Abstract
Purpose
Aqueous levels of vascular endothelial growth factor (VEGF) can be a surrogate marker of intraocular VEGF activity and a measure of efficacy of anti-VEGF treatment in a variety of vasoproliferative retinal disorders, including diabetic retinopathy, age-related macular degeneration, and central retinal vein occlusion. Measurement of the VEGF level may be adversely affected by premeasurement variables, such as freezing and delay, in sample analysis. We aim to evaluate the effect of storage and delayed measurement of human aqueous VEGF levels in these conditions.
Methods
Aqueous samples collected from patients receiving intravitreal injection of bevacizumab for various retinal diseases were divided into two groups. In Group 1, the VEGF levels were analyzed on the same day; in Group 2, the VEGF levels were analyzed after 21 days of freezer storage (−80°C) using immunobead assay. Statistical comparison using a paired t-test was performed between the two groups.
Results
Thirty-one aqueous humor samples were collected, and the VEGF concentration for fresh samples was 7.8 ± 5.9 pg/mL (mean ± SD) compared to 6.5 ± 6.0 pg/mL in frozen samples, resulting in a statistically significant difference (P = 0.03).
Conclusions
Accurate measurement of the VEGF level is a vital component of clinical decision-making. Delayed analysis of VEGF levels in aqueous samples may result in significant sample degradation and lower levels of measured VEGF.
Introduction
Vascular endothelial growth factor (VEGF), an endothelial cell mitogen, is implicated as a key factor in angiogenesis and increases vascular permeability.Citation1,Citation2 Increased levels of VEGF are observed in aqueous and vitreous humor in several ischemic ocular conditions, including diabetic retinopathy, retinal vascular occlusions, and age-related macular degeneration. As such, VEGF levels correlate with the severity of the disease process.Citation3–Citation10 Accurate assessment of VEGF in aqueous samples can be a useful tool in assessing the severity of disease, as well as in titrating the dosage of anti-VEGF therapy.
The most widely used enzyme-linked immunosorbent assay (ELISA) that measures VEGF levels in the aqueous samples analyzes one cytokine and requires a sample volume of at least 100 μL for detection of proteins such as VEGF.Citation11 Recent cytometric bead immunoassay measurement of VEGF levels in aqueous samples has shown superior accuracy in smaller volumes.Citation6,Citation12 Use of bead-based immunoassay is more accurate and cost effective than use of ELISA in measuring VEGF levels.Citation13
Aqueous samples are typically frozen at −80°C (a common clinical practice) if immediate measurement of cytokine is not feasible. Extrinsic factors, such as temperature, can alter the measurement of cytokine levels in biological fluids after storage.Citation14,Citation15 In this study, we compared the VEGF levels estimated in a fresh sample with VEGF levels stored for 21 days at −80°C and evaluated the effect of freezing on sample degradation using bead-based immunoassay.
Methods
Sample collection
The study samples were obtained from 31 patients (mean age ± SD, 73.87 ± 9.29 years; male/female, 15/16) who were receiving intravitreal injection of bevacizumab for various retinal diseases. Of these, 18 patients had macular degeneration, ten had cystoid macular edema, and three had diabetic macular edema. One hundred microliters of aqueous humor samples were obtained from the eye before administration of intravitreal injection. All samples were obtained with informed consent from the patient and approved by the institutional review board at the University of Florida.
Each sample was aliquoted into two subgroups. VEGF analysis was performed on the same day on samples that obtained from participants in Group 1. Samples from Group 2 were stored in a −80°C freezer and analyzed after 3 weeks (21 days). All samples were stored in 1.5-mL self-standing screw cap polypropylene tubes (DNAse, RNAse, and pyrogen free). The samples that were processed on the same day were referred as ‘fresh’, and the samples stored at −80°C were referred as ‘frozen’.
Usage of immunobead assay for VEGF analysis
Both fresh and frozen samples were handled by the same experienced researcher. Samples were processed for VEGF analysis at room temperature (RT) using immunobead assay by Luminex 100™ IS fluoroanalyzer (Tepnel Life Sciences, San Diego, CA). Standards were reconstituted and serially diluted as per manufacturer’s instructions (Invitrogen Corporation, Carlsbad, CA). To each well of 96-well microtiter plates, 25 μL of polystyrene microbeads and wash solutions were added and incubated for 30 sec. After the incubation time, the supernatant was washed off using vacuum manifold, followed by the addition of incubation buffer and 50 μL each of sample and assay diluent. Diluted standards were added to the designated wells and the plate was incubated for 2 h at RT on an orbital shaker at 250–500 rpm. After incubation, the liquid was removed and the excess beads were washed off. Furthermore, 100 μL of detector antibody was added to each well and incubated on an orbital shaker at RT for 1 h. Excess detector antibodies were removed, followed by the addition of substrate complex (Streptavidin R-Phycoerythrin [S-RPE]), and the fluorescence was measured using Luminex 100 IS fluoroanalyzer after 30 min of incubation at RT. Both negative and positive controls were included on each microtiter plate for each reaction. To ensure proper assay performance, a known concentration of human recombinant VEGF was included in each run (positive control).
