Abstract
Background
Pars plana vitrectomy is the only treatment for advanced proliferative diabetic retinopathy (PDR). However, vitrectomy is not always successful despite current progress in vitreoretinal surgical techniques. The aim of our study was to investigate whether the vitreal concentrations of MCP-1, IL-6, IL-8, and VEGF are elevated after unsuccessful vitrectomy in patients with PDR and to investigate whether the altered levels of these cytokines are associated with the cause for the reoperation.
Patients and methods
Vitreous samples were collected from 263 eyes of 233 patients: PDR (n=129 eyes), proliferative vitreoretinopathy (PVR; n=24 eyes) and nondiabetic controls (n=110 eyes) prior to vitrectomy. Vitreous samples were also collected from 14 eyes of 14 patients with PDR before vitrectomy and from the same 14 eyes before a second vitrectomy for reoperation. The levels of MCP-1, IL-6, IL-8, and VEGF were measured by flow cytometry using a cytometric bead array (CBA) assay.
Results
The mean concentrations of vitreal MCP-1, IL-6, IL-8, and VEGF were significantly higher in patients with PDR and PVR (P<0.01). There were significantly high correlations among the concentrations of MCP-1, IL-6, and IL-8, whereas the correlation of VEGF with the other 3 cytokines was lower. Among the 14 patients who required reoperation, the mean vitreal concentrations of MCP-1, IL-6, and IL-8 were higher than that at the time of the initial vitrectomy (P<0.01). At the time of the reoperation vitrectomy, the mean vitreous level of MCP-1, IL-6, and IL-8 in eyes with fibrous proliferation was higher than in those without fibrous proliferation (P<0.05). In contrast, VEGF in eyes with neovascular glaucoma (NVG) or anterior hyaloidal fibrovascular proliferation (AHFVP) was higher than in the eyes without NVG and AHFVP (P<0.05).
Conclusion
The elevated levels of MCP-1, IL-6, and IL-8 may be the cause of the postoperative fibrous proliferation. In contrast, VEGF may be the cause of the neovascularization after unsuccessful vitrectomy in the eyes of PDR patients.
Background
Diabetic retinopathy (DR) is one of the leading causes of blindness in the working age population worldwide.Citation1 At advanced stages, neovascularization, the hallmark of proliferative diabetic retinopathy (PDR), develops,Citation2 which can lead to the formation of abnormal fibrovascular membranes (FVMs) with subsequent tractional retinal detachment (TRD) and blindness.Citation3
Accumulating evidence has suggested that inflammatory processes play a major role in the pathogenesis of DR,Citation4,Citation5 neovascular glaucoma (NVG) associated with DR,Citation6 and age-related macular degeneration.Citation7 Studies including oursCitation8,Citation9 have demonstrated that MCP-1, IL-6, IL-8, and VEGF are the 4 major inflammatory cytokines that are upregulated in eyes with PDR.
Pars plana vitrectomy (PPV) is the only treatment for advanced PDR.Citation10 However, vitrectomy is not always successful despite current progress in vitreoretinal surgical techniques. The rate of reoperations after the initial vitrectomy ranges from 7% to 22%.Citation11,Citation12 The main causes for the reoperations are TRDs due to fibrous proliferation, NVG, and anterior hyaloidal fibrovascular proliferation (AHFVP).Citation13 However, the mechanism(s) causing these complications that develop after the first vitrectomy has not been determined.
Thus, the purpose of this study was to determine the vitreous levels of MCP-1, IL-6, IL-8, and VEGF after unsuccessful vitrectomy in eyes with PDR. In addition, we also determined whether the altered expressions of these inflammatory factors were significantly correlated with the particular cause of the reoperation including fibrous proliferation leading to TRDs and neovascularization in eyes with NVG and AHFVP. To accomplish this, we compared the vitreous levels of MCP-1, IL-6, IL-8, and VEGF proteins in patients with PDR collected before the first vitrectomy to that collected at a second vitrectomy.
Patients and methods
This study was approved by the ethics committees of the Kyushu University Hospital and Fukuoka University Chikushi Hospital, and the surgical specimens were handled in accordance with the Declaration of Helsinki. All patients were informed of the purpose of this study and gave informed consent to undergo the surgery before inclusion in the study. The inclusion and exclusion criteria for participation in this study are shown in .
