Abstract
Objective
The aim of this study was to evaluate the 24-hour intraocular pressure (IOP)-control effect of the tafluprost/timolol fixed combination (TAF/TIM-FC) in patients with primary open-angle glaucoma after they switched from the concomitant use of tafluprost and timolol gel-forming solution.
Patients and methods
Twenty patients with primary open-angle glaucoma (12 male and 8 female; mean ± SD age, 57.0±7.1 years) were included in this study. The patients were treated for 8 weeks with the concomitant administration of tafluprost and timolol gel-forming solution (evening dosing). At the end of this period, the patients underwent 24-hour IOP monitoring (measured at 21:00, 01:00, 05:00, 09:00, 13:00 and 17:00). IOP was measured with Goldmann applanation tonometer (GAT) and Icare PRO at sitting position at all timepoints and additionally, at supine position with Icare PRO tonometer at 01:00 and 05:00. The patients were then all switched to TAF/TIM-FC treatment (evening dosing). After 8 weeks, the 24-hour IOP monitoring was repeated.
Results
Nineteen patients completed the study. The mean 24-hour IOPs in the concomitant and TAF/TIM-FC phases were 13.8±2.7 vs 13.3±2.8 mmHg (P=0.0033) with the GAT in the sitting position and 13.96±2.56 vs 13.48±2.56 mmHg (P=0.0120) with the Icare PRO in habitual positions. In comparison with the concomitant phase, significantly lower IOP was observed for the TAF/TIM-FC phase at 21:00 and 01:00 with the GAT and at 01:00 with the Icare PRO. In addition, the maximum IOP and fluctuations in IOP in habitual positions were lower for the TAF/TIM-FC phase than for the concomitant phase.
Conclusion
TAF/TIM-FC showed a stable 24-hour IOP-lowering effect and was equally or more effective than the concomitant use of tafluprost and timolol gel, both when sitting and when in habitual positions.
Introduction
Glaucoma is a progressive disease characterized by changes to the optic nerve head and visual field defects; its fundamental pathology for functional structural abnormality is glaucomatous optic neuropathy. Currently, the only reliable treatment is intraocular pressure (IOP) reduction therapy, based on the evidence that it slows the progression of visual field defects.Citation1
Over the years, 24-hour IOP control has been the focus of attention.Citation2 Indeed, there are many studies investigating the 24-hour efficacy of various ocular hypotensive medicines.Citation3–Citation8 Beta adrenergic blockers including timolol were reported to show significant mean 24-hour IOP reduction and reduced efficacy at night time.Citation3,Citation4 On the other hand, prostaglandin analogs, which are popular first-line treatment for glaucoma, were found to have potent 24-hour IOP reduction efficacy throughout the day including night time.Citation4–Citation6 Tafluprost, one of the prostaglandin analogs, exhibited significantly lower peak IOP, narrower fluctuation and more efficiency at night compared with latanoprost with primary open angle glaucoma (POAG) in a 24-hour monitoring study using preservative-free formulation.Citation7,Citation8
Generally, a single-agent therapy cannot achieve sufficient reduction of the IOP in some patients, requiring two or more agents to be used in the treatment.Citation9 When multiple drugs are used concomitantly, the main problem is poor adherence to the medication. To improve adherence, a fixed-combination drug is used for patients who need multiple IOP-lowering medications. Tafluprost 0.0015%/timolol 0.5% fixed-combination solution (TAF/TIM-FC), a once-a-day instillation, has been shown to demonstrate three-point diurnal IOP reduction efficacy noninferior to the concomitant use of tafluprost 0.0015% ophthalmic solution once a day and timolol maleate ophthalmic 0.5% solution twice a day in the Phase III study conducted in Japan and Europe.Citation10,Citation11 However, to date, there has been no evaluation of TAF/TIM-FC’s 24-hour IOP-lowering profile and its efficacy has not been compared with that of the concomitant use of the medications.
In this study, we evaluated the 24-hour IOP control efficacy of TAF/TIM-FC, both in sitting and habitual positions (ie, sitting during the day and supine at night), in patients with POAG, after switching from the concomitant use of tafluprost and timolol gel-forming ophthalmic solution 0.5%.
