?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
This retrospective case-matched study aimed to compare visual and refractive outcomes between small incision lenticule extraction (SMILE) and LASIK.
Patients and methods
Patients who underwent SMILE (34 eyes of 23 patients) or LASIK (34 eyes of 24 patients) were enrolled and matched according to preoperative manifest refractive spherical equivalents. The mean preoperative manifest refractive spherical equivalent was −4.69±0.6 and −4.67±0.64 D in the SMILE and LASIK groups, respectively. The safety, efficacy, and predictability were compared 3 months after surgery. Changes in corneal refractive power from the center to peripheral points and their maintenance ratios were analyzed and compared between the two groups.
Results
In the SMILE and LASIK groups, 82.4% and 85.3% of patients, respectively, achieved 20/13 or better uncorrected distance visual acuity (p=1.00). There were no eyes that lost two or more lines of corrected distance visual acuity in either group. The maintenance ratios of corneal refractive power changes at the peripheral points in the SMILE group were significantly higher than those in the LASIK group (p<0.05).
Conclusion
Both groups achieved similar high efficacy and safety. SMILE surgery resulted in higher refractive power correction in the peripheral cornea than LASIK surgery.
Introduction
LASIK was developed by Pallikaris et al in 1990Citation1 and has been widely performed in many countries. Previous studies have confirmed the safety and efficacy of LASIK surgery.Citation2–Citation12 On the other hand, small incision lenticule extraction (SMILE) was developed as a refractive surgery without the use of excimer laser in 2008. Shah et alCitation13 and Sekundo et alCitation14 reported early results with SMILE, and confirmed that it was a highly safe and effective refractive surgical technique.
Generally, in LASIK surgery, a corneal flap is created; excimer laser ablation is then performed on the corneal stromal layer under the flap.Citation15 Although excimer laser can accurately ablate the central part of the cornea, ablation efficiency is reduced at the peripheral cornea.Citation13,Citation16 In contrast, the SMILE surgery corrects refractive error using a femtosecond laser, in which a lenticule is created and extracted from a small incision in the cornea. Because femtosecond lasers can accurately create corneal incisions at specified depths, SMILE surgery is expected to achieve accurate refractive corrections, even at the peripheral cornea.Citation13,Citation17
In the current study, we performed a matched comparison analysis of the visual and refractive outcomes between SMILE and LASIK. Additionally, we compared changes in central and peripheral corneal refractive power between SMILE and LASIK using corneal topography.
To our knowledge, this is the first study to use a matched comparison method to evaluate the visual/refractive outcomes and changes in corneal refractive power after adjusting optical zone size between SMILE and LASIK.
Patients and methods
Patients
This matched comparison study included a total of 68 eyes of 47 patients who underwent SMILE or LASIK surgery for myopia and astigmatism correction between August 2012 and December 2015 at Nagoya Eye Clinic (Nagoya, Japan). Consecutive patients who underwent SMILE were selected and subsequently matched with LASIK patients, based on a preoperative manifest refractive spherical equivalent (SE) difference of within ±0.5 D for each pair. Investigators were blinded to postoperative refractive and visual outcomes when the patients were matched. Ultimately, the patients who underwent SMILE (34 eyes of 23 patients; mean age 32.2±6.8 years) and LASIK (34 eyes of 24 patients; mean age 29.9±6.8 years) were enrolled in the study.
summarizes the demographic information of patients in each group. The preoperative manifest refractive sphere was −4.52±0.54 and −4.45±0.61 D in the SMILE and LASIK groups, respectively. The preoperative manifest refractive cylinder was −0.33±0.38 and −0.45±0.44 D in the SMILE and LASIK groups, respectively. With the exception of planned ablation depth, there were no significant differences among parameters between the two groups.
Table 1 Preoperative patient demographic information
The study was approved by the Institutional Review Board of the Nagoya Eye Clinic and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients after explanation of the nature and possible outcomes of the study.