Table 1 Inclusion and exclusion criteria for patients
All patients underwent a comprehensive ocular examination before vitrectomy and periodically up to 6 months after the surgery. None of our patients had undergone anti-VEGF treatments before or after the first vitrectomy.
Fourteen eyes of 14 patients (6 men, 8 women) required a reoperation vitrectomy because the retinopathy had not been alleviated. The cytokine levels at the time of the reoperation vitrectomy were compared to the levels of the same patient at the first vitrectomy. Their mean age was 55.4±14.4 years (range 39–72 years), and the mean duration of diabetes was 12.4±5.9 years (range 2–20 years). All of the eyes were diagnosed with PDR. The mean HbA1c was 7.8%±1.6% (range 5.7%–10.6%) in these patients. The reasons for the reoperation vitrectomy were TRD (7 eyes), NVG (2 eyes), AHFVP (2 eyes), persistent vitreous hemorrhage (2 eyes), and both NVG and TRD (1 eye). The interval between the first vitrectomy and reoperation was 14–133 days with a mean of 61.6 days.
The clinical characteristics of the PDR patients who required reoperation are presented in .
Table 2 Demographics and surgical characteristics of PDR patients
We also collected vitreous samples from a total of 263 eyes of 233 patients (men 112, women 121) just prior to an initial vitrectomy. The vitrectomy was performed for PDR in 129 patients (men/women =81/48) and for proliferative vitreoretinopathy (PVR) in 24 patients (men/women =9/15). The 14 patients who required reoperation were not included in 129 PDR patients. For nondiabetic controls, vitrectomy was performed on 44 patients for the treatment of a macular hole (MH) and on 66 patients for an epiretinal membrane (ERM). The patients’ age ranged from 36 to 85 years with a mean of 61.1±12.3 years.
Sample collection
Prior to the initial 25 G PPV, samples of undiluted vitreous (0.5–1.0 mL) were aspirated under standardized conditions and were immediately transferred to sterile tubes. The samples were centrifuged for 10 minutes at 3,000 rpm (1,630× g) at 4°C, and the supernatants were divided into aliquots and stored at −70°C until analyses.
During the vitrectomy, the FVMs were delaminated, and the posterior vitreous around the macula was removed. Then, panretinal endolaser photocoagulation (PRP) of the retina was performed up to the ora serrata. We tried to generate the same laser photocoagulation spot size by placing the laser probe at the same distance from the retina. The power and the duration of the photocoagulation were 120 mW and 100 ms. If a retinal detachment was detected or developed, it was treated with an air or 20% sulfur hexafluoride (SF6) tamponade. No silicone oil tamponade was used for any of those patients.
Cytometric bead array (CBA) assay
We measured the concentrations of MCP-1, IL-6, IL-8, and VEGF with a human CBA Kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. The minimum detectable concentration was 1.3 pg/mL for MCP-1, 1.6 pg/mL for IL-6, 1.2 pg/mL for IL-8, and 4.5 pg/mL for VEGF.
Statistical analyses
Statistical analyses were performed with a commercial statistical software package (JMP, V.8.0; SAS Institute Inc., Cary, NC, USA). The data were initially examined by Shapiro–Wilk tests to determine the normality of the distribution. Data that were not normally distributed were analyzed by non-parametric statistics. The significance of the differences between the preoperative and postoperative groups was analyzed by Wilcoxon matched-pairs signed rank tests. The correlation between MCP-1, IL-6, IL-8, and VEGF concentrations and the causes of reoperation were determined by the Spearman coefficient of correlation. The significance of the differences in the MCP-1, IL-6, IL-8, and VEGF levels among the different groups was determined by the Mann–Whitney test. To determine whether a significant correlation existed among MCP-1, IL-6, IL-8, and VEGF, Spearman correlation tests were used. Two-tailed test values of P<0.05 were considered statistically significant. Data are presented as mean ± SD.
Results
Vitreous concentrations of MCP-1, IL-6, IL-8, and VEGF in reoperation cases with PDR
To investigate the changes in the level of MCP-1, IL-6, IL-8, and VEGF after an unsuccessful vitrectomy, we compared the concentrations of MCP-1, IL-6, IL-8, and VEGF in the 14 vitreous samples collected at the initial vitrectomy to the 14 samples from the same patients collected at the reoperation vitrectomy.
