Abstract
Purpose
The aim of study was to evaluate the cross-link using riboflavin and ultraviolet A (UVA) for improving scleral wound healing.
Materials and methods
This was an experimental study involving four New Zealand rabbits (eight eyes). Therapy procedure was chosen for the right eye and control procedure for the left one. UVA irradiation of 365 nm with a surface irradiance of 3 mW/cm2 and a photosensitizer of riboflavin drops were applied for 30 minutes on the right eye at 2 mm from the limbus. Sclerotomy incision was performed at 2 mm from the limbus in both right (on the cross-link-treated area) and left eye. Then, 30 days after surgery, a morphological analysis and histological staining with hematoxylin–eosin and picrosirius red were performed, and the sclerotomy cicatrization of right and left eyes was compared. The variables investigated were as follows: sclerotomy incision pictures and measurements were made using the ImageJ Software. Scleral thickness was measured (employing the anterior optical coherence tomography and the digital caliper). Collagen fiber density stained with picrosirius red staining was measured using the Image Pro Plus software.
Results
The morphological analysis showed that in all samples, the right eye presented sclerotomy closure, and in two eyes, among them, there were no visible edges of the sclerotomies incision. The left eye presented sclerotomy closure and incision edges. The Image Pro demonstrated a higher density of collagen fibers in the right eye when compared to the one. The statistical analysis was significant when compared to the collagen fiber density in the treated eyes with the control eyes.
Conclusion
The cross-link procedure resulted in a better sclerotomy wound healing.
Introduction
The introduction of transconjunctival microincision vitrectomy surgery with 25 or 23 G instrumentation has gained support among vitreoretinal surgeons.Citation1 Its appeal comes from the advantages of the sutureless technique, which include reduced operating times, improved patient comfort, a more rapid visual recovery, and the avoidance of sutures.Citation2 However, potential drawbacks of sutureless surgery are an increased incidence of wound leaks, vitreous incarceration, hypotony, choroidal detachment, retinal detachment, endophthalmitis, and subconjunctival migration of gas and oil.Citation3
Even with recent advances in incision techniques, such as oblique incisions, biplanar cannula insertions, and slit-shaped scleral tunnel incisions, it may be difficult to ensure the sclerotomy wound healing, mainly in longer operating times and reoperation on vitrectomized eye.Citation4,Citation5 In cases of leaking sclerotomies, many have reported placing a suture.Citation6 To limit placing sutures, some described a new technique for the closure of the sclerotomy sites with a bipolar diathermy applied to the wound.Citation7
In recent times, a 27 G instrument system has also been developed. However, further refinements of the functions of the 27 G instrument are required.Citation8
In the current pilot study, a new technique for improving the sclerotomy healing was the use of cross-link (CXL) with the ultraviolet A (UVA) through an experimental study. Riboflavin, excited by UVA radiation, interacts with the terminations of different collagen chains, causing the loss of an amine and the formation of covalent bond between the collagen chains of the sclera.Citation9 Moreover, riboflavin also acts as a filter that reduces UVA penetration to deeper structures.Citation10 This technique is used to improve the scleral collagen fiber density. The authors used histological and morphological analysis in the scleral tissue.
Materials and methods
The current experimental study was approved by the Ethics Committee on The Use of Animals (CEUA) in scientific experimentation of the Center of Health Sciences of the Federal University of Rio de Janeiro (UFRJ). The animals used in this study were treated according to the guidelines proposed by the National Animal Experiment Control Council (CONCEA) from Brazil. The Ethics Committee approval documents (copy) are available for review by the Editor-in-Chief of this journal.
Animals
Four male New Zealand White rabbits (3.5 kg) were submitted to 23 G (Alcon Laboratories, Inc., Fort Worth, TX, USA) incision at 2 mm from the limbus in both eyes. The right eye was chosen as the treated one (sclerotomy plus CXL technique), and the left eye was chosen as the control (sclerotomy only).
