Abstract
Purpose
To investigate the safety and efficacy of selective laser trabeculoplasty (SLT) to reduce intraocular pressure (IOP) in patients with pseudoexfoliation glaucoma (PXFG) compared with primary open-angle glaucoma (POAG).
Design
Non-randomized, prospective, clinical trial.
Methods
Nineteen eyes of 13 patients with POAG and 18 eyes of 13 patients with PXFG were treated with SLT. Patients were followed without antiglaucoma medications until additional medical, laser, or surgical intervention was initiated, at which time they were considered failures, had withdrawn from the study, or underwent a second SLT.
Results
The POAG and PXFG eyes showed similar reductions of IOP over the 49 months of follow-up. At 30 months of follow-up the POAG group showed a mean IOP of 17.6 ± 2.8 mmHg and a mean IOP reduction of 5.7 ± 2.1 mmHg; the PXFG group showed a mean IOP of 18.3 ± 4.7 and a mean IOP reduction of 5.3 ± 3.0 mmHg. Four eyes in the PXFG group and three eyes in the POAG group failed by 30 months. The cumulative probability of success was 74% for the PXFG group and 77% for the POAG group. Four PXFG eyes underwent a second SLT after 30 months of follow-up with a final IOP of 17.6 ± 2.8 mmHg. There were no serious adverse events.
Conclusion
SLT is a safe and effective method to lower IOP in patients with PXFG as initial glaucoma therapy. Both groups showed similar IOP reductions and failure rates.
Introduction
The early work of Worthen and WickhamCitation1,Citation2 established that a continuous-wave argon laser could be used safely to photocoagulate the trabecular meshwork, with significant decreases in intraocular pressure (IOP) that were maintained over a period of months. Several years later, Wise and Witter used lower laser energy in the procedure known as argon laser trabeculoplasty (ALT). They reported a successful reduction of IOP in a population of patients who had failed medical therapy.Citation3
The Glaucoma Laser Trial (GLT)Citation3–Citation6 demonstrated that initial treatment with ALT was shown to have a greater pressure-lowering effect than medication (timolol maleate 0.5%) at 2 years of follow-up. The GLT study indicated that initial treatment with ALT in patients with primary open-angle glaucoma (POAG) was at least as effective as intervention with timolol.Citation6
Latina and ParkCitation7 demonstrated that a 532 nm Q-switched Nd:YAG laser with nanosecond pulse duration is capable of selectively targeting pigmented trabecular meshwork cells without collateral thermal damage to the adjacent nonpigmented trabecular meshwork cells and underlying trabecular beams.
A pilot study evaluating the IOP-lowering effect of selective laser trabeculoplasty (SLT) in 53 patients whose IOP could not be controlled by medications or who had failed traditional ALTCitation8 demonstrated the safety and efficacy of SLT. The patients were followed up for 26 weeks, and a mean IOP reduction of 18.7% (4.6 mmHg) was seen with minimal adverse effects. Furthermore, 66% of patients who had previously failed ALT showed an average of 5.9 mmHg IOP reduction without any significant adverse effects.
The authors embarked on a prospective study to establish the safety and effectiveness of SLT as initial therapy to reduce IOP in patients with POAG and pseudoexfoliation glaucoma (PXFG).
Materials and methods
Patients with POAG or PXFG were considered eligible for this study if they met the following entry criteria: 1) were 18 years of age or older; 2) had two sighted eyes; 3) had one of the following in the eye to be treated (a) uncontrolled POAG or (b) PXFG; 4) had no prior medical, laser, or surgical therapy for treatment of POAG; 5) would be considered a candidate for laser trabeculoplasty; and 6) were willing to provide written informed consent.
