Abstract
Purpose
We report the 2-year outcomes of intravitreal aflibercept (IVA) for exudative age-related macular degeneration (AMD) with good visual acuity (VA) and examine the baseline factors associated with good visual outcome.
Materials and methods
This multicenter, prospective study evaluated 39 eyes (39 AMD patients) enrolled from August 2013 to August 2014 at 12 and 24 months. Only patients with initial best-corrected VA (BCVA) >0.3 logarithm of the minimum angle of resolution (20/40 Snellen) were eligible. Three consecutive monthly IVA injections were followed by 2 monthly injections for 12 months. Thereafter, patients received injections on a treat- and-extend regimen for up to 24 months. Outcome measures included BCVA and central macular thickness (CMT) at 12 and 24 months. Post hoc analysis, BCVA, and CMT were evaluated by AMD types (typical AMD [tAMD], type 1, and type 2 polypoidal choroidal vasculopathy [PCV]). Baseline characteristics and BCVA associations were evaluated with linear regression analysis and Student’s t-test.
Results
Mean age was 69 years and 26 of 39 eyes were male. tAMD, type 1 and type 2 PCV occurred in 18, 12, and 9 eyes, respectively. Baseline mean BCVA was 0.097 logarithm of the minimum angle of resolution (20/25 Snellen) and showed significant improvement to 0.058 (20/22 Snellen, P=0.03) at 12 months and 0.066 (20/23) at 24 months. CMT improved significantly from 320 (99) µm (mean [SD]) to 250 (93) µm (P=0.002) at 12 months and 240 (93) µm (P=0.0005) at 24 months. BCVA and CMT were not significantly different among the three groups. Only subretinal hemorrhage (SRH) was significantly associated with improved BCVA. BCVA change from baseline was −0.12 with SRH and −0.011 without SRH (P=0.017) at 12 months.
Conclusion
IVA showed good efficacy for exudative AMD with good VA at 24 months. tAMD and type 1 and 2 PCV showed similar prognosis. Baseline SRH predicted favorable long-term vision in AMD with good VA.
Introduction
Age-related macular degeneration (AMD), the leading cause of blindness in developed countries,Citation1 has seen several changes in treatment modalities over time. The clinical course of AMD is unfavourable,Citation2 but treatment with anti-vascular endothelial growth factor (anti-VEGF) agents has improved visual acuity (VA), and better long-term prognosis for VA is expected.Citation3–Citation6
Aflibercept was approved in November 2012 for use in Japan and is reported to have long-term therapeutic effects.Citation4 Aflibercept is a recombinant fusion protein comprising two main components: vascular endothelial growth factor (VEGF)-binding portions from the extracellular domains of human VEGF receptors 1 and 2, which are fused to the fragment crystallizable portion of human immunoglobulin G1. This soluble decoy receptor acts by binding to VEGF-A and placental growth factor. Both factors are thought to be involved in the pathologic neovascularization and angiogenesis seen in ophthalmologic diseases such as AMD.Citation7 Aflibercept also binds VEGF-B, which may be involved in ophthalmologic disease. In the VIEW 1 and VIEW 2 studies, which assessed the efficacy and safety for treating AMD, intravitreal aflibercept (IVA) injections were administered once monthly or bimonthly after three administrations during the loading phase for 12 months and, as-needed regimen, but at least every 12 weeks dosing, from 12 to 24 months. Efficacy and safety were found to be similar to those of once-monthly intravitreal injections of ranibizumab.Citation4
Polypoidal choroidal vasculopathy (PCV) accounts for about half of all cases of AMD in East Asia.Citation8 Photodynamic therapy (PDT) is effective in PCV, but not in typical AMD (tAMD; AMD without PCV and retinal angiomatous proliferation).Citation9 Aflibercept is almost equally effective in tAMD and PCV.Citation10 Kawamura et al defined type 1 PCV as multiple polypoidal lesions around a network of vessels and type 2 PCV as a single polypoidal lesion without a network of vessels.Citation11–Citation13 Studies from other Japanese groups proved useful for predicting the treatment outcome of PDT, showing that PDT is not effective in type 1 PCV but effective in type 2 PCV.Citation14 Another report compares the short-term treatment outcomes between type 1 and 2 PCV in patients receiving anti-VEGF therapy.Citation15
There are, however, two important unanswered questions. First, there is the lack of evidence for the use of aflibercept in AMD patients with good VA. Notably, in real-world practice, a substantial proportion of AMD patients present with better VA than those included in the aforementioned clinical trials. Many AMD studies have focused on patients with a best-corrected visual acuity (BCVA) of 0.3 logarithm of the minimum angle of resolution (logMAR) or worse,Citation3–Citation6 and the studies that are available about AMD patients with good VA are retrospective in nature.Citation16–Citation18 Therapies with such a low level of evidence may not be recommended in the context of evidence-based medicine.Citation19 Second, is the classification of AMD (ie, tAMD and type 1 and type 2 PCV) useful in stratifying the risk of visual loss? If not, what other parameters are associated with visual outcome?