Patients and methods
This study was approved by the Ethics Committee of the Medical Corporation Shinanokai Shinanozaka Clinic, and was conducted from August 2016 to January 2017 at Shinanozaka Clinic (Tokyo, Japan) in accordance with the tenets of the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan. All patients were fully informed of the purpose and procedures of this study, and all provided written informed consent to the investigators. This study was registered with the University Hospital Medical Information Network clinical trials registry (ID: UMIN000023883).
Patients
Eligible patients for the study were those ≥20 years of age with unilateral or bilateral POAG and who were expected to visit the clinic according to the observation schedule determined in the study protocol. The exclusion criteria were as follows: 1) any corneal abnormalities or other diseases that could interfere with accurate IOP measurement with a Goldmann applanation tonometer (GAT); 2) a history of corneal refractive surgery; 3) corrected visual acuity of 0.6 or less; 4) advanced visual field defects; 5) active extraocular disease, or ocular or eyelid inflammatory or infectious diseases; 6) a history of anterior or intraocular surgery; 7) a history of glaucoma surgery, including laser treatment; 8) allergy to the ingredients used in this study; 9) pregnancy, lactation, possibly pregnancy, a wish to become pregnant during the study period or unable to conduct appropriate contraception during the study; 10) scheduled medications or therapies prohibited during the study period; 11) contraindications to beta blockers, such as bronchial asthma or poorly controlled cardiac failure and 12) anyone the principal or other investigators considered ineligible for enrollment.
During the study period, the patients were prohibited from using any anti-glaucoma eye drops other than the study drugs, or any oral or intravenous IOP-lowering agents and corticosteroid drugs. Ocular laser surgery and invasive surgery were also prohibited. In addition, contact lens wearing was prohibited during the hospital visits (at weeks 8 and 16). Although the patients were allowed to use drugs and therapies other than those that were prohibited, they did not commence, discontinue or change the dosage of any drugs that could affect IOP, such as antihypertensive drugs or therapies, during the study period.
Procedures
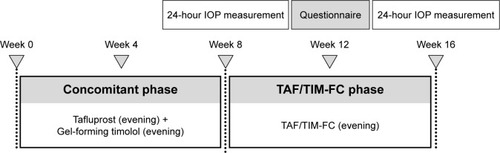
This study was designed as a prospective, single-arm, open-label trial. An outline of the study is presented in .
Figure 1 Study outline. IOP was measured over a 24-hour period at weeks 8 and 16, starting at 21:00.
During the study period, each patient routinely underwent a comprehensive clinical examination that included measuring IOP with a GAT, slit-lamp biomicroscopy, fundoscopy, blood pressure determination, and heart rate measurement. In addition, gonioscopy was conducted at the time of enrollment, and the central corneal thickness was measured 4 weeks later. Visual acuity tests were conducted at the time of enrollment and the end of the study. Prior to the patient’s submission of the informed consent, visual field tests results had been recorded for up to 6 months.
For the first 8 weeks of the study, all patients concomitantly instilled benzalkonium chloride containing tafluprost 0.0015% (Tapros®; Santen Pharmaceutical Co., Ltd., Osaka, Japan) and timolol gel-forming solution 0.5% (Timoptol XE 0.5%®; Santen Pharmaceutical Co., Ltd.) once each evening (the “concomitant phase”). The timolol gel-forming solution was instilled at least 10 minutes after the instillation of tafluprost. At the end of the concomitant phase, patients were admitted to hospital and underwent a 24-hour IOP measurement process. During this, IOP was measured in the sitting position with the Icare® PRO tonometer (Icare Finland Oy, Espoo, Finland) and then with the GAT; a total of six measurements were made at 4-hour intervals (at 21:00, 01:00, 05:00, 09:00, 13:00 and 17:00). In addition, IOP was measured in the supine position with the Icare PRO at 01:00 and 05:00 after the measurements in the sitting position. The maximum acceptable variation in the time of the IOP measurement was ±30 minutes. Blood pressure and heart rate were also measured at the same time as the IOP measurement. For the following 8 weeks, the patients used benzalkonium chloride containing tafluprost 0.0015%/timolol 0.5% fixed-combination solution (Tapcom®, Santen Pharmaceutical Co., Ltd.), instilling one dose each evening (the “TAF/TIM-FC phase”). At the end of this period, the 24-hour IOP measurement protocol was repeated in the hospital. For patients with bilateral POAG with both eyes meeting the eligibility criteria, the eye with the higher mean 24-hour IOP at week 8 was selected as the study eye; if the IOP was equal in both eyes, the right eye was selected for the study.