Anesthesia procedure
For anesthesia, 1.5 mL ketamine hydrochloride 10% (35 mg/kg; Agener União, São Paulo, Brazil) and 0.5 mL xylazine hydrochloride (5 mg/kg; Agener União) were injected intramuscularly. For local anesthesia, proparacaine eye drops (Alcon Laboratories, Inc.) were instilled just once before the CXL procedure. A lid speculum was placed into the fornix.
CXL technique
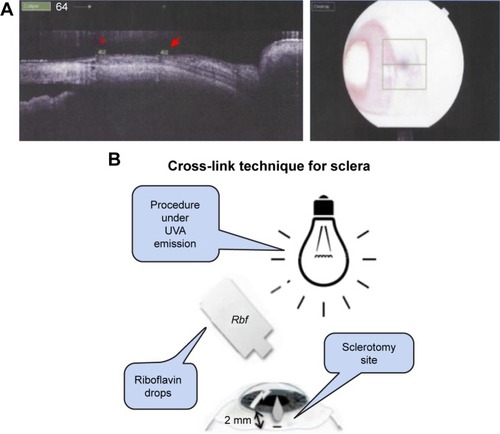
In the right eye (the treated one), a photosensitizer solution containing 0.1% riboflavin-5-phosphate (Ophthalmos, São Paulo, Brazil) was instilled at 2 mm from the limbus, 5 minutes before the irradiation and every 5 minutes during the irradiation. UVA irradiation of 365 nm (CXL; Opto Inc., São Carlos, Brazil) with a surface irradiance of 3 mW/cm2 (at a distance of 5 cm from the sclera) for 30 minutes and a photosensitizer of riboflavin drops were applied for 30 minutes. Sclerotomy procedure with 23 G incision (Alcon Laboratories, Inc.) was performed at 2 mm from the limbus in both right (on the CXL-treated area) and left eyes. After the procedure, 0.5% moxifloxacin drops (Vigamox; Alcon Laboratories, Inc.) were applied four times a day during 7 days. Then, 30 days after surgery, the sclera thickness was evaluated with optical coherence tomography (Topcon 3D-OCT-2000, Topcon, Tokyo, Japan; ). Then, the animals were killed under general anesthesia using an overdose of intravenous sodium phenobarbital (Cristália, São Paulo, Brazil). illustrates how/where the riboflavin was applied and insertion was made.
Figure 1 (A) Anterior OCT: rabbit scleral thickness at the sclerotomy site (red arrow) and scleral thickness at a control site (red asterisk). The image on the right shows the place on the sclera where the OCT scan was made. (B) Schematic representation of CXL procedure details.
Histological routine
The eyes were enucleated, and the part of the sclera with the sclerotomy site was removed and evaluated under slit-lamp biomicroscopy (Topcon SL-D7) through pictures. Then, the scleral thickness was measured using a digital caliper (Mitutoyo, Kawasaki, Japan; ). Next, it was placed in a 4% paraformaldehyde solution for 2 hours. After that, the tissue was placed in a graded sucrose solution series (10, 20, and 30%) for 24 hours. At the end, the sclera was placed in aluminum chambers filled with optimum cutting temperature solution (Tissue-Tek, Delaware, PA, USA). Frozen sections were prepared using Cryostat (Leica CM 3000; Leica Microsystems, Wetzlar, Germany) with 10 μm slices performed until the sclerotomy site was found. Parts of the tissue slices were stained with hematoxylin–eosin (HE) and the other part with picrosirius red (PR).
The specimens stained with the routine HE were evaluated under optical microscope (Carl Zeiss Meditec AG, Jena, Germany) to evaluate cellularity.
Histochemical study
The specimens stained with PR were evaluated under a polarized light microscope (Axioskop 2 plus; Carl Zeiss MicroImaging GmbH, Jena, Germany), and the photomicrographs were acquired with the Axion Vision 3.0 software. The photomicrographs were analyzed using Digital Image Pro Plus 7.0 software (Media Cybernetics Inc., Rockville, MD, USA). PR staining was used to analyze collagen fibers. The Image Pro Plus software evaluated collagen fiber density and morphology in the sclerotomy wound healing site.
