further information about the safety profile of XEN gel stent. However, the implantation of the XEN gel stent can be regarded as a modified TE which results in a bleb, which requires the use of MMC, and thus it shares at least some of the risks of TE. Further long-term studies are needed to provide a full picture on the safety profile of XEN gel stent.
Discussion
Glaucoma therapy includes several different methods to lower IOP, such as medications, laser, and surgery. In many cases, patients need more than one of those treatments to achieve target-pressures low enough to avoid progression. Current management of glaucoma also comprises a variety of different issues. Major issues of medical therapy include treatment adherence and persistence issues,Citation11,Citation46 especially in patients treated concomitantly with different glaucoma medications, the toxicity of preservatives in eye-drops, such as benzalkonium chloride to corneal and conjunctival tissues,Citation47 especially when applied chronically, and a poor tolerance of the applied glaucoma medications.Citation8 MIGS may provide a solution for many of these issues. The different devices lower IOP between 3.2 and 12.6 mmHg. The reduction of IOP seems to be larger in patients with higher baseline IOP levels.Citation19,Citation37 Furthermore, the magnitude of the IOP lowering effect seems to depend on the number of shunts or stents which are implanted.Citation35,Citation36 However, further studies have to confirm these observations. Several limitations to the current findings are discussed in the various publications. These include limited data quality, especially in some studies with retrospective nature of the data, lack of study standardization, concomitant application of different therapies (ie, PE/IOL and micro-stents), limited knowledge on the duration of the IOP lowering effect for some micro-devices, and missing information about the ideal patient for the different MIGS procedures. Concomitant application of different therapies in clinical studies with glaucoma devices frequently makes it difficult to do a proper evaluation and comparison of the results obtained. Direct comparisons of the evidence of the different approaches of MIGS are difficult or even impossible due to the diverse study designs, patient populations, and outcome measures. Thus a standardization of those studies is urgently needed. Key among the recommendations of the American National Standards Institute for those studies, is to apply a medication wash-out both at enrollment and at the follow-up visits, to mask the study for the tonometer reading and to provide a follow-up of at least 2 years.Citation48 Many trials have included cataract surgery, therefore it is important when assessing the available MIGS data to consider the effect of PE/IOL itself on the decrease of IOP and on the number of IOP lowering medications needed after a combined PE/IOL/MIGS procedure.Citation49–Citation51 In the Ocular Hypertension Treatment Study, an initial IOP reduction was observed within the first 12 months. Despite the fact that this IOP lowering effect diminished over time, it remained present for up to 3 years.Citation52 Therefore, clinicians cannot assume that IOP lowering abilities will be similar when micro-shunts are used during stand-alone procedures. In December 2015 the FDA issued guidance which specifies standards which should be met by future MIGS studies.Citation53 These standards include a clear selection of patients with mild to moderate glaucoma by applying specific visual field and optic nerve characteristics, a follow-up period of at least 12 months, selection of glaucoma patients which allows a wash-out period at baseline and follow-up visits, diurnal IOP measurements, and a primary effectiveness endpoint of a ≥20% IOP reduction versus baseline. If future MIGS studies follow these proposed, more standardized study designs, a better understanding of the IOP reducing effects of each MIGS procedure will be possible.
When considering choosing the right patient profile for the different MIGS procedures, a few things besides the IOP lowering effect need be considered: firstly, the mode of action may be one criterion. iStent, iStent inject, and Hydrus work by improving aqueous humor outflow at the structure of the physiological outflow into Schlemm’s canal, while the other options are generating new and thus probably less physiological outflow pathways into the suprachoroi dal space (CyPass and iStent Supra [Glaukos Inc.]) or the subconjunctival space (XEN). Secondly, the safety profile of the different approaches needs to be considered, especially the risk for generating hypotony. Furthermore, the implantation of XEN gel stent can be regarded as a modified ab interno TE with the formation of a bleb and the need for MMC. A result of these considerations may be to use the Schlemm’s canal micro-stents in patients with mild to moderate glaucoma, and the suprachoroidal and subconjunctival devices for the more severe cases of glaucoma ().