Thus, the aim of this prospective study was to assess the efficacy of aflibercept to treat AMD patients with good VA for 24 months. In a post hoc analysis, we also investigated the baseline factors associated with good visual outcomes at 12 months.
Materials and methods
Study design
This multicenter, prospective, non-randomized, interventional exploratory study was performed in an institutional setting.
Institutional review board approval
Institutional review board approval was obtained from Jichi Medical University (A13-23, B14-54) and the Japan Community Health Care Organization (JCHO) Tokyo Shinjuku Medical Center (H25-3, H.26-3).
Patients
Inclusion criteria were adults aged ≥50 years; AMD with exudative signs on indocyanine green (ICG) angiography, fluorescein angiography, or optical coherence tomography (OCT); treatment-naïve status; and initial BCVA over 0.3 logMAR (20/40 Snellen equivalent).
Exclusion criteria were intraocular surgery in the target eye within 3 months; history of vitreous surgery in the target eye; inflammation or infection in the intraocular, outer ocular area, or around either eye; history of uveitis in either eye; hypersensitivity or allergy to fluorescein or ICG; clinically significant drug allergy or known hypersensitivity to therapeutic or diagnostic protein products, or to any of the study drugs or their components; pregnancy, possibility of pregnancy, or lactation; and any history that might affect the interpretation of the results of the study or put the patient at an increased risk of treatment complications.
Primary endpoints were mean change of BCVA from baseline to 12 months and ratio of patients with improved BCVA at 12 months. Secondary endpoints were mean change of BCVA from baseline to 24 months and ratio of patients with improved BCVA at 24 months, mean change of retinal thickness in the central subfield on OCT from baseline to 12 and 24 months, ratio of patients with subretinal fluid at 12 and 24 months, and mean change of greatest linear diameter (GLD) of disease area. Further secondary endpoint of PCV was ratio of patients with regression of polypoidal lesions. Safety outcomes were adverse events and side effects of the procedure.
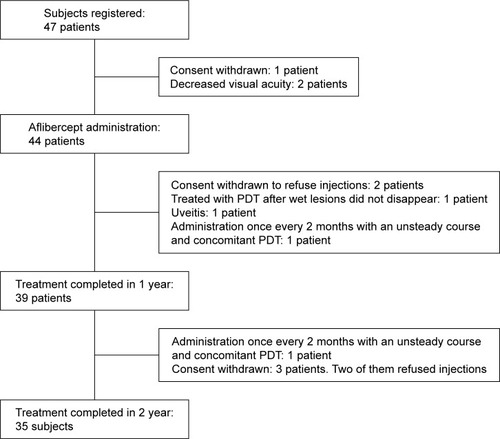
Subjects were 47 AMD patients (47 eyes) with BCVA >0.3 logMAR (). All subjects received their first IVA at either Jichi Medical University or JCHO Tokyo Shinjuku Medical Center between August 2013 and August 2014. Written informed consent was obtained from all patients. The study protocol adhered to the tenets of the Declaration of Helsinki and was registered in the University Hospital Medical Information Network Clinical Trials registry prior to study commencement (UMIN000011337 for the first year and UMIN000016598 for the second year). The protocol was based on the VIEW 1 and VIEW 2 studies.Citation4 First, IVA injections were administered once monthly for three consecutive months and bimonthly thereafter. Treatment continued for 12 months. Second, IVA injections were administered on treat-and-extend regimens from 13 to 24 months. On signs of recurrent choroidal neovascularization (CNV) activity (increased exudative change at any visit after 12 months), IVA was administered and the interval to the next visit was shortened by 1 month versus the last interval. Additionally, on signs of new subretinal hemorrhage (SRH) at any visit after 12 months, IVA was administered and the interval to the next visit was set to 1 month. If there was a minor exudative lesion (residual exudation), IVA was administered and the interval to the next visit remain unchanged. With no sign of recurrent CNV activity, IVA was administered and the interval to the next visit was extended by 1 month versus the last interval. Minimum dosing interval was 1 month, and maximum dosing interval was not regulated.