Patients with the following findings were excluded from the study: 1) those with any of the following in the eye to be treated (a) evidence of glaucoma other than POAG (eg, angle closure, inflammatory, or neovascular), (b) any ocular condition precluding adequate visualization and treatment of the trabecular meshwork, (c) prior treatment with medical therapy, glaucoma surgery, or laser trabeculoplasty other than peripheral iridotomy; 2) any evidence of mental impairment that precluded the patient from understanding the protocol or the inability to sign an informed consent; 3) pregnancy; 4) potential need for other ocular surgery within 6 months of initial treatment; and 5) potential need for treatment with systemic steroids or having a concurrent condition warranting treatment with systemic steroids.
Patients were recruited by Mark A Latina (Reading Health Center, Reading, MA, USA) and Jan Smith (Oslo Eye Institute, Oslo, Norway). The study was approved by the Institutional Review Boards at the participating institutions.
A full ophthalmologic examination of both eyes was conducted 2–6 weeks prior to treatment (first screening examination), which included best corrected visual acuity, two readings of IOP determinations by Goldmann applanation tonometry, slit lamp examination of the anterior segment (conjunctival injection; cornea, iris, and lens appearance; presence of anterior chamber cells and flare), gonioscopy (grading of pigmentation, presence of peripheral anterior synechiae, and evaluation of angle opening), fundus examination (including evaluation of cup:disc ratio and pallor), visual field examination, and the recording of glaucoma medications (type, dosage, and date, and time of last medication administration). Patient demographic information (sex and date of birth) and medical history, including previous ocular surgery, were also recorded.
Each investigator used his or her clinical judgment to determine which eye would be treated based on current IOP and general ocular and visual status of the eye.
The procedure was performed with topical proparacaine for anesthesia. Laser treatment was performed with a Coherent Selecta 7000 Frequency Doubled Nd:YAG ophthalmic laser (Coherent, Inc, Palo Alto, CA, USA). The laser is a frequency-doubled Q-switched Nd:YAG laser that delivers 532 nm wavelength of laser light at a pulse duration of 3 nanoseconds and a spot size of 400 microns. The pulse energy ranges from 0.1 to 1.2 mJ/pulse.
A Goldman three-mirror goniolens was placed on the eye with methylcellulose 1%. The nasal 180 degrees was treated in all eyes. To determine the optimum energy level for SLT for each eye, the laser energy was initially set at 0.8 mJ, and then the energy level was increased by 0.1 mJ until a microcavitation bubble formation was observed. Treatment was then continued in a single pulse mode at the treatment energy level until 50 ± 10 contiguous but nonoverlapping 400 μm laser spots were created along 180 degrees of the nasal side of the trabecular meshwork. Bubble formation was monitored with each pulse. The total number of pulses delivered and the total amount of laser energy delivered were recorded.
Immediately after laser treatment, at the discretion of the treating physician, prednisolone acetate (1%) drops were administered and continued in the treated eye three to four times daily for 3 to 4 days. The IOP in the treated eye was assessed and recorded 1 hour postoperatively. If there was an IOP rise greater than 5 mmHg from the immediate preoperative IOP, the elevation of the IOP was treated with appropriate antiglaucoma medications and recorded. The presence and level of cells in the anterior chamber were recorded. Any immediate postoperative complications were recorded.
Patients were evaluated at 3 weeks, 3 months, 6 months, 9 months, and 15 months, with additional follow-up at 3-month intervals for up to 42 months. At each postoperative visit, the best corrected visual acuity and IOP were measured, and slit lamp examination was performed (noting for conjunctival injection; anterior chamber cell and flare; cornea, lens, and iris appearance). All major and minor complications and complaints were recorded and treated appropriately.
All ocular medications were recorded before surgery and at each subsequent visit. Medication changes or adjustments made at the physician’s discretion were recorded. Patients that were started on antiglaucoma medications following SLT were considered failures and removed from the study.