Investigated variables
Variables used to analyze the sclerotomy wound healing were investigated. These variables were listed as follows: sclerotomy incision pictures and measurements, scleral thickness measurements at sclerotomy site, and collagen fiber density stained with PR.
Sclerotomy incision pictures and measurements
The sclerotomy incision was measured through biomicroscopy pictures by the software ImageJ (images.gif). The software sequence program was analyzed through gif image/set scale/measurement through global area. Data were presented in box plot graphic.
Scleral thickness measurements
Scleral thickness was measured at the sclerotomy incision site. Two methods of measurements were made: a measurement employing an anterior optical coherence tomography (OCT; Topcon 3D-OCT-2000; Nakano, Tokyo, Japan) and a measurement using a digital caliper (). Data were presented in box plot graphic.
Collagen fiber density stained with PR
The collagen fiber density was stained with PR and analyzed through the software Image Pro Plus. This software used the following sequence to analyze the collagen fiber density: tif image/convert to gray scale 8/count size/select measurements/oer area (object/total)/count/view/statistic/sum per area. Data were presented in box plot graphic.
Statistical analysis
To assess the sclerotomy wound healing improvement, statistical tests comparing the treated eye and the control one were used.
The paired Student’s t-test was used to analyze sclerotomy incision pictures, scleral thickness, and collagen fiber density. The intraclass correlation coefficient (ICC) test was used to compare the two different methods of measuring scleral thickness.
The level of significance was established as 5% (P<0.05). The software SPSS version 21.0 (2013; IBM Corporation, Armonk, NY, USA) was employed to statistical analysis.
Results
Sclerotomy incision pictures and measurements
The scleral incision pictures showed that in all samples the right eye (CXL) presented sclerotomy closure and in two eyes the incisions had no edges. The left eye presented sclerotomy closure and incision edges, as shown in .
Figure 2 (A) Biomicroscopy at slit lamp: sclerotomy incision site (red arrows). (B) Box plot chart. Analysis of ImageJ measurements of the sclerotomy incisions. Statistical data of paired Student’s t-test. Significant results (P<0.05). (C) Table with the sclerotomy measurements per area through the ImageJ software.
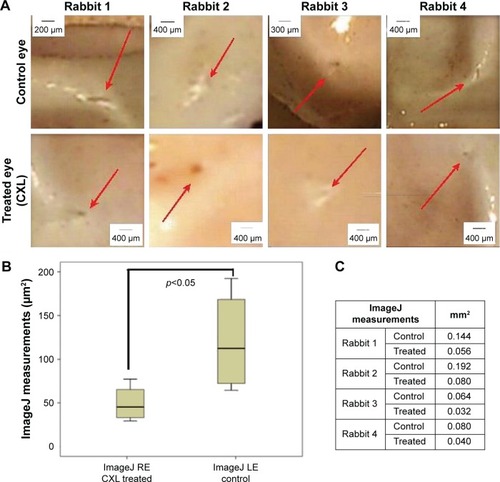
The standard size of the sclerotomy incision 23 G before the healing process is 0.64 mm. The sclerotomy incision size, as shown in box plot graphic () and table (), was bigger in the left eye than in the right one. The mean and standard deviation for measurements of incision dimensions, employing ImageJ (right eye – CXL), was 0.052±0.021 μm and (left eye – control) was 0.120±0.059 μm. This difference between the treated eye and the control one was statistically significant (P=0.028) with the paired Student’s t-test.
Scleral thickness measurements
The measurements regarding scleral thickness are shown in .
Figure 3 (A) Box plot chart. Scleral thickness measurements in treated and control eye with anterior OCT (in vivo) and with the digital caliper (measurement: postmortem). Statistical analysis performed with ICC test revealed showed a significant result. (B) Table with scleral thickness data.