Among the 47 subjects, three dropped out before starting treatment (one withdrew consent and two experienced decreased VA and no longer satisfied the inclusion criteria). Also, five subjects dropped out after the third administration of the loading phase (one demonstrated no effects during the loading phase, withdrew consent, and received PDT; one showed insufficient effects after the first course of maintenance therapy, withdrew consent, and received PDT; one developed uveitis; and two withdrew consent and stopped IVA due to the high treatment costs). At 12 months, 39 subjects completed the study. Of these, four dropped out during the second year (two withdrew consent and stopped IVA due to high treatment costs; one withdrew only study consent and continued treat and extend; and one experienced worsening of exudative lesions with bimonthly injections, withdrew consent, and received PDT). At 24 months, 35 subjects completed the study. A flow diagram of the study is shown in .
Table 1 Enrolled patient characteristics
Measurements
The full analysis set was 39 subjects; four subjects dropped out during the second year. Their missing values up to 24 months were imputed using the last observation carried forward analysis.
On study commencement, we performed comprehensive ophthalmologic examination on all 39 subjects, including measuring decimal BCVA using Landolt rings and performing color fundus photography. Fluorescein angiography and ICG angiography were performed using a confocal scanning laser ophthalmoscope (HRA2; Heidelberg Engineering GmbH, Heidelberg, Germany) at Jichi Medical University and a fundus camera (TRC-50; TOPCON Corporation, Tokyo, Japan) at JCHO Tokyo Shinjuku Medical Center. Swept source OCT (DRI OCT-1 Atlantis, TOPCON) or spectral domain OCT (RS-3000 Lite; NIDEK Co, Ltd, Aichi, Japan) was performed at Jichi Medical University, and spectral domain OCT (Cirrus HD-OCT Model 4000; Carl Zeiss Meditec AG, Oberkochen, Germany) was performed at JCHO Tokyo Shinjuku Medical Center. Decimal BCVA assessment and dilated fundus examination were performed at every visit. Axial length was examined using A-mode ultrasonography (UD-6000; TOMEY Corp., Aichi, Japan).
Diagnosis of AMD was made by experienced retinal specialists using color fundus photography, fluorescein angiography, and ICG angiography. Fluorescein angiography and ICG angiography were done at baseline, 12 months, and 24 months after treatment. PCV was diagnosed based on the presence of characteristic polyp-like aneurysmal dilatations in the choroidal vessels in ICG angiography findings and the presence of orange-red protruding lesions on the ocular fundus in ophthalmoscopy findings. GLD was measured using fluorescein angiography.
We measured the central macular thickness (CMT) manually during OCT between the inner limiting membrane and Bruch’s membrane using an OCT measurement function. Bruch’s membrane was identified by the presence of a highly reflective line photographed under the retinal pigment epithelium in cases with subfoveal pigment epithelial detachment, and using the lower end of the highly reflective line in the retinal pigment epithelium in cases without pigment epithelial detachment.
Subanalysis
Eyes with multiple polypoidal lesions around a network of vessels on ICG angiography were set to type 1 PCV. Eyes with a single polypoidal lesion without a network of vessels were set to type 2 PCV.Citation11 Similarly, we assessed polyp regression on ICG angiography at 12 and 24 months. In this study, only two eyes had type 2 CNV. We could not perform statistical analysis on types 1 and 2 CNV.