At each time point, IOP was measured three times with the GAT and the median value adopted for the analysis. With the Icare PRO, the measurement was made at least six times until “deviation OK” was displayed on the tonometer’s screen, and the mean of these values was adopted. On the day before hospitalization for the 24-hour IOP measurement, the patients were asked to instill the eye drops between 20:00 and 22:00. On the days of hospitalization, the eye drops were administered at 21:00 after the measurements of IOP, blood pressure and heart rate. The same observer made the 24-hour IOP measurements for both the concomitant phase and the TAF/TIM-FC phase.
Adherence to the instillation schedule was recorded at each visit and classified as follows: “complete instillation (100% instillation)”, “sometimes forgot instillation (75%–99% instillation)”, “instilled half of the times as scheduled (25%–74% instillation)” and “instilled a few times as scheduled (<25% instillation).” At week 12, a questionnaire was given to the patients asking for their impression of TAF/TIM-FC in comparison with the concomitant use of medications. They were asked about the frequency of forgetting instillation (increased, decreased or unchanged), its user-friendliness (more user-friendly, less user-friendly or unchanged) and their preference of continuation (prefer the fixed combination, prefer the concomitant use or no preference).
Statistical analysis
Data regarding the backgrounds, questionnaire and profiles of adverse events of all the enrolled patients, and the other data for all the patients who completed the study, were analyzed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). Summary statistics (mean and SD) of the following were calculated for each 24-hour measurement: 24-hour IOP, diurnal IOP (09:00, 13:00, 17:00 hours), nocturnal IOP (21:00, 01:00, 05:00), maximum, minimum and fluctuation (maximum − minimum). A mixed-effect model was used for the analysis of 24-hour, diurnal and nocturnal IOPs and the individual time points, calculating P-values by using least squares estimation and the 95% CI. The changes in the maximum value, the minimum value and the fluctuation during 24 hours in the treatment period between the TAF/TIM-FC phase and the concomitant phase were compared using the paired t-test. Sign tests were used for the analysis of the questionnaire responses. P-values <0.05 were considered significant.
Results
Patients
Initially, 21 patients were enrolled, but deviation from the study criteria was found in 1 patient after the submission of informed consent. The study, therefore, started with 20 patients who complied with the inclusion and exclusion criteria. One patient withdrew during the study period for personal reasons; the remaining 19 patients completed the study. The characteristics of the patients are presented in .
Table 1 Patients’ characteristics
Across the whole study period, 89.5%–100% of the patients reported “perfect adherence to the scheduled instillation (100% instillation)” and none reported “low adherence to the scheduled instillation (25%–74% instillation)” or “poor adherence to the scheduled instillation (<25% instillation)”.
Twenty-four-hour IOP measurement
presents mean values for the patients’ 24-hour IOP profiles measured with the GAT in the sitting position. The mean 24-hour IOP and nocturnal IOP were significantly lower for the TAF/TIM-FC phase than for the concomitant phase (24-hour IOP [mean ± SD]: 13.8±2.7 vs 13.3±2.8 mmHg, P=0.0033; nocturnal IOP: 14.0±3.0 vs 12.9±2.7 mmHg, P<0.0001). For two of the nocturnal time points, 21:00 and 01:00, the IOPs for the TAF/TIM-FC phase were lower than those for the concomitant phase (21:00: 14.5±3.3 vs 13.0±3.2 mmHg, P=0.0009; 01:00: 13.8±3.0 vs 12.8±2.6 mmHg, P=0.0168; ). There were no statistically significant differences in the mean diurnal IOP, maximum IOP, minimum IOP and fluctuations between the two phases.
Figure 2 IOP during the 24-hour measurement periods, measured in the sitting position with a Goldmann Applanation Tonometer.