Surgical techniques
A 500 kHz femtosecond laser system (VisuMax; Carl Zeiss Meditec, Jena, Germany) was used for surgical refractive corrections in patients in the SMILE group. The femtosecond laser parameters were as follows: cap thickness, 120 μm; diameter of cap cut, 7.5 mm; and posterior lenticule diameter, 6.5 mm. In the LASIK group as well, flaps were created using a 500 kHz VisuMax femtosecond laser system. The target flap thickness was 80 μm and a hinge was created at the superior position in all cases. An excimer laser (MEL80, Carl Zeiss Meditec) was used for tissue ablation. The MEL80 parameters were as follows: diameter of optical zone, 6.0 mm and diameter of transition zone, 8.2 mm. The Aberration Smart Ablation and wavefront-guided program with data obtained using the WASCA aberrometer (Carl Zeiss Meditec) were applied to all patients.
Preoperative examinations
Preoperative examination included uncorrected distance visual acuity (UDVA) and corrected distance visual acuity (CDVA), manifest refraction, corneal thickness (Orbscan II; Bausch & Lomb, Rochester, NY, USA), corneal topography (TMS-4; Tomey, Nagoya, Japan), and corneal refractive power.
Postoperative examination
Postoperative examination included UDVA, CDVA, manifest refraction, corneal topography, and corneal refractive power 3 months postoperatively.
Comparison of refractive power correction efficiency
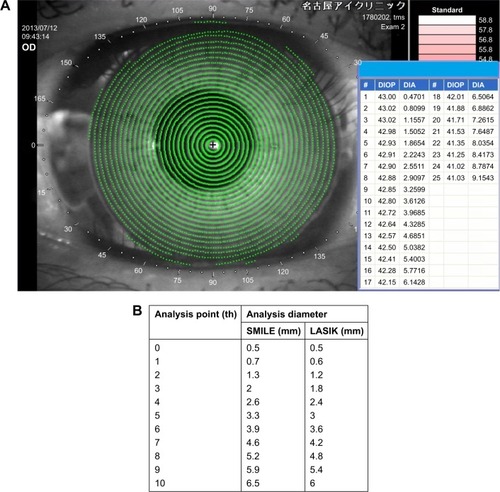
Initially, the “reference” point in both the SMILE and LASIK groups was defined as a position located at 0.5 mm from the measurement center. Because the diameter of the optical zone was different between the SMILE and LASIK groups (SMILE, 6.5 mm; LASIK, 6.0 mm), the diameter of the optical zone was divided into 10 segments, each named 1st–10th from the center to the peripheral cornea. The analysis diameter was rounded up to the first decimal place. The anterior corneal refractive power at each of the selected analysis points (0th–10th; ) was calculated using results obtained with the TMS-4 topographer. Briefly, based on the refractive power and diameter of each Mire ring (), a linear regression equation was created in each case. Then, the refractive power at a specific point (0th–10th) in the LASIK and SMILE groups () was calculated from the equation. The differences in corneal refractive power were then calculated at each point before and after surgery in both the groups. The change at the 0th analysis point was defined as the “reference”. The maintenance ratio of the corneal refractive power changes was calculated using the following equation:
Figure 1 Representative image of corneal topography and analysis points in SMILE and LASIK groups.
Abbreviation: SMILE, small incision lenticule extraction.
The maintenance ratio obtained was compared between the SMILE and LASIK groups.
Statistical analyses
The chi-squared test was applied to compare the sex ratios between the two groups. Spearman’s rank correlation was used to analyze correlations between refractive power correction efficiency and analysis diameters. Fisher’s exact test was performed to compare the ratios of postoperative distribution of refractive power, UDVA, and CDVA between the two groups. Paired t-test was applied to compare age, preoperative UDVA, CDVA, central corneal thickness, planned ablation depth, postoperative refractive power correction efficiency at each analysis point, preoperative and postoperative manifest refractive sphere, manifest refractive cylinder, SE, and mean K. A p-value <5% was considered to be statistically significant.