Success/failure criteria
The primary outcome of this clinical trial was to evaluate the IOP response to SLT in patients without requiring any antiglaucoma medications or additional laser or surgical therapy. Eyes were considered successes as long as the IOP did not return to baseline (<3 mmHg) and/or no further medical, laser, or surgical intervention for the treatment of glaucoma was initiated. Eyes were included in the analysis of each time interval until an intervention occurred, at which point they were considered failures and removed from subsequent follow-up periods. Patients were excluded from analysis at any subsequent follow-up interval if they underwent cataract surgery or failed to reach the specified follow-up exam interval.
Analysis
A Student’s t-test for paired data was used to assess the changes in IOP from baseline values and differences between the groups.
Results
A total of 37 eyes in 26 patients were treated with SLT. Nineteen eyes in 13 patients had the diagnosis of POAG, and 18 eyes in 13 patients had the diagnosis of PXFG. The POAG group consisted of seven males (53.8%) and six females (46.2%) with an average age of 71 years. The PXFG group consisted of nine females (69.2%) and four males (30.8%) with an average age of 74 years. Patients in the POAG group were followed for an average of 27.1 months and 20.4 months in the PXFG group. The demographic attributes, baseline characteristics, and treatment parameters are shown in .
Table 1 Demographics and baseline characteristics
After the 3-month follow-up visit, patients returned at various time intervals with up to 49 months of follow-up. For this reason, analysis of IOPs was performed on the last IOP taken with the time intervals 9–15 months post-treatment, 21–27 months post-treatment, and 30 months or more post- treatment, which allowed at least one IOP measurement for each patient within the specified time interval.
As shown in , , and , the POAG and PXFG groups showed statistically significant mean IOP reductions from baseline at each time interval. At 30–42 months of follow-up, the mean IOP for the POAG group (17.6 ± 2.8 mmHg) was slightly lower than the PXFG group (18.3 ± 4.7 mmHg) (30–32 months follow-up), although this was not significant.
Figure 1 Mean IOP following SLT in patients with POAG and PXFG.
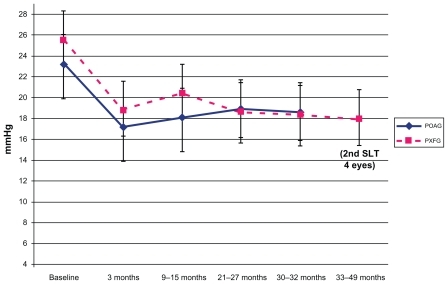
Table 2 IOP reduction from SLT when used as primary therapy for patients with POAG (n = 19 eyes from 13 patients)
Table 3 IOP reduction from SLT when used as primary therapy for patients with PXFG glaucoma (n = 18 eyes from 13 patients)
As noted in , four eyes underwent a second SLT procedure in the PXFG group after the 30- to 32-month follow-up period. All of these eyes remained off medications by the 30- to 32-month follow-up period and were considered successes at that time period. One eye received a second SLT at 32 months and was followed for an additional 12 months.
Two of the eyes received SLT at 32 months with an additional 3 months of follow-up, and one eye at 42 months with follow-up for up to 49 months. The mean IOP at the last follow-up visit for all 10 PXFG eyes without antiglaucoma medications and having undergone either one or two SLT treatments was 17.9 ± 2.1 mmHg with a mean IOP reduction of 5.5 ± 3.3 mmHg.
and demonstrate the number of eyes followed at each time interval, the number of eyes that were determined to be failures, and the number of eyes withdrawn due to loss to follow-up or another intervention such as cataract surgery, which removed them from further follow-up (). The number of eyes reported in and at each time interval represent those eyes that reached the follow-up interval without having been withdrawn at an earlier time period due to failure or another intervention such as cataract surgery.
Figure 2 Kaplan-Meier survival curves for all patients, plotting the cumulative probabilities against time that the IOP did not return to baseline (<3 mmHg) and/or no further medical, laser, or surgical intervention for the treatment of glaucoma was initiated following SLT.