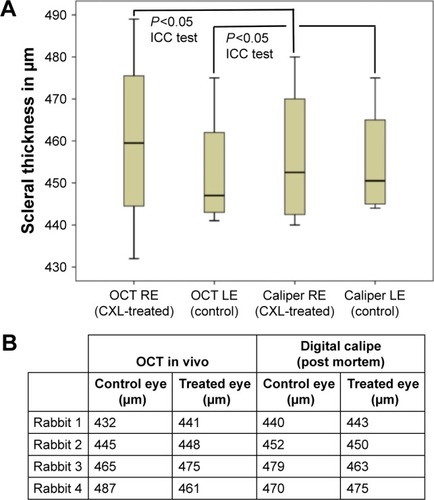
The measurements of mean/standard deviation of anterior OCT at right eye and left eye were 460.0±23.36 μm and 452.50±15.35 μm, respectively. The same measurements regarding scleral thickness measured through digital caliper at right eye and left eye were 456.25±17.96 μm and 455.0±14.15 μm, respectively. The statistical analysis with ICC test was significant at level 0.05 (P=0.012). Its value indicates high similarity between the measurements.
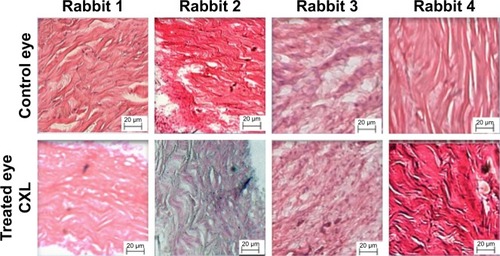
The HE staining showed a few inflammatory cells in the incisions of all treated eyes and controls. The images are shown in .
Figure 4 HE staining. Treated (CXL) and control eyes.
PR staining: PR staining revealed no evidence of type III collagen fibers in the wound healing process of sclerotomy in both eyes; it revealed collagen fibers type I in the wound healing of the incision in both eyes.
Specific evaluation on PR staining revealed less evidence of empty interfibrillar space among the collagen fibers in the right eye (CXL treated) than in the left eye (control), as shown in .
Figure 5 (A) Collagen fibers stained with PR in treated and control eyes. (B) Box plot chart showing the collagen fiber density through Image Pro Plus of the two eyes. Abscise axis measured in density dot pixels (dot pixels per area/total). (C) Image Pro Plus measurements.
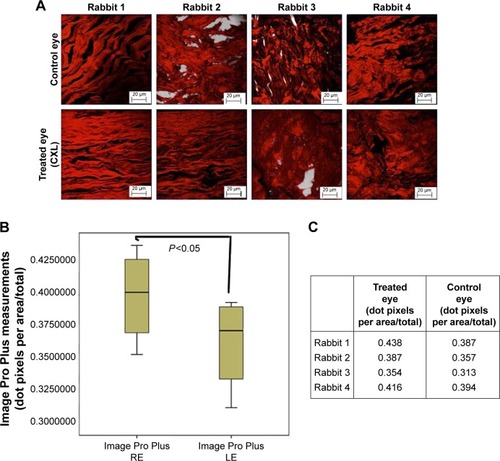
Collagen fiber density stained with PR
The Image Pro Plus demonstrated a higher density of collagen fiber in the treated eyes (0.438, 0.387, 0.354, 0.416) when compared to the fellow control one, respectively (0.387, 0.357, 0.313, 0.394). The paired Student’s t-test was found to be significant (P=0.038) when the collagen fiber density in the right and the left eyes was analyzed.
Discussion
The aim of this study was to evaluate the efficacy of a new technique, cross-linking with riboflavin and UVA, in improving 23 G sclerotomy wound healing. The authors found a higher density of collagen fibers of the sclerotomy incision cicatrization when the CXL technique was used (0.39 treated eye/0.36 control eye).