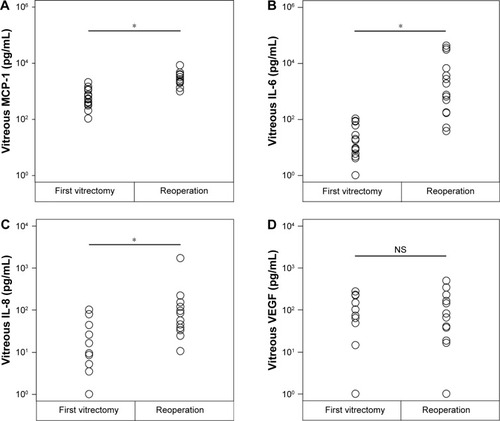
At the time of the reoperation, the mean vitreal concentrations of MCP-1 (3,082.1±574.3 pg/mL; P<0.0001), IL-6 (7,839.6±3,577.4 pg/mL; P<0.0001), and IL-8 (172.9±103.9 pg/mL, P=0.0007) were significantly higher than that at the time of the initial vitrectomy (). In contrast, the vitreal level of VEGF at the time of the reoperation (124.3±35.6 pg/mL) was not significantly different from that at the time of the initial vitrectomy (123.8±30.9 pg/mL; P=0.9849; ).
Figure 1 Vitreous concentrations of MCP-1 (A), IL-6 (B), IL-8 (C), and VEGF (D) in the vitreous samples at the first vitrectomy in PDR patients, and the same vitrectomized PDR patients at the time of the reoperation.
Abbreviations: PDR, proliferative diabetic retinopathy; NS, not significant.
Correlation between cause of reoperation and vitreous levels of MCP-1, IL-6, IL-8, and VEGF
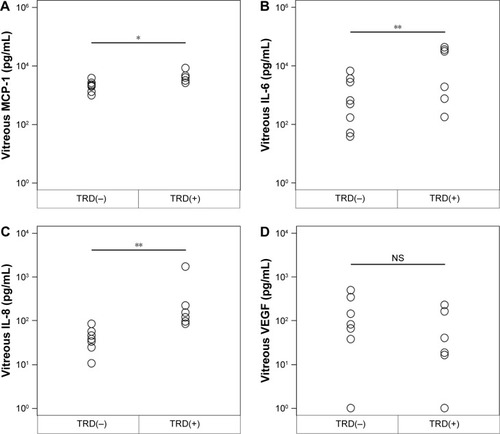
In the 14 eyes that required reoperation, 5 had TRDs due to fibrous proliferation, and the average vitreal levels of MCP-1, IL-6, and IL-8 at the time of the reoperation were 5,118.9±1,036.7, 19,671.4±8,030.8, and 397.5±264.7 pg/mL, respectively. In the 9 eyes without fibrous proliferation, the average levels were 1,913.1±308.0, 1,330.0±808.0, and 38.5±7.4 pg/mL. These differences from the eyes with TRD were statistically significant (P=0.0039, P=0.0454, P=0.0027, ). The VEGF concentration in the 5 eyes with fibrous proliferation (77.1±38.0 pg/mL) was not significantly different from that in 9 patients without fibrous proliferation (157.7±57.3 pg/mL; ).
Figure 2 Intravitreous levels of MCP-1, IL-6, IL-8, and VEGF at the time of reoperation vitrectomy and their correlations with the presence or absence of tractional retinal detachment (TRD) due to fibrous proliferation.
Abbreviation: NS, not significant.
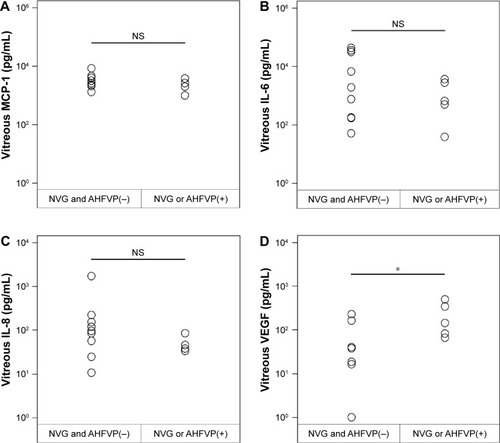
In contrast, the vitreal level of VEGF at the time of the reoperation vitrectomy was significantly higher in the 5 eyes with neovascularization, NVG, or AHFVP (225.9±83.9 pg/mL; P=0.0492), than in the 9 eyes without neovascularization (74.9±31.1 pg/mL; ). However, the concentrations of MCP-1, IL-6, and IL-8 at the time of reoperation surgery in the 5 eyes with neovascularization (2,220.8±457.7, 1,490.6±699.3, and 47.6±9.5 pg/mL, respectively) were not significantly different from those in 9 patients without neovascularization (3,682.8±848.2, 11,787.2±5,420.7, 249.4±164.4 pg/mL, respectively; ).