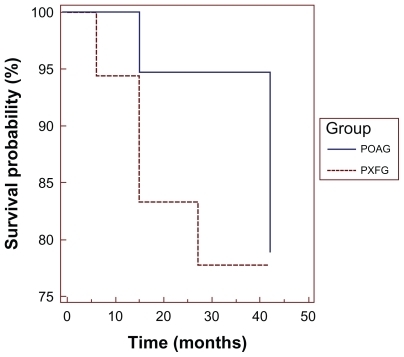
Table 4 Kaplan-Meier life table analysis of time to failure after SLT POAG group without medications
Table 5 Kaplan-Meier life table analysis of time to failure after SLT PXFG group without medications
By 30 months of follow-up, four eyes (22%) in the PXFG group and three eyes (16%) in the POAG group required initiation of medical or additional laser therapy and were classified as failures. As shown in and , following initial therapy with SLT, the probability of successfully remaining off medications without a second SLT at 30 months is 77% for the POAG group and 74% for the PXFG group. It should be noted that 3/4 eyes failed within the first 9–15 months in the PXFG group with a cumulative probability of success of 0.83 compared with the POAG eyes, which tended to fail by the 30-month follow-up period.
In the PXFG group, one eye required further surgical intervention at 6 months, although the IOP reduction following SLT was greater than 3 mmHg. Two eyes were started on antiglaucoma medications at the 9–15 months post-treatment interval because the IOP returned to baseline. At the 21-month follow-up period, one eye underwent ALT and was considered a failure.
A total of six eyes in the PXFG group were withdrawn up to the 30- to 32-month follow-up period for the following: one eye underwent cataract surgery before the 9th month follow-up period and was removed from the study; two eyes were not followed for longer than 16 months and one eye underwent cataract surgery at 17 months (these were considered successes, although they were not included in the follow-up periods beyond the 9–15 month period); and two eyes did not have IOPs measured at the 30-month follow- up period but remained off medications until 42 months, at which time one of the eyes underwent an additional SLT treatment and the other eye was followed for 45 months. For this reason, eight eyes are reported at 30–32 months without medications. However, 10 eyes are reported beyond the 30- to 32-month follow-up period.
In the POAG group, failures were accounted for as follows: medical therapy was initiated in one eye at 9 months and in one eye following the 21-month follow-up period and one eye underwent ALT at 30 months and was removed from the study. A total of five eyes were withdrawn from follow-up over the course of the study for the following: one patient was followed for 12 months; two eyes were followed for 17 months; one patient had an iridotomy at 18 months and was removed from further follow-up; and one eye underwent cataract surgery at 22 months and was removed from the study. The remaining 11 patients were followed for at least 30 months without antiglaucoma medications.
There were no significant adverse events in any patients studied other than 1 hour postoperative pressure spikes, which responded to medical treatment with Iopidine® (Alcon, Forth Worth, TX, USA). In the PXFG group, 5 of 18 patients (28%) showed an IOP spike of greater than 5 mmHg, although only one patient (5.5%) showed an IOP spike of greater than 10 mmHg. In the POAG group, only 4 of 19 patients (21%) showed a postoperative IOP spike of greater than 5 mmHg, and none showed a postoperative IOP spike greater than 10 mmHg. None of the patients received preoperative treatment with antiglaucoma medications.
Discussion
This study was designed to determine the effects of SLT on IOP reduction in patients with PXFG glaucoma compared with POAG when used as initial or primary therapy. The results of this clinical trial demonstrate that SLT is a safe and effective method in lowering IOP, with mean IOP reductions of 5.7 mmHg for the POAG group and 5.3 mmHg for the PXFG group over a follow-up period of 30 months.
