Abstract
Purpose
To compare the short-term effects of three monthly intravitreal bevacizumab (IVB) injections to single dexamethasone (DEX) implantation in treatment-naïve patients with cystoid macular edema (CME) secondary to branch (BRVO) and central retinal vein occlusion (CRVO).
Design
A retrospective single-center study.
Subjects
A total of 135 eyes of 135 patients with BRVO (n=83) and CRVO (n=52).
Methods
Changes in clinical parameters were recorded before treatment and at the first and third month after commencement of IVB (n=121) and DEX (n=14).
Main outcome measures
Central retinal thickness (CRT), intraocular pressure (IOP), and best-corrected visual acuity (BCVA).
Results
The baseline parameters were comparable between IVB and DEX groups. After the first month, CRT decreased by 131.3±42.9 μm in IVB and by 266.9±48.3 μm in DEX (mean ± SEM; p=0.047). IOP change was –0.29±0.39 mmHg in IVB and +3.70±2.34 mmHg in DEX (p=0.005). IOP elevation to ≥25 mmHg and ≥5 mmHg from the baseline was observed in two of the DEX- and in none of the IVB-treated eyes (p=0.010). After the third month, no differences regarding CRT and IOP were observed between the treatment modalities. Moreover, BCVA gain was comparable between IVB (0.37±0.05 logarithm of minimum angle of resolution [logMAR] units) and DEX (0.33±0.30 logMAR units) groups.
Conclusion
DEX was associated with faster resolution of CME, but had greater probability for short-term IOP elevation when compared to IVB. After the third month, treatments were comparably effective. Anatomical outcomes and adverse drug reactions of IVB versus DEX should be considered case specifically in patients having CME secondary to BRVO/CRVO.
Introduction
Retinal vein occlusion (RVO) is the second most common retinal vascular disease after diabetic retinopathy. The prevalence of RVO in people over 40 years of age is 1%–2%, and branch retinal vein occlusion (BRVO) is four times more common than central retinal vein occlusion (CRVO).Citation1 RVO may result from mechanical damage in vascular wall or a local inflammatory process causing thrombosis, hypercoagulation, and stasis. One-third of the RVO cases develop to an ischemic form with worse prognosis.Citation2,Citation3
Disturbances in blood flow after RVO are associated with significant upregulation of vascular endothelial growth factor (VEGF) expression, vascular permeability and neovascularization, cystoid macular edema (CME), and loss of visual acuity (VA). Laser photocoagulation for macular edema in RVO, used to destroy the exuding blood vessels or to induce chorioretinal venous anastomosis, has at most marginal effect on improving VA.Citation4,Citation5 Numerous randomized clinical trials with antithrombotic therapy, fibrinolytic agents, anticoagulants, or with hemodilution have proved to be unsatisfactory in either results or because of their adverse effects.Citation5
Intravitreally administered anti-VEGF agents are a well-established treatment for CME secondary to RVO,Citation6–Citation9 as well as intravitreal corticosteroids (triamcinolone acetonide and dexamethasone [DEX] implants).Citation10,Citation11 Besides partial VEGF inhibition, corticosteroids have an anti-inflammatory and blood–retinal barrier stabilizing effect on the retina by reducing the production of various inflammatory agents, such as tumor necrosis factor (TNF)-α and matrix metalloproteinases (MMPs), expression of intercellular adhesion molecule (ICAM)-1 on choroidal endothelial cells, and increasing the production of anti-inflammatory agents such as pigment epithelium-derived factor (PEDF).Citation12,Citation13 The choice of treatment modality between anti-VEGF agents and intravitreal corticosteroids may affect the patient’s quality of life, workload of the treating clinic, total costs of care and the drug, and drug delivery-related adverse effects, most notably an increase in intraocular pressure (IOP), cataract progression, and endophthalmitis.Citation14
The goal of this study was to compare the short-term treatment response and drug-related adverse effects of intravitreal bevacizumab (IVB) injections and DEX intravitreal implant in treatment-naïve BRVO and CRVO patients.