Figure 3 Intravitreous levels of MCP-1, IL-6, IL-8, and VEGF at the time of reoperation vitrectomy and their correlations with the presence or absence of neovascular glaucoma (NVG) and/or anterior hyaloidal fibrovascular proliferation (AHFVP).
Abbreviation: NS, not significant.
Vitreal concentrations of MCP-1, IL-6, IL-8, and VEGF in eyes with PDR and PVR
In the 129 eyes with PDR, the mean concentration of MCP-1 was 1,753.1±211.5 pg/mL, IL-6 was 587.2±369.0 pg/mL, IL-8 was 112.5±27.6 pg/mL, and VEGF was 228.7±26.1 pg/mL (). These concentrations were significantly higher than that in the 110 nondiabetic controls of 542.8±38.2 pg/mL for MCP-1 (P<0.0001), 13.3±3.4 pg/mL for IL-6 (P<0.0001), 11.6±3.2 pg/mL for IL-8 (P<0.0001), and 3.0±1.3 pg/mL for VEGF (P<0.0001).
Figure 4 Vitreous concentrations of MCP-1 (A), IL-6 (B), IL-8 (C), and VEGF (D) in patients with an idiopathic epiretinal membrane or a macular hole (nondiabetic controls; n=110), and also eyes with proliferative diabetic retinopathy (PDR; n=129), and proliferative vitreoretinopathy (PVR; n=24).
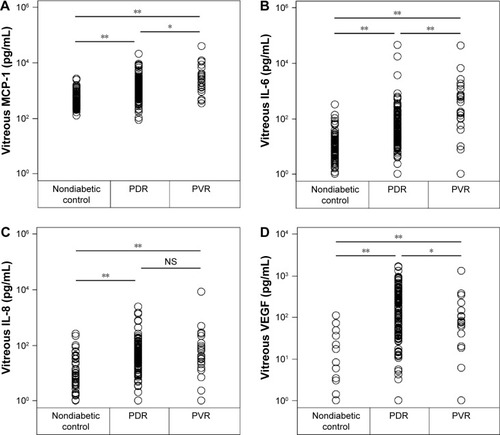
Similarly in the 24 eyes with PVR, the concentration of MCP-1 was 4,761.0±1,647.3 pg/mL, IL-6 was 587.2± 369.0 pg/mL, IL-8 was 439.6±347.97 pg/mL, and VEGF was 126.5±55.2 pg/mL (). These concentrations were also significantly higher than in the 110 nondiabetic controls at 542.8±38.2 pg/mL for MCP-1 (P<0.0001), 13.3±3.4 pg/mL for IL-6 (P<0.0001), 11.6±3.2 pg/mL for IL-8 (P<0.0001), and 3.0±1.3 pg/mL for VEGF (P<0.0001).
The mean concentration of vitreal MCP-1 and IL-6 was significantly higher in eyes with PVR than that with PDR (P<0.01 and P<0.001, respectively). The vitreal concentration of IL-8 in eyes with PDR was not significantly different from that in eyes with PVR (P=0.351). The mean concentration of vitreal VEGF was significantly lower in eyes with PVR than that with PDR (P<0.01).
Correlations of MCP-1, IL-6, IL-8, and VEGF concentrations
The correlations between the vitreal concentrations of MCP-1 and IL-6 in eyes with PDR (r=0.7329, P<0.0001; Spearman correlation; ) and those with PVR (r=0.8078, P<0.0001) were significant. The correlation between the vitreal concentrations of MCP-1 and IL-8 was also significant in eyes with PDR (r=0.7765, P<0.0001) and those with PVR (r=0.5687, P<0.0001). The correlation between the IL-6 and IL-8 concentrations in the vitreous of eyes with PDR (r=0.6158, P<0.0001) and PVR patients (r=0.5687, P<0.0001) was also significant. In contrast, there were weak but significant correlations between the vitreous concentrations of VEGF and IL-6 (r=0.2493, P=0.0044) and VEGF and IL-8 (r=0.3400, P<0.0001) in the vitreous of PDR patients. No significant correlation was found between the VEGF concentration and the other 3 cytokines in the vitreous of eyes with PVR.