Abbreviations: IOP, intraocular pressure; TAF/TIM-FC, tafluprost/timolol fixed combination.
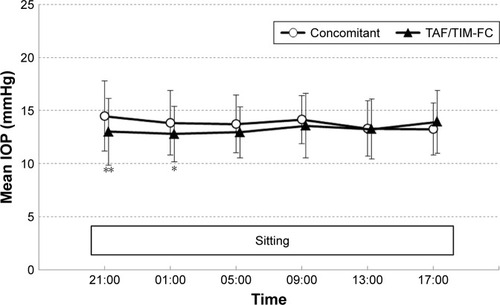
Table 2 Twenty-four-hour IOP profiles for the concomitant and TAF/TIM-FC treatments measured using a Goldmann Applanation Tonometer
Measured with the Icare PRO tonometer, the mean 24-hour IOP in the sitting position was lower in the TAF/TIM-FC phase than in the concomitant phase (13.68±2.45 vs 12.99±2.41 mmHg, P=0.0005). The mean 24-hour IOP, diurnal IOP and nocturnal IOP were in good agreement with those measured with the GAT (data not shown). Significantly lower IOPs in the sitting position were observed for the TAF/TIM-FC phase than for the concomitant phase at two time points, 01:00 (14.62±2.88 vs 12.57±2.42 mmHg, P<0.0001) and 05:00 (14.42±2.18 vs 13.25±2.13 mmHg, P=0.0126).
The 24-hour IOP profile in the habitual positions (sitting during the day and supine at night) measured with the Icare PRO is given in . Similar to the measurements made in the sitting position, the mean 24-hour IOP and nocturnal IOP were significantly lower in the TAF/TIM-FC phase than in the concomitant phase (24-hour IOP: 13.96±2.56 vs 13.48±2.56 mmHg, P=0.0120; nocturnal IOP: 14.74±2.53 vs 13.81±2.68 mmHg, P=0.0006). With the concomitant therapy, IOP increased slightly during the nocturnal period, whereas with the TAF/TIM-FC therapy, it remained nearly constant throughout the 24-hour period (). At 01:00, the mean IOP was significantly lower in the TAF/TIM-FC phase than in the concomitant phase (16.33±2.56 vs 14.53±2.83 mmHg, P=0.0001). In addition, the maximum IOP and fluctuation were lower in the TAF/TIM-FC phase than in the concomitant phase (P=0.0011 and 0.0097, respectively).
Figure 3 IOP measured in the habitual positions during the 24-hour measurement periods.
Abbreviations: IOP, intraocular pressure; TAF/TIM-FC, tafluprost/timolol fixed combination.
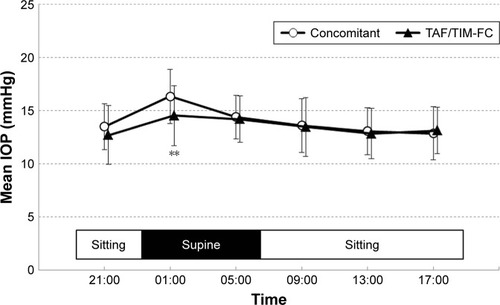
Table 3 Twenty-four-hour IOP profiles for the concomitant and TAF/TIM-FC treatments, measured in the sitting or supine position with an Icare PRO tonometer
Blood pressure and heart rate
There were no significant differences in 24-hour systolic blood pressure, diastolic blood pressure or heart rate between the two phases, either over the whole 24-hour period or at any time point (). Indeed, no remarkable changes in values were observed throughout the day (data not shown).
Table 4 Mean blood pressure and heart rate during the 24-hour measurement
Questionnaire results
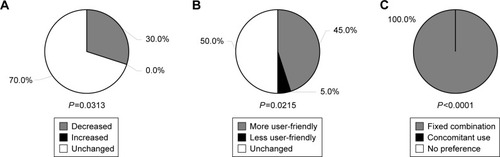
In their responses to the questionnaire on the use of the eye drops, 6 of the 20 patients (30.0%) answered that their frequency of instillation forgetfulness decreased after switching to TAF/TIM-FC (P=0.0313 vs the number of those who answered “increased”); the remaining 14 patients (70.0%) reported no change after switching, with none reporting increased forgetfulness (). Nine of the patients felt the treatment after switching to be more user-friendly (P=0.0215 vs the number of those who answered “less user-friendly”), whereas 10 (50.0%) reported no difference in user-friendliness between the two treatments. All preferred to continue with the fixed-combination drug than return to the concomitant treatment (P<0.0001 vs the number of those who chose “concomitant use”).