Analysis of VEGF concentration
We measured the mean fluorescence of associated S-RPE with 100 beads that were bound to the VEGF in the sample. The resulting data was interpreted by curve-fitting algorithm using InStat data analysis software (GraphPad, San Diego, CA). VEGF levels were estimated based on the intensity of emitted fluorescence in each sample. Sample concentrations were expressed as pg/mL based on standard curves plotted using protein standards supplied with the kits (minimal detectable limit is 5.7 pg/mL; Invitrogen Corporation). The effect of dilution was corrected in determining final values.
Statistical analysis
Statistical analysis was done using GraphPad InStat and Minitab 13 (Minitab, State College, PA) statistical packages. A paired t-test was used to compare the VEGF concentration between the fresh and the frozen samples. The level of statistical significance was set at P < 0.05.
Results
In our study, we analyzed 31 fresh and frozen aqueous humor samples to observe the difference in VEGF concentration. Using this immunobead assay, we were able to detect the VEGF levels in all the samples (). The mean VEGF concentration in the fresh samples was 7.8 ± 5.9 pg/mL (mean ± SD; range: 1.12–25.4 pg/mL) compared with 6.5 ± 6.0 pg/mL (range: 0.60–23.74 pg/mL) for the frozen samples (average of 17% reduction after freezing for 21 days).
Table 1 VEGF levels in aqueous measured fresh and after storage at −80°C for 21 days
Of the 31 samples analyzed, 24 samples showed more than 10% variation in VEGF levels after storage at −80°C compared with the fresh samples. Nineteen samples showed decreased VEGF levels and 12 samples showed increased VEGF levels after storage at −80°C. The observed difference was statistically significant in frozen samples in comparison with VEGF concentration in the fresh samples (P = 0.03; ). shows the fresh and frozen aqueous individual and mean VEGF levels based on the clinical diagnoses.
Figure 1 Comparative analysis of vascular endothelial growth factor (VEGF) concentration between fresh and frozen samples.
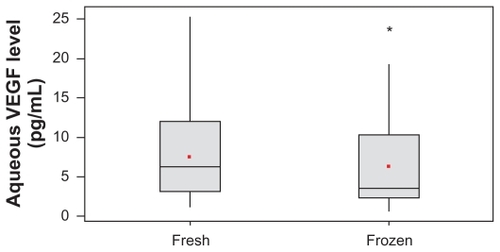
Table 2 Vascular endothelial growth factor (VEGF) levels in aqueous samples measured fresh and after storage at −80°C for 21 days based on clinical diagnosis
Discussion
Anti-VEGF therapy, in the form of intravitreal injection, is the mainstay of therapy in the management of ischemic ocular conditions. VEGF levels in ocular fluids provide indirect evidence of severity of disease process and help in monitoring the therapeutic effects of anti-VEGF agents. Vitreous levels of secreted proteins or drugs have been used as a surrogate for retinal and choroidal levels, but vitreous taps or biopsies are not risk free and are usually obtained from patients undergoing surgery for different reasons. The use of an anterior chamber tap, at the time of intravitreal injection, to decompress the vitreous cavity has been found to be well tolerated. Studies are evaluating the potential usefulness of incorporating anterior chamber taps into clinical trials for measurement of molecular targets and drug levels (personal communication, DRCR.net).
Traditionally, ELISA is used to measure VEGF levels in the aqueous samples. Bead-based assay is fast replacing ELISA for use in laboratories to quantify cytokines in biological samples, such as serum, as bead-based assay is quicker to perform and is more efficient and cost effective.Citation11,Citation12 The increased surface area provided by the microbeads significantly reduces the required sample volume. In addition, the sensitivity of the test is superior because fluorescence, rather than colorimetric measurement, is utilized. In our study, all the VEGF measurements were conducted using bead-based assay with standard VEGF concentrations as a positive control, which enhanced the validity of our results.
Recently, numerous studies evaluated the correlation between VEGF levels in the aqueous samples and severity of ocular ischemic process.Citation3,Citation16–Citation18 The next step after finding an appropriate correlation is to test whether the levels could be used to monitor the disease activity and response to treatment.Citation15,Citation16 Cytokines are usually not considered to be very stable after collection, which might artificially interfere with test results.Citation15 Detected levels of cytokines may be affected by methods of storage or repeated freezing–thawing.Citation14
However, there have been no studies evaluating the effect of storage on measurement of VEGF levels in aqueous samples. Our study is the first of its kind to test the stability of VEGF in aqueous humor samples and provides evidence of variation in VEGF measurement after 3 weeks of storage. This is consistent with results reported by Rosenling et alCitation19 who observed a significant change in proteome-derived peptide profile in cerebrospinal fluid after storage for a week or 1 month, and with those of Aziz et alCitation20 who reported a significant change in tumor necrosis factor alpha levels in plasma after storage, at 4°C or at −70°C for 20 days in comparison to samples stored at 24°C. This study’s results are also consistent with earlier study results on fresh versus frozen urine sample peptide concentration.Citation21
We can only speculate as to the mechanism of variation in VEGF measurements in aqueous samples. One possible mechanism is the presence of different proteases in the aqueous sample, which could cause a true decrease in VEGF measurement.Citation22 Another possible mechanism could be the result of alteration of the binding of detector antibody used during the assay, possibly from degradation, and a change in configuration of amino acids in VEGF protein due to delayed measurements.Citation19 The presence of soluble VEGF receptors in aqueous samples and the possibility of alteration in protein folding after freezing can increase the competitive binding affinity of other VEGF isoforms and increase VEGF levels observed in some samples.