Materials and methods
Study design
The study design was an institutional, retrospective, register-based, observational study. Patients were admitted for the management of CME secondary to BRVO or CRVO in the Department of Ophthalmology, Kymenlaakso Central Hospital, Kotka, Finland. All patients included in the retrospective analysis were treatment-naïve regarding intravitreal medication. The diagnosis of BRVO/CRVO was carried out by a physician specialized in its diagnosis and treatment. The choice of therapies between three monthly anti-VEGF injections with bevacizumab (Avastin®; Genentech, Inc., South San Francisco, CA, USA) or single DEX implant (Ozurdex®; Allergan, Plc, Dublin, Ireland) was at the discretion of the treating physician. Main outcome measures were central retinal thickness (CRT), IOP, and best-corrected visual acuity (BCVA) at 1 and 3 months. The study was conducted as monitoring of clinical practice, and therefore informed patient consent was not required. The study was approved by the Institutional Review Board of the Research Director and Chief Medical Officer of the Kymenlaakso Central Hospital. Confidentiality of the patient records was maintained when the clinical data were entered into a computer-based standardized data entry for analysis.
Patients
A total of 135 eyes of 135 treatment-naïve patients who were treated for CME secondary to BRVO/CRVO either with IVB injections or DEX implantation between January 1, 2011 and December 31, 2015, were included in the study. The inclusion criteria were: 1) CME from BRVO/CRVO and 2) follow-up of at least 3 months after commencement of the intravitreal therapy. Exclusion criteria were: ischemic BRVO/CRVO or less than three anti-VEGF injections given.
The patients were divided into two groups according to the type of the primary intravitreal treatment. Of the 135 study eyes, 121 were treated with three monthly bevacizumab injections and 14 were treated with single DEX implantation. The procedures were performed in the operation room by ophthalmologists or residents in ophthalmology. All patients were assessed before injections/implantations. VA, tonometry, and CRT derived from optical coherence tomography (OCT) imaging were recorded. Patients with DEX implantation were routinely followed by ophthalmologists at months 1 and 3. No postoperative endophthalmitis was observed in our study. The baseline variables are presented in .
Table 1 Baseline variables according to primary treatment for RVO-related CME
Clinical evaluation
Clinical examination included bilateral VA testing at standardized light conditions, biomicroscopy, tonometry, and examination of the fundus. The classification for very low VA was on a semi-quantitative scale such as counting fingers (CF) and hand motion (HM). For statistical purposes, the Snellen values were transformed to the equivalent logarithm of minimum angle of resolution (logMAR) units. The very low VA measurements were converted as follows: CF 1.9, HM 2.3 logMAR. None of the study patients had light perception or no light perception VA.
The mean CRT was recorded by spectral-domain OCT by an experienced ophthalmic nurse. At follow-up, 30-frame scans were performed with AutoRescan™ software and OCT analyses were compared to those done prior to intravitreal treatment (Heidelberg Eye Explorer version 1.9.10.0 and HRA/SPECTRALIS® Viewing Module version 6.0.9.0; Heidelberg Engineering GmbH, Heidelberg, Germany).
Statistical analysis
Data are given as mean ± SEM and range (min–max) except the absolute number and proportion for nominal scale. IBM SPSS Statistics 23 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. For two-group comparisons, qualitative data were analyzed by two-factor χ2 test (or with the Fisher’s exact test when values in any of the cells of a contingency table were below five) and continuous variables by Student’s t-test. A p-value ≤0.05 was considered statistically significant.
Results
Baseline patient and ophthalmic characteristics in bevacizumab and DEX treatment groups
The gender distribution, mean age at the time of initiation of primary intravitreal treatment, type of RVO, BCVA, CRT, lens status, incidence of glaucoma, and IOP did not differ between the bevacizumab and DEX intravitreal implant treatment groups ().