Statistical analysis
Statistical analysis was performed using JMP Pro software version 11.2.0 (SAS Institute, Cary, NC, USA). Decimal BCVA was converted to logMAR. BCVA and CMT were compared with the first visit by single-sided paired t-test. Improvement of BCVA and CMT were compared between type 1 and type 2 PCV by double-sided t-test. Power analysis was performed, and the least significant number, which was needed for analysis of significance, was calculated. A P-value of <0.05 was considered statistically significant. The equality test of BCVA prognosis was calculated with logMAR 0.15 as clinically equivalent. Maximum value of an upper P-value and a lower P-value was used. Analysis of variance was used to evaluate the baseline characteristics of AMD and type 1 and type 2 PCV, except sex and GLD, which were analyzed by Fisher’s exact test and Kruskal–Wallis test, respectively. Since this research is an explanatory study, Bonferroni correction was not done.
Associations between the baseline factors and good visual prognosis were examined. Influence of baseline characteristics (age, axial length, logMAR BCVA, GLD, CMT and numbers of injections) for logMAR BCVA was evaluated by linear regression analysis. We also examined the baseline characteristics (sex, presence of posterior vitreous detachment, types of CNV, intraretinal fluid, subretinal fluid, SRH, pigment endothelial detachment, and hemorrhagic pigment endothelial detachment) by Student’s t-test. Correlations were evaluated between statistically significant baseline characteristics and awareness ratio of VA drop at the first visit to local ophthalmologists and SRH recurrences in 1 year.
Results
A total of 47 eyes in 47 subjects were analyzed (). At 12 months of treatment, we were able to analyze the clinical course of 39 eyes of 39 subjects (25 men and 14 women) of mean (SD) age 67 (9) years with an age range of 52–88 years. Mean CMT was 250 (93) µm. There were 18 eyes with tAMD (16 type 1 CNV and 2 type 2 CNV) and 21 eyes with PCV (12 type 1 PCV and 9 type 2 PCV). There were no significant differences between tAMD, type 1 PCV, and type 2 PCV in age, sex, BCVA, GLD, and CMT (). Representative PCV cases are shown in . BCVA at the start of treatment was 0.10 (0.11) logMAR. At the end of 2 years of treatment, the mean CMT was 240 (93) µm.
Table 2 Baseline characteristics of tAMD, type 1 PCV, and type 2 PCV
Figure 2 Indocyanine green angiogram of representative cases of type 1 and 2 PCV.
Abbreviation: PCV, polypoidal choroidal vasculopathy.
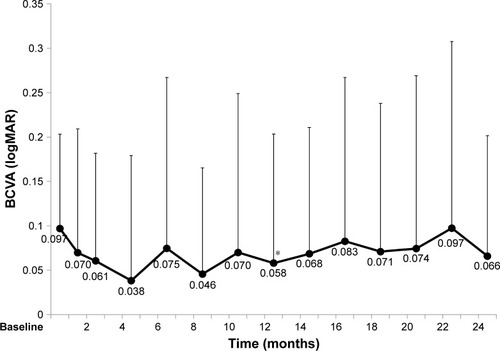
At 12 months, the BCVA was 0.06 (0.15) logMAR, indicating improvement (P=0.03; ). There were 5/39 patients (12.8%) in whom the BCVA improved by 0.2 logMAR or more, 34 patients (87.2%) in whom there was no change, and no cases in which there was worsening of 0.2 logMAR or more. At 24 months, the BCVA was 0.07 (0.14) logMAR, indicating no significant improvement (P=0.09).
Figure 3 BCVA over 2 years of treatment (mean, SD).
Abbreviations: BCVA, best-corrected visual acuity; IVA, intravitreal aflibercept; logMAR, logarithm of minimum angle of resolution.
Mean CMT after 12 and 24 months was 250 (93) µm (P=0.002) and 240 (93) µm (P=0.0005), indicating a significant reduction, respectively (). The mean number of aflibercept injections during the 12 months was 6.7±0.72 (39 cases) and from 13 to 24 months was 4.8±1.8 (35 cases). There was no significant difference in BCVA or CMT between the AMD cases with and without PCV ().
Figure 4 CMT over 2 years of treatment (mean, SD).
Abbreviation: CMT, central macular thickness.
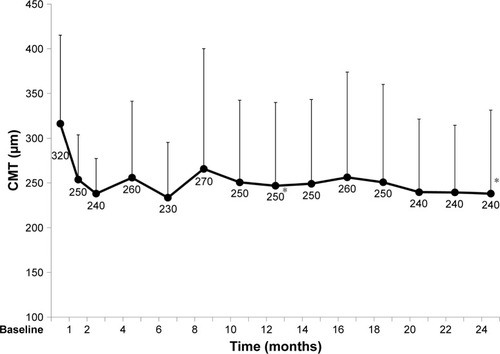
Figure 5 BCVA and CMT over 2 years of treatment in tAMD (tAMD [AMD] without PCV and retinal angiomatous proliferation) and PCV (mean, SD).