Safety
summarizes the adverse events experienced by the patients related with the two study treatments. Both treatments were well tolerated. No serious adverse events occurred during the study period.
Table 5 Adverse events related to the study treatment
Discussion
The aim of this study was to investigate the 24-hour IOP-lowering efficacy of TAF/TIM-FC, in both the sitting and habitual positions, after switching from the concomitant use of tafluprost and timolol gel. To the best of our knowledge, this is the first study to evaluate the 24-hour efficacy of TAF/TIM-FC and compare this with the concomitant use. When measured in the sitting position with the GAT, IOP was stable at a low level in both the concomitant phase and the TAF/TIM-FC phase throughout the 24-hour testing period. In addition, TAF/TIM-FC demonstrated small but statistically significant reduction of the 24-hour and nocturnal IOP after switching from the concomitant use (0.5 and 1.1 mmHg, respectively). Because the Early Manifest Glaucoma Trial has shown that a difference of 1 mmHg in IOP can offer a 10% protection against the progression of glaucoma, we consider that the reductions shown in this study might also be clinically significant.Citation12
Previous studies have compared fixed combinations of prostaglandin analogs and timolol with the concomitant use of the ingredients,Citation13–Citation17 with most of them reporting lower efficacy for the fixed-combination drugs.Citation18 These results were mostly explained as resulting from the declining number of instillations of timolol in fixed-combination drugs than when the drugs were used concomitantly. Timolol gel 0.5% was provided as a once-daily dosing formulation, and it showed the same efficacy as 0.5% timolol solution for POAG or ocular hypertension because of the extended precorneal residence time.Citation19–Citation21 Indeed, in a randomized parallel-group study of 90 POAG patients conducted by Özyol and Özyol, the mean diurnal IOP with the concomitant use of evening-dosed latanoprost and morning-dosed timolol gel was significantly lower than that with evening-dosed latanoprost/timolol maleate fixed combination after 8 weeks of treatment.Citation22
It is unclear why there were statistically significant differences between the two treatments in this study. TAF/TIM-FC was designed for improving timolol penetration into the eye to compensate for the possible diminished effect of timolol arising from the decreased instillation frequency from twice daily with the concomitant use to once daily. Indeed, timolol penetration into the eye with TAF/TIM-FC has been reported to be higher than that with the timolol mono-preparation.Citation23,Citation24 The reason for the lowered IOP with TAF/TIM-FC in this study may be partly because of the good penetration of timolol into the anterior chamber.
In this study, IOP was measured with the Icare PRO tonometer in addition to the GAT. The Icare is a user-friendly, handheld rebound tonometer with a disposable probe that prevents infection during the measurement. Icare PRO allows IOP to be measured in the supine position as well as in the normal upright position. It has been reported to show good reproducibility of measurements made with the GAT in the sitting position.Citation25 This study also demonstrated that the mean 24-hour IOP and IOP at individual time points measured with the Icare PRO in the sitting position were similar to those measured with the GAT (data not shown). In this study, IOP was also measured with Icare PRO in the habitual positions (sitting during the day and supine at night). The mean value of 24-hour IOP was in rough agreement with that measured by the GAT in the sitting position for both treatment phases. Furthermore, maximum IOP and the fluctuation in IOP when measured in the habitual positions were statistically lower in the TAF/TIM-FC phase than in the concomitant phase. This result suggests that TAF/TIM-FC treatment could result in lower IOP for POAG patients even at night in the supine position; thus, TAF/TIM-FC may be an appropriate treatment for careful 24-hour IOP control in daily life.