Results
Comparison of efficacy of refractive surgery
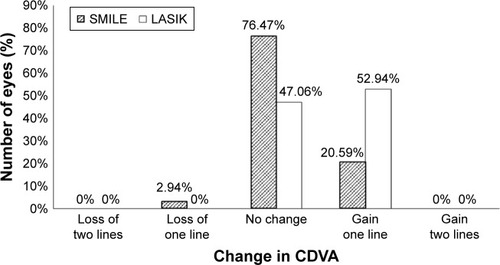
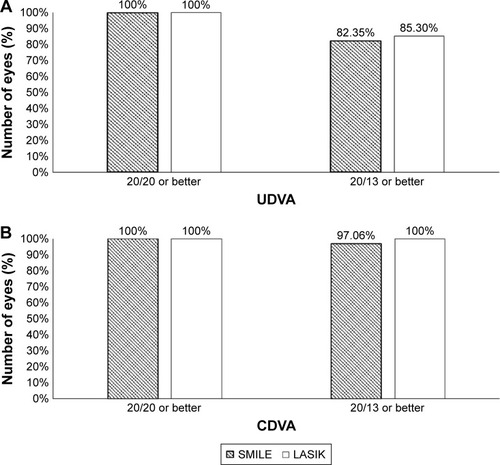
In the SMILE and LASIK groups, 82.35% and 85.3% of patients, respectively, achieved 20/13 or better UDVA (p=1.00). All patients in both groups exhibited UDVA 20/20 or better ().
Figure 2 Visual outcomes in SMILE and LASIK groups.
Abbreviations: CDVA, corrected distance visual acuity; SMILE, small incision lenticule extraction; UDVA, uncorrected distance visual acuity.
Comparison of safety of refractive surgery
In the SMILE and LASIK groups, 97.06% and 100% of patients, respectively, achieved 20/13 or better CDVA (p=1.00). All patients in both groups exhibited 20/20 or better CDVA (). There were no eyes that lost two or more lines of CDVA in either group ().
Comparison of predictability of refractive surgery
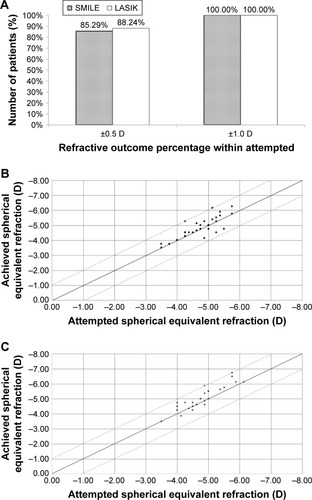
Postoperative manifest refractive sphere 3 months after surgery was +0.17±0.41 and +0.32±0.34 D in the SMILE and LASIK groups, respectively. There were no significant differences between the two groups (p=0.155). Postoperative manifest cylinder 3 months after surgery was −0.13±0.25 and −0.15±0.26 D in the SMILE and LASIK groups, respectively. There were no significant differences between the two groups (p=0.829; ).
Table 2 Comparison of postoperative refractive outcomes between SMILE and LASIK groups
Ratio corrected within ±0.5 D of target refraction was 85.29% and 88.24% in the SMILE and LASIK groups, respectively; this was not significantly different (p=1.00; ). All cases in both groups were corrected to within ±1.00 D of target refraction.
Figure 4 Comparison of the predictability between the SMILE and LASIK groups.
Abbreviation: SMILE, small incision lenticule extraction.