Abbreviations: AMD, age-related macular degeneration; BCVA, best-corrected visual acuity; CMT, central macular thickness; logMAR, logarithm of minimum angle of resolution; PCV, polypoidal choroidal vasculopathy; tAMD, typical age-related macular degeneration.
![Figure 5 BCVA and CMT over 2 years of treatment in tAMD (tAMD [AMD] without PCV and retinal angiomatous proliferation) and PCV (mean, SD).](/cms/asset/3216806e-e9a6-4408-a2df-5ce293d89367/doph_a_160961_f0005_c.jpg)
At 12 months, the polypoidal lesions had completely regressed in 12/21 PCV patients (57%), partially regressed in 2 (10%), and enlarged in 2 (10%). In type 1 PCV, the polypoidal lesions had completely regressed in 6/12 PCV patients (50%), partially regressed in 2 (17%), and enlarged in 2 (17%). In type 2 PCV, the polypoidal lesions had completely regressed in 6/9 PCV patients (67%). There was no statistical difference in the ratio of complete regression between type 1 and 2 PCV (P=0.66, Fisher’s exact test).
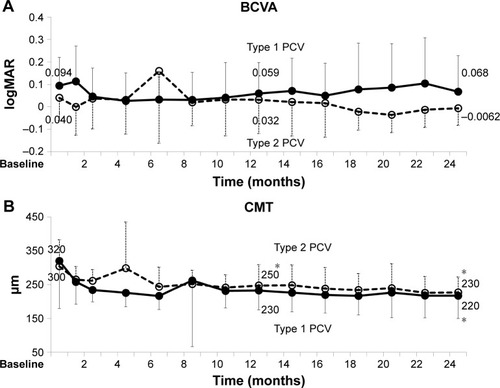
At 24 months, the polypoidal lesions had regressed completely in two additional type 1 PCV cases and were unchanged in type 2 CNV from 12 months. Ratio of complete regression between type 1 and 2 PCV (P=0.66, Fisher’s exact test) did not differ significantly. Mean 1- and 2-year prognosis of BCVA and CMT was −0.035 and −0.027 logMAR, 87 and 103 µm in type 1 PCV, 0.0086 and 0.046 logMAR, and 57 and 77 µm in type 2 PCV, respectively. There were no significant differences between type 1 and type 2 PCV in BCVA prognosis (P=0.29 and 0.79, least significant number: 72 at 1 year and 1,100 at 2 years, respectively) or CMT prognosis (P=0.56 and 0.68, respectively). It was rejected that there were over 0.15 logMAR differences between type 1 and type 2 PCV in terms of BCVA prognosis (P=0.024 and 0.043, respectively; ).
Figure 6 BCVA and CMT over 2 years of treatment in type 1 PCV and type 2 PCV (mean, SD).
Abbreviations: BCVA, best-corrected visual acuity; CMT, central macular thickness; logMAR, logarithm of minimum angle of resolution; PCV, polypoidal choroidal vasculopathy.
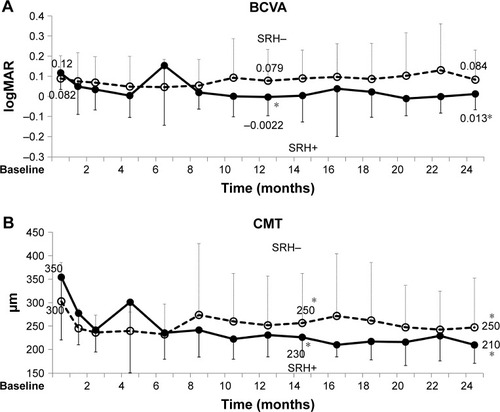
Baseline SRH was a significantly good factor for BCVA prognosis at 12 months. Change of logMAR BCVA at 12 months was −0.12 with SRH and −0.011 without SRH (P=0.017; ; ). Baseline logMAR BCVA correlated with the prognosis of logMAR BCVA at 12 months (R=−0.44, P=0.005). Other factors were not significant. Baseline SRH was correlated with the awareness ratio of VA drop at the first visit to local ophthalmologists (11/12 eyes with SRH, 16/35 eyes without SRH, P=0.006, Pearson’s chi-square test) and SRH recurrences in 1 year (mean: 1.6 versus 0.12 times, P<0.0001). AMD was pointed out in most patients without self-awareness by local ophthalmologists during the visits for common eye diseases, such as cataract or dry eye.