BCVA gain in short term is similar between the bevacizumab and DEX treatment groups
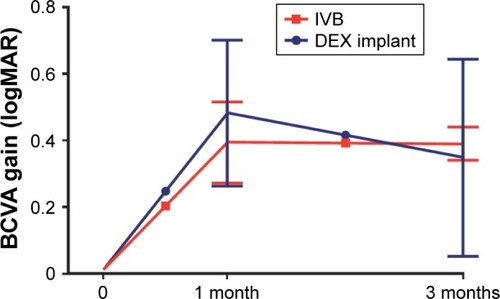
The patients were treated either with three monthly injections of bevacizumab, the final follow-up was 1 month after the last injection in this group, or with single DEX implant, the follow-ups were at months 1 and 3 in this group. The BCVA gain in logMAR units was comparable between the treatment groups at these time points ().
Figure 1 Three-month BCVA gain of the treatment-naïve CME eyes secondary to retinal vein occlusion.
Abbreviations: BCVA, best-corrected visual acuity; CME, cystoid macular edema; MAR, minimum angle of resolution; DEX, dexamethasone.
DEX implantation results in faster resolution of CME compared to bevacizumab treatment group
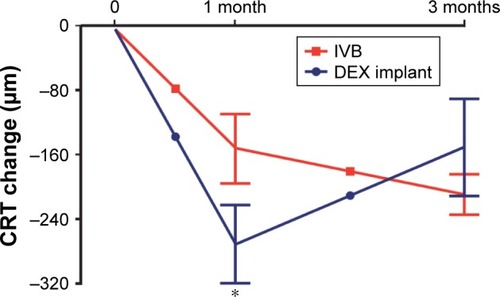
Next, we analyzed the effect of bevacizumab and DEX treatment on the mean CRT at months 1 and 3. At month 1, DEX treatment group resulted in greater CME resolution when compared to the bevacizumab treatment group (p=0.047, ). However, at 3 months, the CRT change was comparable between the treatment groups ().
Figure 2 Three-month CRT decrease of the treatment-naïve CME eyes secondary to retinal vein occlusion.
Abbreviations: CRT, central retinal thickness; CME, cystoid macular edema; DEX, dexamethasone.
DEX implantation results in short-term but significant IOP increase compared to bevacizumab treatment group
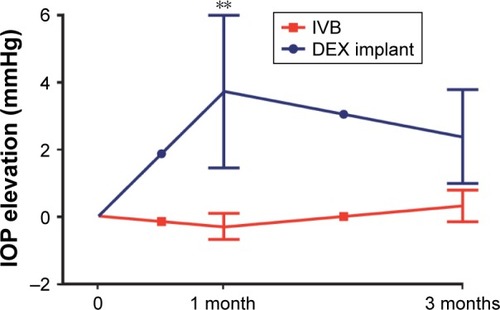
Finally, we analyzed the effect of bevacizumab and DEX treatment on IOP at months 1 and 3. At month 1, DEX treatment resulted in IOP increase when compared to the bevacizumab treatment group (p=0.005, ). IOP ≥25 mmHg and elevation ≥5 mmHg from the baseline were observed more frequently in the DEX (2 of 14) than bevacizumab (0 of 121) treated eyes (p<0.010, data not shown). Topical IOP-lowering medication was temporarily prescribed for both patients (one with prostaglandin analogue and one with combination of carbonic anhydrase inhibitor and beta blocker). At 3 months, the IOP levels did not significantly differ between the study groups ().
Figure 3 Three-month IOP change of the treatment-naïve CME eyes secondary to retinal vein occlusion.
Abbreviations: IOP, intraocular pressure; CME, cystoid macular edema; DEX, dexamethasone.
Discussion
In RVO eyes, primary treatment with DEX implant resulted in more effective resolution of CME at month 1, but with a greater probability for IOP elevation when compared to IVB. At 3 months, these treatment modalities were equally effective regarding BCVA gain, CME resolution, and IOP change.