Comparison of refractive power correction efficiency
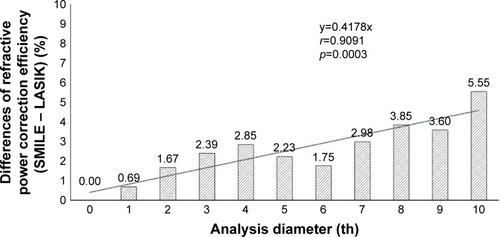
The average maintenance ratios of refractive power changes at the 1st to the 10th point of the cornea are shown in . There were no significant differences in the maintenance ratios between the two groups at the 7th or inner analysis points. However, the maintenance ratios at the 8th to 10th analysis points in the SMILE group (8th, 83.71%±8.44%; 9th, 74.03%±7.9%; and 10th, 63.25%±8.06%) were significantly higher than those in the LASIK group (8th, 79.86%±5.83%; 9th, 70.44%±6.11%; and 10th, 57.7%±6.37%; the p-values being 8th, p=0.0346; 9th, p=0.0392; and 10th, p=0.0016). In addition, subtraction of maintenance ratio of LASIK from SMILE revealed that the difference in maintenance ratios between the SMILE and LASIK groups increased at the peripheral points of the cornea (r=0.91, p=0.0003; ).
Figure 5 Changes in the refractive power correction efficiency (1st–10th analysis diameter).
Abbreviation: SMILE, small incision lenticule extraction.
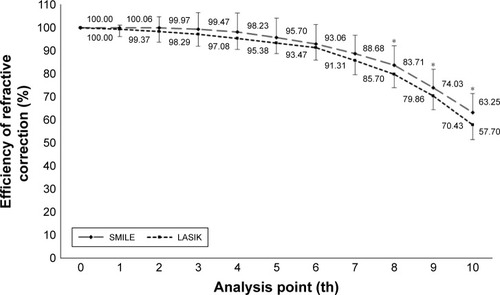
Figure 6 Changes in differences in refractive power correction efficiency (1st–10th analysis diameter) between SMILE and LASIK.
Abbreviation: SMILE, small incision lenticule extraction.
Discussion
The current study found no significant differences in postoperative UDVA and CDVA between the SMILE and LASIK groups, which is consistent with several previous reports.Citation15,Citation18–Citation20 However, Ganesh and GuptaCitation21 reported that their SMILE group achieved a higher rate of UCVA (20/20 or better) than the LASIK group. In our study, all patients in both groups achieved UDVA and CDVA 20/20 or better at 3 months after surgery. The discrepancies with our investigation may be due to study design or the number of cases included. In our study, no cases lost two or more lines of CDVA in either group. Our results confirm that both LASIK and SMILE are highly effective and safe as corneal laser refractive surgical techniques.
Similarly, significant differences were not found in the postoperative refractive sphere, refractive cylinder, and prediction accuracy between SMILE and LASIK groups in our study. A comparative study by Lin et alCitation18 reported no differences in postoperative SEs between the SMILE and LASIK groups. Although Ganesh and GuptaCitation21 found that the postoperative SEs in their SMILE group were smaller than those in the LASIK group, the differences between the two groups were small.
For accurate correction of astigmatism, accurate alignment of the cylinder axis is essential. A previous study showed that iris registration system was effective in performing accurate astigmatism correction in LASIK.Citation22 Because the current SMILE procedure does not have an iris registration system, we evaluated astigmatism correction separately. In our study, we found that there was no significant difference in postoperative manifest cylinder between the two groups. Ivarsen and HjortdalCitation23 reported that cylinder correction in SMILE was 87% per D (low astigmatism) and 84% per D (high astigmatism). Furthermore, Pedersen et alCitation24 reported that cylinder correction in SMILE was 89% per D at 1 year postoperatively. Based on the results from previous reports and our current analysis, we conclude that SMILE does not have limitations for astigmatism correction, even without an iris registration system.
In the current study, we found that the refractive power correction efficiency in the SMILE group was better than the LASIK group in the outer 70% area of the optical zone.