In conclusion, this pilot study showed a statistically significant decrease in VEGF levels after storage for 3 weeks. Estimation of VEGF immediately after sample collection is recommended for accuracy.
Disclosure
The authors report no conflicts of interest in this work.
References
- ShweikiDItinASofferDKeshetEVascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesisNature199235963988438551279431
- DvorakHFBrownLFDetmarMDvorakAMVascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesisAm J Pathol19951465102910397538264
- AdamisAPMillerJWBernalMTIncreased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathyAm J Ophthalmol199411844454507943121
- HeraRKeramidasMPeoc’hMMouillonMRomanetJPFeigeJJExpression of VEGF and angiopoietins in subfoveal membranes from patients with age-related macular degenerationAm J Ophthalmol2005139458959615808152
- Pe’erJFolbergRItinAGnessinHHemoIKeshetEVascular endothelial growth factor upregulation in human central retinal vein occlusionOphthalmology199810534124169499769
- FunkMKriechbaumKPragerFIntraocular concentrations of growth factors and cytokines in retinal vein occlusion and the effect of therapy with bevacizumabInvest Ophthalmol Vis Sci20095031025103219060280
- FunatsuHYamashitaHNomaHAqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patientsGraefes Arch Clin Exp Ophthalmol200524313815258777
- RohMIKimHSSongJHLimJBKohHJKwonOWConcentration of cytokines in the aqueous humor of patients with naïve, recurrent and regressed CNV associated with AMD after bevacizumab treatmentRetina200929452352919262441
- RohMILimSJAhnJMLimJBKwonOWConcentration of cytokines in age-related macular degeneration after consecutive intravitreal bevacizumab injectionGraefes Arch Clin Exp Ophthalmol2010248563564019997926
- LaiTYLiuDTChanKPLukFOPangCPLamDSVisual outcomes and growth factor changes of two dosages of intravitreal bevacizumab for neovascular age-related macular degeneration: a randomized, controlled trialRetina20092991218122619934816
- ElshalMFMcCoyJPMultiplex bead array assays: performance evaluation and comparison of sensitivity to ELISAMethods200638431732316481199
- YoungSHAntoniniJMRobertsJRErdelyADZeidler-ErdelyPCPerformance evaluation of cytometric bead assays for the measurement of lung cytokines in two rodent modelsJ Immunol Methods20083311–2596818089291
- ChalamKVBalaiyaSMurthyRKAccurate estimation of vascular endothelial growth factor levels in microsamples with a low-cost bead-based assayRetina201030581581920084052
- FlowerLAhujaRHHumphriesSEMohamed-AliVEffects of sample handling on the stability of interleukin 6, tumour necrosis factor-alpha and leptinCytokine200012111712171611052823
- FriebeAVolkHDStability of tumor necrosis factor alpha, interleukin 6, and interleukin 8 in blood samples of patients with systemic immune activationArch Pathol Lab Med2008132111802180618976019
- CampochiaroPAChoyDFDoDVMonitoring ocular drug therapy by analysis of aqueous samplesOphthalmology2009116112158216419700195
- AielloLPAveryRLArriggPGVascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disordersN Engl J Med199433122148014877526212
- WellsJAMurthyRChibberRLevels of vascular endothelial growth factor are elevated in the vitreous of patients with subretinal neovascularisationBr J Ophthalmol19968043633668703891
- RosenlingTSlimCLChristinCThe effect of preanalytical factors on stability of the proteome and selected metabolites in cerebrospinal fluid (CSF)J Proteome Res20098125511552219845411
- AzizNNishanianPMitsuyasuRDetelsRFaheyJLVariables that affect assays for plasma cytokines and soluble activation markersClin Diagn Lab Immunol19996189959874670
- FiedlerGMBaumannSLeichtleAStandardized peptidome profiling of human urine by magnetic bead separation and matrix-assisted laser desorption/ionization time-of-flight mass spectrometryClin Chem200753342142817272489
- RichardsonMRPriceMOPriceFWProteomic analysis of human aqueous humor using multidimensional protein identification technologyMol Vis2009152740275020019884