The riboflavin–UVA CXL has been applied in the treatment of keratoconus in clinical practice in the past decades.Citation11 The cross-linking has also been a promising alternative for the treatment of infective keratitis not responding to antimicrobial drugs.Citation12 The CXL technique using riboflavin and UVA still remains in animal experimental phase for myopic progression and other sclera diseases.Citation10
For corneal use, the UVA irradiance density is 0.18 mW/cm2 for 370 nm wavelength at a depth of 400 μm, which is less than half of the toxic level for endothelium. For this reason, the safety thickness of the cornea that can be treated by the standard protocol is 400 μm.Citation13
There are some experimental studies evaluating the use of riboflavin and UVA cross-linking to treat myopia. The thickness of the sclera in myopic eyes is thinner when compared to normal eyes, which could increase the toxicity of the procedure.Citation14,Citation15 Wollensak and SpoerlCitation16 showed that CXL enhanced the stiffness of porcine and human sclera. Besides, Wollensak et alCitation9 also demonstrated retinal damage in rabbit eyes submitted to 0.1% riboflavin and 370 nm wavelength at 4.2 mW/cm2 for 30 minutes.
The current study revealed an improvement in sclerotomy wound healing using the riboflavin–UVA CXL technique. The Image Pro Plus software demonstrated an increase in the collagen fiber density on the site treated with CXL when compared to the control eye. The study used UVA irradiation of 365 nm with a surface irradiance of 3 mW/cm2 for 30 minutes and the riboflavin as a photosensitizer. Zhang et alCitation17 irradiated the rabbit sclera and found that the optimal UVA irradiation time for the rabbit sclera was 40 minutes, under UVA wavelength of 365 nm (energy density of 3 mW/cm2). This condition showed efficiency without retinal damages. In the current study, the irradiated area target is the pars plana (2 mm from the limbus) that has no retinal tissue in humans.
Wollensak et alCitation9 found no obvious changes of scleral collagen fibers, especially no signs of a heat effect like coagulation scars with the use of riboflavin–UVA CXL technique. They found minor damage to the scleral fibrocytes, showing the low sensitivity of these cells under UVA light.
Wollensak and IomdinaCitation18 evaluated the cross-linking long-term effect. They used UVA irradiation (370 nm) with a surface irradiance of 3 mW/cm2 for 30 minutes at a distance of 1 cm from the sclera. Their study had confirmed an increase stiffening effect of cross-linking by riboflavin and UVA on in vivo rabbit sclera.
The main collagen component of the sclera is collagen type I, which can be cross-linked efficiently with UVA and riboflavin.Citation19 The current study found just collagen fiber type I in both eyes (the treated and the control ones) when the scleral tissues wound healing sclerotomies when the PR staining was used. The study did not find the collagen fiber type III, which is the immature collagen fiber that occurs at the beginning of the cicatrization process. The combination of PR and polarized light microscopy is widespread for detection and analysis of collagen.Citation20
Wollensak and IomdinaCitation18 found moderate signs of inflammation. The current study detected a few inflammatory cells in both eyes with HE staining.
The current study demonstrated an improvement in sclerotomy wound healing. However, surgeons would have to prolong the surgery by 30 minutes to cross-link the sclerotomy. The fact is that although the transconjunctival microincision vitrectomy surgery with 25 or 23 G made surgery safer, patient care and compliance remain the main targets of technological research.Citation8 Recently, the 27 G system has been introduced and reported that no eyes presented wound sealing-related complications.Citation21 However, similar to the recent evolution in 23 and 25 G systems, further development and refinements of the functions of 27 G instrument are required.Citation8
In the current study, the authors stressed that the CXL has clinical value to improve scleral wound healing in selected cases.
Limitations of the study
Rabbits have been widely used in ophthalmological experimental studies lately. Despite its ocular similarities with the human eyes, their eyes have some differences that could interfere with the results of the study. This limitation was reduced with the control eye.
Another limitation was the surgery itself. In the current study, the authors did not perform all the steps of the pars plana vitrectomy but just the sclerotomies incisions.
The small sample size is the most important limitation of the study; nevertheless, the authors used the control eye to balance it. The results demonstrated a great improvement of the sclerotomy wound healing using the CXL technique. Those additional studies will be certainly necessary to verify its toxicity, albeit the pars plana in humans has no retina tissue.