Table 3 Correlations among vitreous concentration of MCP-1, IL-6, IL-8 and VEGF in eyes with proliferative diabetic retinopathy (PDR) and proliferative vitreoretinopathy (PVR)
Discussion
Our results showed that the mean vitreous concentrations of MCP-1, IL-6, and IL-8 were significantly higher in eyes after unsuccessful vitrectomy, ie, eyes requiring a reoperation, than at the first vitrectomy in patients with PDR (). In contrast, the vitreous level of VEGF was not significantly different from that at the first vitrectomy. Our observations are consistent with the earlier findings that vitrectomy can induce significant inflammation and a breakdown of the blood:ocular barrier.Citation14
The possible mechanisms that can cause an increase in the levels of MCP-1, IL-6, and IL-8 after vitrectomy are recruitment and activation of immune cells in the vitreous that produce the cytokines, breakdown of blood:ocular barrier, possibly by creation of retinal holes and tears, and the secretion of the cytokines by surrounding cells.Citation15–Citation17
We have previously demonstrated that there was an increase in the intravitreal concentrations of MCP-1 (1,189.6±103.33 vs 929.5±69.65 pg/mL) and IL-6 (90.95±32.66 vs 23.59±3.05 pg/mL), in spite of a significant decrease in IL-8 (30.19±2.39 vs 49.64±7.01 pg/mL) and VEGF (240.28±330.01 vs 964.92±1,358.57 pg/mL) even after successful vitrectomy.Citation18 In addition, the level of MCP-1 after vitrectomy was significantly correlated with the presence of postoperative diabetic macular edema in vitrectomized eyes. However, in the present study, the concentrations of MCP-1 (3,082.1±574.3 pg/mL), IL-6 (7,839.6±3,577.4 pg/mL), and IL-8 (172.908±103.90 pg/mL) after unsuccessful vitrectomy were much higher than that after successful vitrectomy for eyes with PDR.
From all of these findings, we hypothesize that the invasiveness of vitrectomy surgery triggers the production of the inflammatory cytokines such as MCP-1 and IL-6 even after successful vitrectomy. This modest upregulation of MCP-1 and IL-6 may cause the postoperative diabetic macular edema. However, in the presence of inappropriately high and/or persistent upregulation of the inflammatory cytokines that come in contact with retinal cells, such as the Muller cells and retinal pigment epithelium cells, there is an overshoot of the normal transient repair process leading to TRD due to fibrous proliferation.
In those eyes of patients who required a reoperation, the mean vitreous level of MCP-1, IL-6, and IL-8 in eyes with TRD due to fibrous proliferation was significantly higher than in those without TRD (). In contrast, the mean vitreal level of VEGF in eyes with NVG or AHFVP due to neovascularization was significantly higher than that in eyes without NVG and AHFVP (). These results indicate the differential actions of the elevated inflammatory cytokines after unsuccessful vitrectomy in patients with PDR. The elevated levels of MCP-1, IL-6, and IL-8 may cause postoperative fibrous proliferation and VEGF can cause neovascularization. This is consistent with previous reports that showed that chemokines play a role in the inflammatory pathways involved in the epiretinal fibrous proliferations.Citation19,Citation20 MCP-1 recruits leukocytes and is present in the vast majority of eyes affected by PVR.Citation21 IL-6 is secreted by T cells and macrophages to stimulate the immune response after tissue damage leading to inflammation. IL-6 stimulates the proliferation of glial cells and fibroblasts and promotes wound healing.Citation22 In contrast, the VEGF level was elevated in patients with NVG and AHFVP.Citation23,Citation24
Interestingly, there was a significant correlation among the concentrations of MCP-1, IL-6, and IL-8, whereas the correlation of VEGF with the other 3 cytokines was lower in the vitreous of patients with PDR (). Moreover, the mean concentration of vitreal VEGF was significantly lower in eyes with PVR only containing fibrous membranes than that with PDR with both fibrous and neovascular tissues (). These results further support the hypothesis on the distinct and differential roles played by the 3 inflammatory cytokines and VEGF in the proliferative vitreoretinal diseases, postoperative fibrous proliferation, and neovascularization. Therefore, we should consider different treatment modalities to inhibit unfavorable manifestations after vitrectomy in terms of inhibiting the inflammatory cytokines involved in postoperative proliferations. One is the postoperative steroids that suppress MCP-1 and IL-6 induction for inhibiting fibrous proliferations,Citation25 and the other is anti-VEGF therapies for NVG and AHFVP.