Table 4 Implants used during micro-invasive glaucoma surgery procedures – summary of mode of action, possible risks, and potential use
Schlemm’s canal is a special feature. Within the scope of the concept of a segmental flow, the trabecular bypass operations may not achieve very low IOP levels as compared to TE. This is probably due to the fact that in patients with POAG, the number of opened collector channels remains the same with increased IOP and therefore may be the cause of an increased outflow resistance.Citation54,Citation55 Suprachoroidal devices may also be considered if trabecular stents fail or if the target pressure cannot be achieved by these stents. Further standardized studies are needed to gain more specific information on which patients will benefit most from each of these micro-devices. These data will help to individualize the management of glaucoma patients and to choose the best option MIGS for the individual glaucoma patient. Further information is also needed to choose the best medical approach with an appropriate primary mode of action (decrease aqueous humor production, increase of trabecular or uveoscleral outflow) in cases where adjunctive medication is needed.
Acknowledgments
Medical writing of this review was financially supported by Glaukos Inc., San Clemente, USA.
Disclosure
Medical writing of this review was done by eyecons (F Kimmich) with financial support from Glaukos Inc. San Clemente, USA. C Erb and A Jünemann are speakers for Glaukos Inc. L Pillunat has no conflicts of interest in this work.
References
- QuigleyHABromanATThe number of people with glaucoma world-wide in 2010 and 2020Br J Ophthalmol200690326226716488940
- ChauhanBCMikelbergFSBalasziAGLeBlancRPLeskMRTropeGECanadian Glaucoma Study GroupCanadian Glaucoma Study: 2. risk factors for the progression of open-angle glaucomaArch Ophthalmol200812681030103618695095
- ActisAGVersinoEBrogliattiBRolleTRisk factors for primary open angle glaucoma (POAG) progression: a study ruled in TorinoOpen Ophthalmol J20161012913927347249
- No authors listedThe Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS InvestigatorsAm J Ophthalmol2000130442944011024415
- HeijlALeskeMCBengtssonBHymanLBengtssonBHusseinMEarly Manifest Glaucoma Trial GroupReduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma TrialArch Ophthalmol2002120101268127912365904
- European Glaucoma SocietyTerminology and Guidelines for Glaucoma4th edEditrice Publicomm s.r.l2014
- ErbCGastUSchremmerDGerman register for glaucoma patients with dry eye. I. Basic outcome with respect to dry eyeGraefes Arch Clin Exp Ophthalmol2008246111593160118648841
- JaenenNBaudouinCPouliquenPManniGFigueiredoAZeyenTOcular symptoms and signs with preserved and preservative-free glaucoma medicationsEur J Ophthalmol200717334134917534814
- KassMAHeuerDKHigginbothamEJThe Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open- angle glaucomaArch Ophthalmol2002120670171312049574
- TsaiJCMcClureCARamosSESchlundtDGPichertJWCompliance barriers in glaucoma: a systematic classificationJ Glaucoma200312539339814520147
- TsaiJCA comprehensive perspective on patient adherence to topical glaucoma therapyOphthalmology200911611 SupplS30S3619837258
- FriedmanDSQuigleyHAGelbLUsing pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the glaucoma adherence and persistency study (GAPS)Invest Ophthalmol Vis Sci200748115052505717962457
- NordstromBLFriedmanDSMozaffariEQuigleyHAWalkerAMPersistence and adherence with topical glaucoma therapyAm J Ophthalmol2005140459860616226511
- SahebHAhmedIIMicro-invasive glaucoma surgery: current perspectives and future directionsCurr Opin Ophthalmol20122329610422249233