VEGF is a pivotal target to reduce CME in patients with RVO. In addition, various cytokines, vascular destabilizing and fibroproliferative factors such as angiopoietin (Ang)-2, platelet derived growth factor (PDGF)-AA, transforming growth factor (TGF)-β1, and matrix MMPs were reported to be elevated in aqueous and vitreous specimens of RVO eyes.Citation15–Citation18 Clinical experience has shown that some of the eyes with refractory CME are bad responders or resistant to anti-VEGF treatment. At the moment, lack of clinical parameters or reliable diagnostic tools to distinguish eyes with a prominent inflammatory component limit decision making in regard to primary treatment modality.
Administration of intravitreal triamcinolone acetonide or DEX implant reduced plural inflammatory cytokines in RVO eyes.Citation19,Citation20 Treatment with DEX implant has become an alternative for anti-VEGF agents, due to its long efficacy in CME resolution and visual improvement,Citation11,Citation21 especially when administered early after the onset of disease.Citation22–Citation24 DEX implants have shown to be superior to anti-VEGF treatment regarding short-term CME resolution and VA recovery of treatment-naïve RVO patients.Citation25,Citation26 These findings are in accordance with our study, suggesting that short-term CME resolution is greater with DEX implant. The effect of DEX implant on CME resolution seemed to be waning by 3 months, particularly for CME resolution. This has been reported in previous studies.Citation21,Citation27
Retinal vascular disorders (including RVO) have direct impact on the costs of health care by increasing patient visits and medication and on other costs such as travel expenses and social services, eg, home care. They also cause indirect costs related to the lowered functional ability of the patient and the time and effort required by the treatment. DEX implant as a primary treatment for RVO is justified for patients in whom there is a reason to minimize the number of treatment and follow-up visits. On the other hand, the possible rise in IOP may increase the number of follow-up visits and precipitated development of cataract may lead to earlier cataract surgery. It has been reported before that combination treatment with both anti-VEGF and DEX implant may have stronger positive effect to either monotherapy, and thus could be a viable treatment option for some RVO patients.Citation27
This study has several limitations. First, the patient selection may be biased, as the decision of primary treatment modality was nonrandomized and performed by the treating clinician. On the other hand, baseline patient and ophthalmic parameters did not significantly differ between the study groups. Second, longitudinal follow-up after the 3-month time-point was not systematic and thus not included in the analysis. Despite the limitations, our study emphasizes that primary treatment with IVB and single DEX implantation results in comparable BCVA gain. Anatomical outcomes and adverse drug reactions between intravitreal anti-VEGF and corticosteroid treatments should be case specifically considered in RVO patients.
Acknowledgments
The study was supported by grants from the Helsinki University Hospital Specific Catchment Area Clinical Research Grants, the Finnish Eye Foundation, Finnish Ophthalmological Society, the Nissi Foundation, and the Waldemar von Frenckell Foundation, Helsinki, Finland.
Disclosure
The authors report no conflicts of interest in this work.