Gyldenkerne et alCitation25 also compared corneal shape changes between SMILE and LASIK groups. They found that the corneal sagittal curvature in the SMILE group was constant within the central 4 mm diameter, while the LASIK group showed a gradual steepening with increasing diameter. However, they compared different optical zones between the two groups. Some cases in the SMILE group had a larger diameter than the cases in the LASIK group. Since we were interested in the detailed refractive power correction at the peripheral cornea, we divided the optical zone into 10 regions and measured the refractive power correction efficiency at each diameter. Moreover, to compare the refractive correction efficiency between the two groups in an equitable manner, we compared the same region between the two groups.
Lin et alCitation18 compared higher-order aberrations (HOAs) between SMILE and LASIK groups and found that spherical, coma, and total HOAs were significantly lower in the SMILE group than in the LASIK group. Another study also compared HOAs and contrast sensitivity between the two groups and found that postoperative HOAs were significantly lower and contrast sensitivity was significantly better in the SMILE group compared with the LASIK group.Citation21 Because increased HOA is a known cause of night vision disturbances such as glare, halos, and decreased contrast sensitivity,Citation26–Citation30 we speculate that corneal refractive power changes at the peripheral cornea may have affected these differences between the LASIK and SMILE groups.
Previous studies have reported that LASIK surgery changed the prolate shape of the cornea to an oblate shape and, therefore, increased spherical aberrations after surgery.Citation31–Citation33 These changes are considered to be due to decreased ablation efficiency on the peripheral cornea in LASIK surgery. Generally, excimer laser is applied perpendicularly at the central cornea, but not at the peripheral cornea; therefore, ablation efficiency is reduced at the peripheral part of the cornea.Citation34,Citation35 In addition, because excimer laser ablation is performed after the corneal flap is lifted, water content of the corneal stroma and humidity of the surgical suite could affect ablation efficiency.Citation36,Citation37 On the other hand, SMILE surgery is performed using a femtosecond laser. One of the advantages is that it can disrupt the corneal tissue accurately at the peripheral cornea.Citation38–Citation41 From this principle, SMILE surgery is expected to achieve high ablation efficiency, even at the peripheral cornea. Our results suggest that the difference in principles between SMILE and LASIK surgery affects the refractive power correction efficiency at the peripheral cornea.
The MEL80 Aberration Smart Ablation program was applied in all cases in this study. This program was designed to maintain the prolate shape of the cornea from −3 to −6 D of sphere correction.Citation42,Citation43 In addition, the wavefront-guided LASIK program was applied for all cases in the LASIK group; SMILE does not have such a program. If an aspheric lenticule or a wavefront-guided lenticule creation protocol was available for SMILE surgery, the difference between the SMILE and LASIK groups could potentially be larger.
The current study had several limitations, the first of which was its retrospective design. Prospective comparative studies are needed to evaluate refractive power correction efficiency. In addition, we did not measure HOAs and contrast sensitivity in the current study. Because peripheral corneal refractive power affects HOA and contrast sensitivity in dim light conditions, questionnaires regarding glare and halo should be used in future studies.
In conclusion, we found that both SMILE and LASIK were equally effective, safe, and highly predictable refractive surgical techniques. We demonstrated that SMILE surgery resulted in more accurate refractive error correction at the peripheral cornea than LASIK.
Acknowledgments
This study was orally presented at the Annual Meeting of the Japan Orthoptic Congress held in Osaka, October 15–16, 2016.
Disclosure
The authors report no conflicts of interest in this work.