- CaprioliJKimJHFriedmanDSSpecial commentary: supporting innovation for safe and effective minimally invasive glaucoma surgery: Summary of a Joint Meeting of the American Glaucoma Society and the Food and Drug Administration, Washington, DC, February 26, 2014Ophthalmology201512291795180125881513
- WellikSRDaleEAA review of the iStent(®) trabecular micro-bypass stent: safety and efficacyClin Ophthalmol2015967768425931808
- VoskanyanLGarcía-FeijoóJBeldaJIFeaAJünemannABaudouinCSynergy Study GroupProspective, unmasked evaluation of the iStent® inject system for open-angle glaucoma: synergy trialAdv Ther201431218920124452726
- PfeifferNGarcia-FeijooJMartinez-de-la-CasaJMA randomized trial of a Schlemm’s canal microstent with phacoemulsification for reducing intraocular pressure in open-angle glaucomaOphthalmology201512271283129325972254
- HoehHVoldSDAhmedIKInitial clinical experience with the CyPass micro-stent: safety and surgical outcomes of a novel supraciliary microstentJ Glaucoma201625110611225304276
- LewisRAAb interno approach to the subconjunctival space using a collagen glaucoma stentJ Cataract Refract Surg20144081301130624943904
- GeddeSJSchiffmanJCFeuerWJHerndonLWBrandtJDBudenzDLTube Versus Trabeculectomy Study GroupThree-year follow-up of the tube versus trabeculectomy studyAm J Ophthalmol2009148567068419674729
- JohnsonM‘What controls aqueous humour resistance?’Exp Eye Res200682454555716386733
- SaccàSCGandolfiSBagnisAThe outflow pathway: A tissue with morphological and functional unityCell Physiol20162311918761893
- ZeimerRCGieserDKWilenskyJTNothJMMoriMMOdunukweEEA practical venomanometer. Measurement of episcleral venous pressure and assessment of the normal rangeArch Ophthalmol19831019144714496615314
- TorisCBYablonskiMEWangYLCamrasCBHumor dynamics in the aging human eyeAm J Ophthalmol1999127440741210218693
- SultanMBlondeauPEpiscleral venous pressure in younger and older subjects in the sitting and supine positionsJ Glaucoma200312437037312897584
- SelbachJMPosielekKSteuhlKPKremmerSEpiscleral venous pressure in untreated primary open-angle and normal-tension glaucomaOphthalmologica2005219635736116286795
- FeaAMPhacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma: randomized double-masked clinical trialJ Cataract Refract Surg201036340741220202537
- SamuelsonTWKatzLJWellsJMDuhYJGiamporcaroJEUS iStent Study GroupRandomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataractOphthalmology2011118345946720828829
- CravenERKatzJLWellsJMGiamporcaroJEiStent Study GroupCataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-upJ Cataract Refract Surg20123881339134522814041
- Arriola-VillalobosPMartínez-de-la-CasaJMDíaz-ValleDFernández-PérezCGarcía-SánchezJGarcía-FeijoóJCombined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: a long-term studyBr J Ophthalmol201296564564922275344
- NeuhannTHTrabecular micro-bypass stent implantation during small-incision cataract surgery for open-angle glaucoma or ocular hypertension: Long-term resultsJ Cataract Refract Surg201541122664267126796447
- PatelIde KlerkTAAuLManchester iStent study: early results from a prospective UK case seriesClin Exp Ophthalmol201341764865223448425
- Fernández-BarrientosYGarcia-FeioóJMartinez-de-la-CasaJMPabloLEFernández-PérezCGarcía SánchezJFluorophotometric study of the effect of the glaukos trabecular microbypass stent on aqueous humor dynamicsInvest Ophthalmol Vis Sci20105173327333220207977
- BelovayGWNagiAChanBJRatebMAhmedIIUsing multiple trabecular micro-bypass stents in cataract patients to treat open-angle glaucomaJ Cataract Refract Surg201238111911191722980724
- KatzLJErbCCarcellerAProspective, randomized study of one, two, or three trabecular bypass stents in open-angle glaucoma subjects on topical hypotensive medicationClin Ophthalmol201592313232026715834