The results of the questionnaire on switching to the TAF/TIM-FC treatment revealed that almost all the patients experienced either no change or improved in their frequency of instillation and user-friendliness after switching treatments, and all patients preferred to continue using TAF/TIM-FC. This suggests that a fixed-combination therapy can improve patient adherence because of the reduced number of daily instillations. The clinical practice guidelines for glaucoma treatment in Japan note that poor compliance is one of the most important factors for the progression of glaucomatous optic neuropathy.Citation1 The optimal control of IOP requires topical therapy for glaucoma treatment to continue over a long time. The selection of user-friendly eye drops could be useful for the long-term control of IOP.
This study had some limitations: First, there was no washout period between the two treatment periods. However, the patients had been treated for 8 weeks before the 24-hour hospital stay for IOP measurements, so the impact of the prior treatment is considered minimal. Second, this trial evaluated switching from one treatment to the other, so an order effect bias was inevitable. The questionnaire focused only on how patients felt after switching, and the results may have contained some bias because of the order effect. In addition, baseline 24-hour IOP measurements were not made, so the reduction in IOP from the previous untreated period could not be established. Other limitations include this being a single-center trial, the small study population and the short-term follow-up.
Conclusion
This study examined the effects of TAF/TIM-FC over 24 hours after switching from the concomitant use of tafluprost and timolol gel-forming solution in POAG patients, and demonstrated TAF/TIM-FC maintained 24-hour IOP equal to or lower than that of the concomitant use, in both the sitting and habitual positions. This showed that TAF/TIM-FC instilled at one drop a day could be beneficial for patients, allowing their IOP to be maintained at a level as low as with the concomitant use.
Acknowledgments
This study was funded by Santen Pharmaceutical Co., Ltd. (Osaka, Japan). The funding organization participated in the design of the study, interpretation of the data, review and approval of the manuscript, and had no role in the data collection and analysis of data derived from this study. Data management and statistical analysis were conducted by Biostatistical Research Corporation (Tokyo, Japan). Etsuyo Miyamoto and Izumi Furutani are employees of Santen Pharmaceutical Co., Ltd. Etsuyo Miyamoto contributed toward drafting and revising the protocol and reviewing the paper. Izumi Furutani contributed toward reviewing the paper.
Disclosure
Kenji Nakamoto received consultant fee from Santen Pharmaceutical Co., Ltd. Naomi Otsuka, Hiroko Hizaki, Hiromi Akai and Masayo Hashimoto are employees of Santen Pharmaceutical Co., Ltd. The other authors report no conflicts of interest in this work.
References
- The Japan Glaucoma SocietyGuidelines for Glaucoma 3rd editionJ Jpn Ophthalmol Soc20121161346 Japanese
- KonstasAGQuarantaLBozkurtB24-h Efficacy of Glaucoma treatment optionsAdv Ther201633448151726909513
- KonstasAGMantzirisDACateEAStewartWCEffect of timolol on the diurnal intraocular pressure in exfoliation and primary open-angle glaucomaArch Ophthalmol199711589759799258218
- OrzalesiNRossettiLInvernizziTBottoliAAutelitanoAEffect of timolol, latanoprost, and dorzolamide on circadian IOP in glaucoma or ocular hypertensionInvest Ophthalmol Vis Sci20004192566257310937568
- StewartWCKonstasAGNelsonLAKruftBMeta-analysis of 24-hour intraocular pressure studies evaluating the efficacy of glaucoma medicinesOphthalmology200811571117112218082886
- NakamotoKYasudaNNannoMKinohiraYMuraiKFukudaTEffect of latanoprost on diurnal variations of intraocular pressure in normal-tension glaucomaNippon Ganka Gakkai Zasshi20031079530534 Japanese14531313
- KonstasAGBoboridisKGKapisP24-Hour efficacy and ocular surface health with preservative-free tafluprost alone and in conjunction with preservative-free dorzolamide/timolol fixed combination in open-angle glaucoma patients insufficiently controlled with preserved latanoprost monotherapyAdv Ther201734122123527913991