Table 3 Analysis of baseline characteristics and mean change of logMAR BCVA
Figure 7 BCVA and CMT over 2 years of treatment in eyes with and without baseline SRH (mean, SD).
Abbreviations: BCVA, best-corrected visual acuity; CMT, central macular thickness; logMAR, logarithm of minimum angle of resolution; PCV, polypoidal choroidal vasculopathy; SRH, subretinal hemorrhage.
Among the 44 subjects who started this study, three withdrew consent due to inadequate effects of monthly or bimonthly IVA and requested PDT combination therapy in anticipation of reducing the frequency of IVA. One patient had type 1 PCV and the others had type 1 CNV. All three patients did not receive IVA after PDT up to month 24, and only one CNV patient experienced VA loss over 0.15 logMAR.
The other four patients withdrew consent and stopped injections due to high costs. One patient had type 1 PCV, one had type 2 PCV, and two had type 1 CNV. Three of the four patients continued to visit the hospital up to month 24 and received IVA once in the second year, and one of the three patients experienced VA loss of over 0.15 logMAR.
One patient developed endophthalmitis 2 days after IVA at 24 months. However, treatment was successful and did not affect visual prognosis. No other serious adverse events were observed due to physical adverse drug reactions, such as cardiovascular events.
Discussion
In Japan, the treatment guidelines for AMD recommend anti-VEGF monotherapy for AMD patients with good BCVA.Citation11 However, there is no direct prospective evidence supporting this. Hence, we planned this study and found that aflibercept monotherapy could maintain good BCVA. Here, we observed maintenance of BCVA and improvement in CMT at 12 and 24 months after the start of the planned aflibercept regimen, even in favorable cases with BCVA >0.3 logMAR. Because untreated AMD is a progressive disease, treatment should be started early, even in patients with good BCVA.Citation2
In the VIEW 1 and VIEW 2 studies, AMD subjects received their first treatment with BCVA ranging from 0.3 to 1.2 logMARCitation4 (Early Treatment Diabetic Retinopathy Study acuity: 25–73 letters). Improvement was observed in both studies. In the group that received aflibercept 2 mg bimonthly after three monthly administrations during the loading phase, mean BCVA was 53.6 before treatment, with an 8.4-letter improvement at 12 months. Between 12 and 24 months, the same group received aflibercept 2 mg as needed with mandatory dosing at least every 12 weeks, and mean BCVA showed a 7.6-letter improvement at 24 months. Our subjects had good BCVA before treatment, so we observed an improvement of only 0.04 and 0.03 logMAR at 12 and 24 months, respectively, due to ceiling effect. Few cases showed improvement, but there was only one case that worsened. Three of the patients who dropped out developed residual serous retinal detachment and were treated with PDT, so worsening can occur even in patients with good VA.
Previous reports have indicated that treating AMD with anti-VEGF agents may not always be effective,Citation20–Citation23 and investigation of treatments for such cases is necessary. Consideration should be given as to whether to continue monthly administration, switch to another anti-VEGF agent, or use an anti-VEGF agent in combination with PDT. In this study, baseline SRH and baseline good BCVA were independently associated with improved BCVA at 12 months. Notably, even though eyes with initial SRH tend to have recurrent SRH, eyes with initial SRH had good prognosis. Thus, eyes with baseline SRH might likely receive IVA earlier than eyes without initial SRH, because most eyes with SRH had visual symptoms themselves, whereas most without SRH were found to have exudative AMD lesions incidentally.