References
- RogersSMcIntoshRLCheungNThe prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and AustraliaOphthalmology20101172313319 e31120022117
- LaatikainenLKohnerEMFluorescein angiography and its prognostic significance in central retinal vein occlusionBr J Ophthalmol1976606411418952814
- McIntoshRLMohamedQSawSMWongTYInterventions for branch retinal vein occlusion: an evidence-based systematic reviewOphthalmology2007114583585417397923
- No authors listedEvaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The Central Vein Occlusion Study Group M reportOphthalmology199510210142514339097788
- MohamedQMcIntoshRLSawSMWongTYInterventions for central retinal vein occlusion: an evidence-based systematic reviewOphthalmology2007114350751952417324695
- BrownDMCampochiaroPASinghRPRanibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III studyOphthalmology2010117611241133.e112120381871
- KingeBStordahlPBForsaaVEfficacy of ranibizumab in patients with macular edema secondary to central retinal vein occlusion: results from the sham-controlled ROCC studyAm J Ophthalmol2010150331031420591399
- EpsteinDLAlgverePVvon WendtGSeregardSKvantaABevacizumab for macular edema in central retinal vein occlusion: a prospective, randomized, double-masked clinical studyOphthalmology201211961184118922424833
- TanMHMcAllisterILGilliesMERandomized controlled trial of intravitreal ranibizumab versus standard grid laser for macular edema following branch retinal vein occlusionAm J Ophthalmol20141571237247 e23124112635
- IpMSScottIUVanVeldhuisenPCA randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5Arch Ophthalmol200912791101111419752419
- HallerJABandelloFBelfortRJrRandomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusionOphthalmology2010117611341146.e113320417567
- FlomanNZorUMechanism of steroid action in ocular inflammation: inhibition of prostaglandin productionInvest Ophthalmol Vis Sci19771616973319076
- WangKWangYGaoLLiXLiMGuoJDexamethasone inhibits leukocyte accumulation and vascular permeability in retina of streptozotocin-induced diabetic rats via reducing vascular endothelial growth factor and intercellular adhesion molecule-1 expressionBiol Pharm Bull20083181541154618670086
- GarwegJGZandiSRetinal vein occlusion and the use of a dexamethasone intravitreal implant (Ozurdex(R)) in its treatmentGraefes Arch Clin Exp Ophthalmol201625471257126527178087
- TuuminenRLoukovaaraSIncreased intravitreal angiopoietin-2 levels in patients with retinal vein occlusionActa Ophthalmol2014922e164e16523786639
- JungSHKimKASohnSWYangSJAssociation of aqueous humor cytokines with the development of retinal ischemia and recurrent macular edema in retinal vein occlusionInvest Ophthalmol Vis Sci20145542290229624398091
- TuuminenRLoukovaaraSHigh intravitreal TGF-beta1 and MMP-9 levels in eyes with retinal vein occlusionEye20142891095109924946846
- EhlkenCGrundelBMichelsDIncreased expression of angiogenic and inflammatory proteins in the vitreous of patients with ischemic central retinal vein occlusionPLoS One2015105e012685925978399
- SohnHJHanDHLeeDYNamDHChanges in aqueous cytokines after intravitreal triamcinolone versus bevacizumab for macular oedema in branch retinal vein occlusionActa Ophthalmol2014923e217e22423889803
- Rezar-DreindlSEibenbergerKPollreiszAEffect of intravitreal dexamethasone implant on intra-ocular cytokines and chemokines in eyes with retinal vein occlusionActa Ophthalmol2017952e119e12727417275
- BezatisASpitalGHohnFFunctional and anatomical results after a single intravitreal Ozurdex injection in retinal vein occlusion: a 6-month follow-up – the SOLO studyActa Ophthalmol2013915e340e34723638803
- YehWSHallerJALanzettaPEffect of the duration of macular edema on clinical outcomes in retinal vein occlusion treated with dexamethasone intravitreal implantOphthalmology201211961190119822361318
- MayerWJRemyMWolfAComparison of intravitreal bevacizumab upload followed by a dexamethasone implant versus dexamethasone implant monotherapy for retinal vein occlusion with macular edemaOphthalmologica2012228211011622739239
- EterNMohrAWachtlinJDexamethasone intravitreal implant in retinal vein occlusion: real-life data from a prospective, multicenter clinical trialGraefes Arch Clin Exp Ophthalmol201725518927957602
- ChiquetCDupuyCBronAMIntravitreal dexamethasone implant versus anti-VEGF injection for treatment-naive patients with retinal vein occlusion and macular edema: a 12-month follow-up studyGraefes Arch Clin Exp Ophthalmol2015253122095210225673251
- YumusakEBuyuktortopNOrnekKEarly results of dexamethasone implant, ranibizumab, and triamcinolone in macular edema due to branch retinal vein occlusionEur J Ophthalmol2016261545926109021
- MayerWJWolfAKerntMTwelve-month experience with ozurdex for the treatment of macular edema associated with retinal vein occlusionEye201327781682223598674