- AhmedIIKatzLJChangDFProspective evaluation of microinvasive glaucoma surgery with trabecular microbypass stents and prostaglandin in open-angle glaucomaJ Cataract Refract Surg20144081295130025088627
- DonnenfeldEDSolomonKDVoskanyanLA prospective 3-year follow-up trial of implantation of two trabecular microbypass stents in open-angle glaucomaClin Ophthalmol201592057206526604675
- FergusonTJBerdahlJPSchweitzerJASudhagoniRGClinical evaluation of a trabecular microbypass stent with phacoemulsification in patients with open-angle glaucoma and cataractClin Ophthalmol2016101767177327695280
- SeiboldLKGamettKMKennedyJBOutcomes after combined phacoemulsification and trabecular microbypass stent implantation in controlled open-angle glaucomaJ Cataract Refract Surg20164291332133827697252
- KlamannMGonnermannJPahlitzschMiStent inject in phakic open angle glaucomaGraefes Arch Clin Exp Ophthalmol2015253694194725912085
- FeaAMBeldaJIRekasMProspective unmasked randomized evaluation of the iStent inject® versus two ocular hypotensive agents in patients with primary open-angle glaucomaClin Ophthalmol2014887588224855336
- García-FeijooJRauMGrisantiSSupraciliary micro-stent implantation for open-angle glaucoma failing topical therapy: 1-year results of a multicenter studyAm J Ophthalmol201515961075108125747677
- VoldSAhmedIICravenERTwo-year COMPASS trial results: Supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataractsOphthalmology2016123102103211227506486
- Pérez-TorregrosaVTOlate-PérezÁCerdà-IbáñezMCombined phacoemulsification and XEN45 surgery from a temporal approach and 2 incisionsArch Soc Esp Oftalmol201691941542126995503
- LafumaASalmonJFRobertJBerdeauxGTreatment persistence and cost-effectiveness of latanoprost/latanoprost-timolol, bimatoprost/bimatoprost-timolol and travoprost/travoprost-timolol in glaucoma: an analysis based on the United Kingdom general practitioner research databaseClin Ophtlmol20115361367
- BaudouinCLabbéALiangHPaulyABrignole-BaudouinFPreservatives in eyedrops: the good, the bad and the uglyProg Retin Eye Res201029431233420302969
- American National Standards InstituteANSI Z80.27-2001(R2014). Ophthalmics: Aqueous shunts for Glaucoma ApplicationAlexandria, VAAmerican National Standards Institute2001
- ShingletonBJGamellLSO’DonoghueMWBaylusSLKingRLong-term changes in intraocular pressure after clear corneal phacoemulsification: normal patients versus glaucoma suspects and glaucoma patientsJ Cataract Refract Surg199925788589010404361
- HayashiKHayashiHNakaoFHayashiFEffect of cataract surgery on intraocular pressure control in glaucoma patientsJ Cataract Refract Surg200127111779178611709251
- PoleyBJLindstromRLSamuelsonTWLong-term effects of phacoemulsification with intraocular lens implantation in normotensive and ocular hypertensive eyesJ Cataract Refract Surg200834573574218471626
- ManbergerSLGordonMOJampelHEffect of cataract surgery on intraocular pressure control in glaucoma patientsOphthalmology201211991826183122608478
- Food and Drug AdministrationPremarket Studies of Implantable Minimally Invasive Glaucoma Surgical (MIGS) Devices: Draft Guidance for Industry and Food and Drug Administration StaffRockvilleFood and Drug Administration2015 Available from: http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM433165.pdfAccessed: May 11, 2017
- HannCRVercnockeAJBentleyMDJorgensenSMFautschMPAnatomic changes in Schlemm’s canal and collector channels in normal and primary open-angle glaucoma eyes using low and high perfusion pressuresInvest Ophthalmol Vis Sci20145595834584125139736
- SwaminathanSSOhDJKangMHRheeDJAqueous outflow: segmental and distal flowJ Cataract Refract Surg20144081263127225088623