To our knowledge, only three papers have described treatment outcomes of anti-VEGF therapy in AMD with good VA. Compared with previous reports,Citation16–Citation18 BCVA was favorable after 24 months, although more injections were administered owing to a fixed regimen and modified treat-and-extend regimen (). Axer-Siegel et al reported that mean BCVA in 150 cases of AMD did not change from the start of treatment (0.22 logMAR) after administration of a mean 11.3 bevacizumab injections over a mean 20.2 months.Citation16 Furthermore, a report on ranibizumab injections by Lee et al indicated worsening of mean BCVA. In 754 eyes with AMD, mean BCVA was 0.23 logMAR before treatment and 0.31 logMAR after 5.7 injections over 12 months.Citation18 According to Kato et al, mean BCVA in 36 eyes with AMD showed no significant change from 0.11 logMAR at treatment initiation to 0.12 logMAR after 3.3 injections over 12 months.Citation17 In this study, we treated 39 eyes with AMD. BCVA improved, ranging from −0.08 to 0.26 logMAR (mean 0.097), and from −0.08 to 0.40 logMAR at 12 months (mean 0.058, P=0.03), and was maintained at −0.08 to 0.52 logMAR at 24 months (mean 0.066, P=0.09), after a mean of 6.7±0.72 injections of aflibercept over 12 months and 4.8±1.8 injections from 13 to 24 months.
This study included 21 cases of PCV (54%). Good short-term outcomes have been reported when PCV is treated with aflibercept.Citation24 Here, we found no significant differences in BCVA or CMT between AMD cases with and without PCV. We also found no significant differences in BCVA, CMT prognosis, or polypoidal regression ratio between type 1 and 2 PCV. In some cases of type 1 PCV, polypoidal lesions may enlarge with bimonthly aflibercept. We believe IVA should be administered during the early stages of treatment in cases with good VA, irrespective of the presence of type 1 or 2 PCV. Also, the ratio of disappearance of polypoidal lesions was reported as 69%Citation10 and 55%Citation24 in treatment-naïve PCV patients. Here, we found the ratio was almost the same − 57% at 12 months and 67% at 24 months, respectively – in treatment-naïve PCV patients with good BCVA.
We recognize some limitations of this study. It was an exploratory study and the sample size was small for the ad hoc analysis. Further large-scale studies are needed to identify prognostic imaging biomarkers for the treatment.
Conclusion
We observed improved BCVA and CMT at 12 months and no worsening of BCVA and improved CMT after 24 months of planned administration of aflibercept in patients with AMD with a mean baseline BCVA of ≥0.3 logMAR. Our patients received monthly administrations over 3 months, followed by bimonthly administration up to 12 months and administrations with a treat-and-extend regimen from 13 to 24 months. There was no significant difference in BCVA or CMT prognosis between AMD with and without PCV or between type 1 and type 2 PCV. There was no significant difference in the regression ratio of polypoidal lesions between type 1 and type 2 PCV. Baseline SRH was a good indicator of change of logMAR BCVA after 1 year, but was not a significant factor after 2 years.
Data sharing statement
All dataset files are available from the Figshare database (https://doi.org/10.6084/m9.figshare.5734857).
Author contributions
SS: analysis, writing, and editing. HT: data collection, analysis, writing, and editing. YA, SI, NK, YF, TT, HK, and YY: data collection, editing, and review of manuscript. YI: data collection, analysis, editing, and review of manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This paper has been presented in part at the 54th Annual Meeting of the Japanese Retina and Vitreous Society, Tokyo, December 2015. We thank Hironobu Tampo, Satoko Tominaga, Aya Sato, and Mikiko Takezawa for their participation in this study. This study was funded by Bayer Yakuhin, Osaka, Japan. The study sponsor had no role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; and in the decision to submit the paper for publication.
Disclosure
SS, YA, SI, and NK: none. YY: lecturer’s fees from Novartis Pharmaceuticals, Bayer Healthcare, and Santen Pharmaceuticals outside this work. HT: lecturer’s fees from Kowa Pharmaceuticals, Novartis Pharmaceuticals, Bayer Yakuhin, and Santen Pharmaceuticals; grants from Novartis Pharma outside this work. YI: lecturer’s fees from Kowa Pharmaceuticals, Novartis Pharmaceuticals, Bayer Yakuhin, and Santen Pharmaceuticals outside this work. YF: lecturer’s fees from ALCON Japan and Otsuka Pharmaceuticals outside this work. TT: lecturer’s fees from ALCON Japan outside this work. HK: lecturer’s fees from Kowa Pharmaceuticals, Novartis Pharmaceuticals, and Santen Pharmaceuticals outside this work. The authors report no other conflicts of interest in this work.