There are limitations to our analyses. First, the sample size was too small to determine the reasons for the reoperation vitrectomy. Because the cause of complications requiring reoperation is likely to be multifactorial, despite statistically significant differences, physiological differences might not be so remarkable.
However, the conclusions are most likely correct because our results are consistent with those of previous reports.Citation19,Citation23,Citation24 An analysis of a larger number of reoperation cases will be necessary to confirm the differential association of elevated inflammatory cytokines with postoperative fibrous proliferation and neovascularization after unsuccessful vitrectomy.
The second limitation is that we compared vitreous samples obtained at the first vitrectomy to vitreous samples obtained at a reoperation vitrectomy. When the vitreous gel is replaced with less viscous saline, the transport of all cytokines is facilitated.Citation26 Therefore, theoretically, the concentration in the second vitreous samples for cytokine analysis could be lower than that of the first sample. However, the rate of the clearance of the inflammatory cytokines may be different due to the intrinsic nature of molecules in the vitreous after vitrectomy.Citation18,Citation27,Citation28 For instance, because oxygen transport to ischemic retinal areas is improved after vitrectomy, the clearance of hypoxia-dependent molecules such as VEGF and IL-8 could be more rapid than relatively hypoxia-independent molecules. Finally, there have been no data published on how the vitreous matrix influences cytokine measurements as compared to vitreous fluid samples taken after gas tamponades.
Conclusion
We showed that the mean vitreous level of MCP-1, IL-6, and IL-8 was significantly higher in eyes with TRDs due to fibrous proliferation than in eyes without fibrous proliferation after unsuccessful vitrectomy. In contrast, the mean vitreous levels of VEGF in eyes with NVG or AHFVP were significantly higher than in those without NVG and AHFVP. These findings indicate that the elevated levels of MCP-1, IL-6, and IL-8 may cause postoperative fibrous proliferation, whereas VEGF can cause neovascularization after unsuccessful vitrectomy in the eyes of PDR patients.
Author contributions
Conception and design: SY and YS. Acquisition of data: SY. Analysis of data: SY and YK. Interpretation of data: SY, SN, TH, YI, and YO. Drafting of the manuscript: SY. Revision of the manuscript for important intellectual content: SY, TK, TI, and KS. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We thank Drs Yuki Kubo, Takahito Nakama, Keijiro Ishikawa, and Yusuke Murakami for their fruitful discussions. We also thank Ms Masayo Eto for her excellent technical assistance. This work was supported in part by JSPS KAKENHI Grant Numbers 26293374, 26670757, 15H04995, and 16K15734.
Disclosure
The authors report no conflicts of interests in this work.