References
- PallikarisIGPapatzanakiMEStathiEZFrenschockOGeorgiadisALaser in situ keratomileusisLasers Surg Med19901054634682233101
- MancheEEHawWWWavefront-guided laser in situ keratomileusis (Lasik) versus wavefront-guided photorefractive keratectomy (Prk): a prospective randomized eye-to-eye comparison (an american ophthalmological society thesis)Trans Am Ophthalmol Soc201110920122022253488
- SchallhornSCVenterJAHannanSJHettingerKAClinical outcomes of wavefront-guided laser in situ keratomileusis to treat moderate–to–high astigmatismClin Ophthalmol201591291129826203219
- XiaLYuJChaiGWangDLiYComparison of the femtosecond laser and mechanical microkeratome for flap cutting in LASIKInt J Ophthalmol20158478479026309880
- XiaLKMaJLiuHNShiCHuangQThree-year results of small incision lenticule extraction and wavefront-guided femtosecond laser-assisted laser in situ keratomileusis for correction of high myopia and myopic astigmatismInt J Ophthalmol201811347047729600182
- DjodeyreMRBeltranJOrtega-UsobiagaJGonzalez-LopezFRuiz-RizaldosAIBavieraJLong-term evaluation of eyes with central corneal thickness <400 μm following laser in situ keratomileusisClin Ophthalmol20161053554027099459
- KohnenTBührenJKühneCMirshahiAWavefront-guided LASIK with the Zyoptix 3.1 system for the correction of myopia and compound myopic astigmatism with 1-year follow-up: clinical outcome and change in higher order aberrationsOphthalmology2004111122175218515582071
- Al-ZeraidFMOsuagwuULInduced higher-order aberrations after Laser In Situ Keratomileusis (LASIK) performed with wavefront-guided intralase femtosecond laser in moderate to high astigmatismBMC Ophthalmol2016162927000109
- LiuTXChenYTDanTTShiRLinghuSRLiHXFour-year follow-up of corneal aberrations and visual functions of myopic patients after laser in situ keratomileusisPak J Med Sci20153161453145626870114
- MeidaniATzavaraCComparison of efficacy, safety, and predictability of laser in situ keratomileusis using two laser suitesClin Ophthalmol2016101639164627601880
- SajjadiVGhoreishiMJafarzadehpourERefractive and aberration outcomes after customized photorefractive keratectomy in comparison with customized femtosecond laserMed Hypothesis Discov Innov Ophthalmol20154413614127800501
- AlmahmoudTMungerRJacksonWBEffects of advanced surface ablations and intralase femtosecond LASIK on higher order aberrations and visual acuity outcomeSaudi J Ophthalmol201125327528023960936
- ShahRShahSSenguptaSResults of small incision lenticule extraction: all-in-one femtosecond laser refractive surgeryJ Cataract Refract Surg201137112713721183108
- SekundoWKunertKSBlumMSmall incision corneal refractive surgery using the small incision lenticule extraction (SMILE) procedure for the correction of myopia and myopic astigmatism: results of a 6 month prospective studyBr J Ophthalmol201195333533920601657
- BlumMKunertKGilleASekundoWLASIK for myopia using the Zeiss VisuMax femtosecond laser and MEL 80 excimer laserJ Refract Surg200925435035619431925
- SekundoWKunertKRussmannCFirst efficacy and safety study of femtosecond lenticule extraction for the correction of myopia: six-month resultsJ Cataract Refract Surg20083491513152018721712
- ReinsteinDZArcherTJGobbeMSmall incision lenticule extraction (SMILE) history, fundamentals of a new refractive surgery technique and clinical outcomesEye Vis (Lond)20141326605350
- LinFXuYYangYComparison of the visual results after SMILE and femtosecond laser–assisted LASIK for myopiaJ Refract Surg201430424825424702576
- LazaridisADroutsasKSekundoWPetrakMSchulzeSCorneal clarity and visual outcomes after small-incision lenticule extraction and comparison to femtosecond laser-assisted in situ keratomileusisJ Ophthalmol20172017564639028396803
- JinYWangYXuLComparison of the optical quality between small incision lenticule extraction and femtosecond laser LASIKJ Ophthalmol20162016250797327957338