The magnitude of the IOP reduction achieved in this study was similar to the IOP reductions we achieved in our initial multicenter pilot clinical study to investigate the IOP-lowering effects of SLT in patients on maximum medical therapy. In that study, the mean IOP reduction at 6 months was 5.8 mmHg in those patients who responded to SLT, with at least a 3 mmHg IOP reduction. In a longer-term randomized prospective clinical study comparing the IOP effects of SLT with ALT in patients with POAG on medications, Damji et alCitation9 found a mean IOP reduction of 6.5 mmHg at 12 months in those patients treated with SLT over 180 degrees. Eyes treated with ALT achieved an IOP reduction of 6.03 mmHg, which was not statistically different from the SLT-treated eyes.
In this study, the cumulative probability of success for patients with either POAG or PXFG to remain off medications for 2.5 years following SLT was approximately 75%. This result compares favorably with the GLT study, although the sample size in this study is relatively small and study conditions were not as rigid as the GLT study.Citation5,Citation6 Furthermore, patients received ALT over 360 degrees in the GLT study, whereas in this study the cumulative probability of success was reported only for SLT performed over 180 degrees.
The mean IOPs obtained in this study using SLT as initial therapy and probability of success also compared favorably with a 10-year follow-up study of the long-term efficacy of ALT by Shingelton et al.Citation10 They reported a mean IOP at 30 months of 18.1 ± 3.8 mmHg and demonstrated a cumulative probability of success of 65% at 2.5 years following ALT treatment, where success was deemed as at least a 3 mmHg reduction of IOP from baseline, with stable visual field and nerve and no further laser or surgical intervention.
Comparing the rate of failure in the PXFG and POAG groups, this study suggests that eyes with PXFG fail sooner than eyes with POAG, which is similar to what is seen with ALT. Furthermore, a second SLT treatment was performed in four of the PXFG eyes. Although follow-up for these eyes was limited, all eyes responded with at least a 3 mmHg reduction of IOP and were maintained off medications for an additional 3–17 months.
In summary, the results of this study indicate that SLT is a safe and effective modality to treat eyes with both PXFG and POAG as initial therapy, with a 30-month cumulative probability of successfully remaining off medications of 70% without significant adverse effects. Although only a small number of patients underwent a second SLT in this study, those eyes were able to extend the duration off medications.
Further large-scale randomized clinical trials are indicated to demonstrate the repeatability of SLT and its long-term effectiveness in patients with POAG and PXFG.
Disclosure
Authors Shazly and Smith declare no conflicts of interest in relation to this paper. Mark A Latina has financial interest in SLT technology and receives patent royalties related to it.
References
- WorthenDMWickhamMGLaser trabeculotomy in monkeysInvest Ophthalmol Vis Sci197312707711
- WorthenDMWickhamMGArgon laser trabeculotomyTrans Am Acad Ophthalmol Otolaryngol197478371375
- WiseJBWitterSLArgon laser therapy for open-angle glaucoma: a pilot studyArch Ophthalmol1979197319322575877
- Glaucoma Laser Trial Research GroupAcute effects of argon laser trabeculoplasty on intraocular pressureArch Ophthalmol198930711351142
- Glaucoma Laser Trial Research GroupThe glaucoma laser trial, VI: treatment group differences in visual field changesAm J Ophthalmol199512010227611312
- Glaucoma Laser Trial Research GroupVII: The glaucoma laser trial (GLT) and glaucoma laser trial follow-up study resultsAm J Ophthalmol19951207187318540545
- LatinaMAParkCSelective targeting of trabecular meshwork cells: in vitro studies of pulsed and CW laser interactionsExp Eye Res1995603593727789416
- LatinaMASibayanSASliinDHNoeckerRJMarcellinoGQ-switched 532-nm Nd: YAG laser trabeculoplasty (selective laser trabeculoplasty)Ophthalmology1998105208220909818610
- DamjiKRShahKCRockWJBainsHSHodgeWGSelective laser trabeculoplasty v argon laser trabeculoplasty: a prospective randomised clinical trialBr J Ophthalmol19998371872210340983
- ShingeltonBJRichterCUDharmaSKLong-term efficacy of argon laser trabeculoplasty: a 10 year follow-up studyOphthalmology1993100132413298371919