- KonstasAGQuarantaLKatsanosATwenty-four hour efficacy with preservative free tafluprost compared with latanoprost in patients with primary open angle glaucoma or ocular hypertensionBr J Ophthalmol201397121510151523681371
- KassMAHeuerDKHigginbothamEJThe ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucomaArch Ophthalmol2002120670173012049574
- KuwayamaYDE-111 collaborative trial group. [Phase III double-masked study of fixed combination tafluprost 0.0015%/timolol 0.5% (DE-111) versus tafluprost 0.0015% alone or given concomitantly with timolol 0.5% in primary open angle glaucoma and ocular hypertension]J Eye (Atarashii Ganka)201330811851194 Japanese
- HollóGHommerAAntón LópezARopoAEfficacy, safety, and tolerability of preservative-free fixed combination of tafluprost 0.0015%/timolol 0.5% versus concomitant use of the ingredientsJ Ocul Pharmacol Ther201430646847524738883
- LeskeMCHeijlAHusseinMFactors for glaucoma progression and the effect of treatment: the early manifest glaucoma trialArch Ophthalmol20031211485612523884
- DiestelhorstMLarssonLIEuropean Latanoprost Fixed Combination Study GroupEuropean latanoprost fixed combination study group. A 12 week study comparing the fixed combination of latanoprost and timolol with the concomitant use of the individual components in patients with open angle glaucoma and ocular hypertensionBr J Ophthalmol200488219920314736774
- DiestelhorstMLarssonLIEuropean-Canadian latanoprost fixed combination study groupA 12-week, randomized, double-masked, multicenter study of the fixed combination of latanoprost and timolol in the evening versus the individual componentsOphthalmology20061131707616263174
- SchumanJSKatzGJLewisRAEfficacy and safety of fixed combination of travoprost 0.004%/timolol 0.5% ocular hypertensionAm J Ophthalmol2005140224225016086946
- HughesBABacharachJCravenERA three-month, multicenter, double-masked study of the safety and efficacy of travoprost 0.004%/timolol 0.5% ophthalmic solution compared to travoprost 0.004% ophthalmic solution and timolol 0.5% dosed concomitantly in subjects with open angle glaucoma or ocular hypertensionJ Glaucoma200514539239916148589
- NucciCVaresiCMartucciAEfficacy of timolol 0.1% gel and a prostaglandin analog in an unfixed combination compared to the corresponding fixed combinationsEur J Ophthalmol201323568368923640513
- QuarantaLBiagioliERivaIProstaglandin analogs and timolol-fixed versus unfixed combinations or monotherapy for open-angle glaucoma: a systematic review and meta-analysisJ Ocul Pharmacol Ther201329438238923231442
- KitazawaYAzumaITsukaharaSKomemushiSClinical evaluation of Timolol GS once-a-day ophthalmic solution in primary open-angle glaucoma and ocular hypertension. Phase III comparative study with Timolol twice-a-day ophthalmic solution as control drugJ Eye (Atarashii Ganka)1996131143154 Japanese
- RolleTCurtoDAlovisiCFranzoneMBrogliattiBGrignoloFMTimogel vs timolol 0.5% ophthalmic solution: efficacy, safety, and acceptanceEur J Ophthalmol2012221283322167540
- EastyDLNemeth-WasmerGVounatsosJPComparison of a non-preserved 0.1% T-Gel eye gel (single dose unit) with a preserved 0.1% T-Gel eye gel (multidose) in ocular hypertension and glaucomatous patientsBr J Ophthalmol200690557457816622086
- ÖzyolEÖzyolPThe efficacy of a latanoprost/timolol fixed combination versus latanoprost and timolol gel-forming solution unfixed combination on daytime intraocular pressureJ Glaucoma201625213513925264990
- UedaKTonouchiAFukanoYAsadaHKawazuKTafluprost 0.0015%/timolol 0.5% combination ophthalmic solution (DE-111 ophthalmic solution) formulation design and intraocular penetration in ratsJ Eye (Atarashii Ganka)2013301217611766 Japanese
- FuwaMUedaKAkaishiTAdvantages of efficacy and safety of fixed-dose tafluprost/timolol combination over fixed-dose latanoprost/timolol combinationPLoS One2016117e015879727383260
- Moreno-MontañésJMartínez-de-la-CasaJMSabaterALMorales-FernandezLSáenzCGarcia-FeijooJClinical evaluation of the new rebound tonometers Icare PRO and Icare ONE compared with the Goldmann tonometerJ Glaucoma201524752753224844537