- GaneshSGuptaRComparison of visual and refractive outcomes following femtosecond laser–assisted lasik with smile in patients with myopia or myopic astigmatismJ Refract Surg201430959059625250415
- KhalifaMEl-KatebMShaheenMSIris registration in wavefront-guided LASIK to correct mixed astigmatismJ Cataract Refract Surg200935343343719251134
- IvarsenAHjortdalJCorrection of myopic astigmatism with small incision lenticule extractionJ Refract Surg201430424024724702575
- PedersenIBIvarsenAHjortdalJChanges in astigmatism, densitometry, and aberrations after SMILE for low to high myopic astigmatism: a 12-month prospective studyJ Refract Surg2017331111728068441
- GyldenkerneAIvarsenAHjortdalJØComparison of corneal shape changes and aberrations induced By FS-LASIK and SMILE for myopiaJ Refract Surg201531422322925751842
- NepomucenoRLBoxer WachlerBSScruggsRFunctional optical zone after myopic LASIK as a function of ablation diameterJ Cataract Refract Surg200531237938415767162
- SanoYCarrJDTakeiKThompsonKPStultingRDWaringGO3rdVideokeratography after excimer laser in situ keratomileusis for myopiaOphthalmology2000107467468410768328
- YamaneNMiyataKSamejimaTOcular higher-order aberrations and contrast sensitivity after conventional laser in situ keratomileusisInvest Ophthalmol Vis Sci200445113986399015505046
- TaberneroJKlyceSDSarverEJArtalPFunctional optical zone of the corneaInvest Ophthalmol Vis Sci20074831053106017325146
- ZhangJZhouYHLiRTianLVisual performance after conventional LASIK and wavefront-guided LASIK with iris-registration: results at 1 yearInt J Ophthalmol20136449850423991386
- SálesCSMancheEEOne-year outcomes from a prospective, randomized, eye-to-eye comparison of wavefront-guided and wavefront-optimized LASIK in myopesOphthalmology2013120122396240223778091
- OshikaTKlyceSDApplegateRAHowlandHCEl DanasouryMAComparison of corneal wavefront aberrations after photorefractive keratectomy and laser in situ keratomileusisAm J Ophthalmol19991271179932992
- Moreno-BarriusoELlovesJMMarcosSNavarroRLlorenteLBarberoSOcular aberrations before and after myopic corneal refractive surgery: LASIK-induced changes measured with laser ray tracingInvest Ophthalmol Vis Sci20014261396140311328757
- HershPSFryKBlakerJWSpherical aberration after laser in situ keratomileusis and photorefractive keratectomy. Clinical results and theoretical models of etiologyJ Cataract Refract Surg200329112096210414670417
- MelloGRRochaKMSanthiagoMRSmadjaDKruegerRRApplications of wavefront technologyJ Cataract Refract Surg20123891671168322906449
- PatelSAlióJLArtolaAChanges in the refractive index of the human corneal stroma during laser in situ keratomileusis. Effects of exposure time and method used to create the flapJ Cataract Refract Surg20083471077108218571072
- DoughertyPJWellishKLMaloneyRKExcimer laser ablation rate and corneal hydrationAm J Ophthalmol199411821691768053462
- SoongHKMaltaJBFemtosecond lasers in ophthalmologyAm J Ophthalmol2009147218919718930447
- ZhangCCheJYuJYuLYuDZhaoGUsing femtosecond laser to create customized corneal flaps for patients with low and moderate refractive error differing in corneal thicknessPLoS One2015103e012129125807232
- KimCYSongJHNaKSChungSJooCFactors influencing corneal flap thickness in laser in situ keratomileusis with a femtosecond laserKorean J Ophthalmol201125181421350688
- KymionisGDKankariyaVPPlakaADReinsteinDZFemtosecond laser technology in corneal refractive surgery: a reviewJ Refract Surg2012281291292023231742
- DauschDDauschBWottkeMSluyterman van LangeweydeGComparison of clinical outcomes in PRK with a standard and aspherical optimized profile: a full case analysis of 100 eyes with 1-year follow-upClin Ophthalmol201482251226025473256
- MeyerBSluyterman van LangeweydeGWottkeMRefractive outcomes of an advanced aspherically optimized profile for myopia corrections by LASIK: a retrospective comparison with the standard aspherically optimized profileClin Ophthalmol2015937939225750516