References
- SivaprasadSGuptaBCrosby-NwaobiREvansJPrevalence of diabetic retinopathy in various ethnic groups: a worldwide perspectiveSurv Ophthalmol201257434737022542913
- HiscottPWongDGriersonIChallenges in ophthalmic pathology: the vitreoretinal membrane biopsyEye200014pt 454955911040899
- IshikawaKYoshidaSKobayashiYMicroarray analysis of gene expression in fibrovascular membranes excised from patients with proliferative diabetic retinopathyInvest Ophthalmol Vis Sci201556293294625604687
- TangJKernTSInflammation in diabetic retinopathyProg Retin Eye Res201130534335821635964
- KastelanSTomicMGverovic AntunicaASalopek RabaticJLjubicSInflammation and pharmacological treatment in diabetic retinopathyMediators Inflamm2013201321313024288441
- HouXRMiaoHTaoYLiXXWongIYExpression of cytokines on the iris of patients with neovascular glaucomaActa Ophthalmol2015932e100e10425041566
- FauserSViebahnUMuetherPSIntraocular and systemic inflammation-related cytokines during one year of ranibizumab treatment for neovascular age-related macular degenerationActa Ophthalmol201593873473826016605
- OwenLAHartnettMESoluble mediators of diabetic macular edema: the diagnostic role of aqueous VEGF and cytokine levels in diabetic macular edemaCurr Diab Rep201313447648023649946
- YoshimuraTSonodaKHSugaharaMComprehensive analysis of inflammatory immune mediators in vitreoretinal diseasesPLoS One2009412e815819997642
- BlankenshipGWMachemerRLong-term diabetic vitrectomy results. Report of 10 year follow-upOphthalmology19859245035062582329
- SchiffWMBarileGRHwangJCDiabetic vitrectomy: influence of lens status upon anatomic and visual outcomesOphthalmology2007114354455017169431
- OshimaYShimaCWakabayashiTMicroincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachmentOphthalmology2009116592793819269033
- YorstonDWickhamLBensonSBunceCSheardRCharterisDPredictive clinical features and outcomes of vitrectomy for proliferative diabetic retinopathyBr J Ophthalmol20089236536818303158
- InoueYKadonosonoKYamakawaTSurgically-induced inflammation with 20-, 23-, and 25-gauge vitrectomy systems: an experimental studyRetina200929447748019262433
- NishiONishiKOhmotoYEffect of interleukin 1 receptor antagonist on the blood-aqueous barrier after intraocular lens implantationBr J Ophthalmol199478129179207819176
- VujosevicSMiceraABiniSBertonMEspositoGMidenaEProteome analysis of retinal glia cells-related inflammatory cytokines in the aqueous humour of diabetic patientsActa Ophthalmol2016941566426268591
- YoshidaSKobayashiYNakamaTIncreased expression of M-CSF and IL-13 in vitreous of patients with proliferative diabetic retinopathy: implications for M2 macrophage-involving fibrovascular membrane formationBr J Ophthalmol201599562963425355804
- YoshidaSKuboYKobayashiYIncreased vitreous concentrations of MCP-1 and IL-6 after vitrectomy in patients with proliferative diabetic retinopathy: possible association with postoperative macular oedemaBr J Ophthalmol201599796096625631486
- RickerLJKijlstraAde JagerWLiemATHendrikseFLa HeijECChemokine levels in subretinal fluid obtained during scleral buckling surgery after rhegmatogenous retinal detachmentInvest Ophthalmol Vis Sci20105184143415020335622
- WeberKSNelsonPJGroneHJWeberCExpression of CCR2 by endothelial cells: implications for MCP-1 mediated wound injury repair and in vivo inflammatory activation of endotheliumArterioscler Thromb Vasc Biol19991992085209310479649
- DeshmaneSLKremlevSAminiSSawayaBEMonocyte chemoattractant protein-1 (MCP-1): an overviewJ Interferon Cytokine Res200929631332619441883
- MorescalchiFDuseSGambicortiERomanoMRCostagliolaCSemeraroFProliferative vitreoretinopathy after eye injuries: an overexpression of growth factors and cytokines leading to a retinal keloidMediators Inflamm2013201326978724198445
- ItakuraHKishiSKotajimaNMurakamiMPersistent secretion of vascular endothelial growth factor into the vitreous cavity in proliferative diabetic retinopathy after vitrectomyOphthalmology2004111101880188415465550
- KobayashiTMachidaSFujiwaraTIshibeTKurosakaDVitreous levels of vascular endothelial growth factor in eyes with anterior hyaloidal fibrovascular proliferationClin Ophthalmol201041043104620922040
- SohnHJHanDHKimITChanges in aqueous concentrations of various cytokines after intravitreal triamcinolone versus bevacizumab for diabetic macular edemaAm J Ophthalmol2011152468669421782151
- StefanssonEPhysiology of vitreous surgeryGraefes Arch Clin Exp Ophthalmol2009247214716319034481
- YoshidaSIshikawaKMatsumotoTYoshidaAIshibashiTKonoTReduced concentrations of angiogenesis-related factors in vitreous after vitrectomy in patients with proliferative diabetic retinopathyGraefes Arch Clin Exp Ophthalmol2010248679980420135140
- YoshidaSNakamaTIshikawaKAntiangiogenic shift in vitreous after vitrectomy in patients with proliferative diabetic retinopathyInvest Ophthalmol Vis Sci201253116997700322977138