References
- BresslerNMAge-related macular degeneration is the leading cause of blindnessJAMA20042911900190115108691
- LimLSMitchellPSeddonJMHolzFGWongTYAge-related macular degenerationLancet20123791728173822559899
- BrownDMKaiserPKMichelsMRanibizumab versus verteporfin for neovascular age-related macular degenerationN Engl J Med20063551432144417021319
- Schmidt-ErfurthUKaiserPKKorobelnikJFIntravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of VIEW studiesOphthalmology201412119320124084500
- RofaghaSBhisitkulRBBoyerDSSaddaSRZhangKSEVEN-UP Study GroupSeven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP)Ophthalmology20131202292229923642856
- RosenfeldPJBrownDMHeierJSRanibizumab for neovascular age-related macular degenerationN Engl J Med20063551419143117021318
- StewartMWAflibercept (VEGF Trap-eye): the newest anti-VEGF drugBr J Ophthalmol2012961157115822446028
- MarukoIIidaTSaitoMNagayamaDSaitoKClinical characteristics of exudative age-related macular degeneration in Japanese patientsAm J Ophthalmol2007144152217509509
- OginoTTakedaMImaizumiHOkushibaUPhotodynamic therapy for age-related macular degeneration in Japanese patients: results after one yearJpn J Ophthalmol20075121021517554484
- OishiATsujikawaAYamashiroKOne-year result of aflibercept treatment on age-related macular degeneration and predictive factors for visual outcomeAm J Ophthalmol201515985386025634529
- KawamuraAYuzawaMMoriRHaruyamaMYuzawaTKIndocyanine green angiographic and optical coherence tomographic findings support classification of polypoidal choroidal vasculopathy into two typesActa Ophthalmol201391e474e48123848133
- TanCSNgoWKLimLWLimTHA novel classification of the vascular patterns of polypoidal choloidal vasculopathy and its relation to clinical outcomesBr J Ophthalmol2014981528153324997181
- CoscasGLupidiMCoscasFToward a specific classificatio of polypoidal choroidal vasculopathy: idiopathic disease or subtype of age-related macular degenerationInvest Ophthalmol Vis Sci2015563187319526024102
- HondaSMikiAYanagisawaSMatsumiyaWNagaiTTsukaharaYComparison of the outcomes of photodynamic therapy between two angiographic subtypes of polypoidal choroidal vasculopathyOphthalmologica2014232929624993059
- JeongSSagongMShort-term efficacy of intravitreal aflibercept depending on angiographic classification of polypoidal choroidal vasculopathyBr J Ophthalmol201710175876327597740
- Axer-SiegelRBorEBourlaDHWeinbergerDMimouniKIntravitreal bevacizumab treatment for exudative age-related macular degeneration with good visual acuityRetina2012321811182022825407
- KatoAYasukawaTSugaKIntravitreal ranibizumab for patients with neovascular age-related macular degeneration with good baseline visual acuityOphthalmologica2015233273425412682
- LeeAYLeeCSButtTUK AMD EMR USERS group report V: benefits of initiating ranibizumab therapy for neovascular AMD in eyes with vision better than 6/12Br J Ophthalmol2015991045105025680619
- TakahashiKOguraYIshibashiTShiragaFYuzawaMTreatment guidelines for age-related macular degenerationNippon Ganka Gakkai Zasshi20121161150115523379205
- ArcinueCAMaFBarteselliGSharpstenLGomezMLFreemanWROne-year outcomes of aflibercept in recurrent or persistent neovascular age-related macular degenerationAm J Ophthalmol201515942643625461263
- ChangAALiHBroadheadGKIntravitreal aflibercept for treatment-resistant neovascular age-related macular degenerationOphthalmology201412118819224144450
- KuehleweinLBansalMLenisTLOptical coherence tomography angiography of type 1 neovascularization in age-related macular degenerationAm J Ophthalmol201516073974826164826
- NomuraYYanagiYIntravitreal aflibercept for ranibizumab-resistant exudative age-related macular degeneration with choroidal vascular hyperpermeabilityJpn J Ophthalmol20155961265
- YamamotoAOkadaAAKanoMOne-year results of intravitreal aflibercept for polypoidal choroidal vasculopathyOphthalmology20151221866187226088619