Conclusion
The scleral wound healing showed an absence of type III collagen (immature fibers) in the sclerotomy site 30 days after surgery. The CXL procedure resulted in a higher type I collagen fiber density in the sclerotomy cicatrization site. In addition, the CXL technique increased the collagen fiber density, improving the sclerotomy incision cicatrization process. This technique has the potential of becoming a factor to improve sclerotomy wound healing in eyes under high-risk factors of postoperative wound leaking.
Acknowledgments
The authors thank Bryan Hudson Hossy for the contribution to the histological and morphological analysis of this study. The authors also thank Professor Paulo Schor and Professor Mauricio Pereira for the analytical comments and the final scientific analysis of this study. This study was not financially supported by institutions.
Disclosure
The authors report no conflicts of interest in this work.
References
- EckardtCTransconjunctival sutureless 23-gauge vitrectomyRetina20052520821115689813
- FineHFIranmeshRIturraldeDSpaideRFOutcomes of 77 consecutive cases of 23-gauge transconjunctival vitrectomy surgery for posterior segment diseaseOphthalmology200711461197120017544779
- ChiehJJRogersAHWiegandTWBaumalCRReichelEDukerJSShort-term safety of 23-gauge single-step transconjunctival vitrectomy surgeryRetina200929101486149019696698
- WooSJParkKHHwangJMKimJHYuYSChungHRisk factors associated with sclerotomy leakage and postoperative hypotony after 23-gauge transconjunctival sutureless vitrectomyRetina200929445646319174725
- InoueMShinodaKHirakataATwenty-three gauge cannula system with microvitreoretinal blade trocarBr J Ophthalmol201094449850219828517
- LinALGhateDARobertsonZMO’SullivanPSMayWLChenCJFactors affecting wound leakage in 23-gauge sutureless pars plana vitrectomyRetina20113161101110821386764
- BosciaFBesozziGRecchimurzoNCauterization for the prevention of leaking sclerotomies after 23-gauge transconjunctival pars plana vitrectomy: an easy way to obtain sclerotomy closureRetina20113198899021487333
- MitsuiKKogoJTakedaHComparative study of 27-gauge vs 25-gauge vitrectomy for epiretinal membraneEye201630453854426742862
- WollensakGIomdinaEDittertDDCross-linking of sclera collagen in the rabbit using riboflavin and UVAActa Ophthalmol Scand200583447748216029274
- ZhangXTaoXCZhangJA review of collagen cross-linking in cornea and scleraJ Ophthalmol2015201528946725922758
- AlhayekALuPRCorneal collagen crosslinking in Keratoconus and other eye diseaseInt J Ophthalmol20158240741825938065
- AnwarHMEl-DanasouryAMHashemANCorneal collagen crosslinking in the treatment of infectious KeratitisClin Ophthalmol201151277128021966201
- KoppenCGobinLTassignonMJThe absorption characteristics of the human cornea in ultraviolet – a crosslinkingEye Contact Lens2010362778020107418
- McBrienNAGentleARole of the sclera in the development and pathological complications of myopiaProg Retin Eye Res200322330733812852489
- AwetissowESThe role of the sclera in the pathogenesis of progressive myopiaKlin Monatsbl Augenheilkd198017657777816449621
- WollensakGSpoerlECollagen crosslinking of human and porcine scleraJ Cataract Refract Surg200430368969515050269
- ZhangYZouCLiuLEffect of irradiation time on riboflavin-ultraviolet-A collagen crosslinking in rabbit scleraJ Cataract Refract Surg20133981184118923796623
- WollensakGIomdinaELong-term biomechanical properties of rabbit Sclera after collagen crosslinking using riboflavin and ultraviolet A (UVA)Acta Ophthalmol200987219319818803623
- BaileAJStructure, function and ageing of the collagens of the eyeEye198711751833308525
- JalilJEDoeringCWJanickiJSStructural Vs. contractile protein remodeling and myocardial stiffness in hypertrophied rat left ventricleJ Mol Cell Cardiol19882012117911872470910
- OshimaYWakabayashiTSatoTA 27-gauge instrument system for transconjunctival sutureless microincision vitrectomy surgeryOphthalmology20101179310219880185
