Abstract
Purpose
To compare the efficacy and safety of a preservative-free, multi-ingredient formulation of carboxymethylcellulose 0.5%, hyaluronic acid 0.1%, and organic osmolytes (CMC-HA), to preservative-free carboxymethylcellulose 0.5% (CMC) in the management of postoperative signs and symptoms of dry eye following laser-assisted in situ keratomileusis (LASIK).
Methods
This was a double-masked, randomized, parallel-group study conducted in 14 clinical centers in Canada and Australia. Subjects with no more than mild dry eye instilled CMC-HA or CMC for 90 days post-LASIK. Ocular Surface Disease Index© (OSDI; primary efficacy measure), corneal staining, tear break-up time (TBUT), Schirmer’s test, acceptability/tolerability surveys, and visual acuity were assessed at screening and days 2, 10, 30, 60, and 90 post-surgery. Safety analyses included all enrolled.
Results
A total of 148 subjects (CMC-HA, n=75; CMC, n=73) were enrolled and assigned to receive treatment, and 126 subjects completed the study without any protocol violations. Post-LASIK, dry eye signs/symptoms peaked at 10 days. OSDI scores for both groups returned to normal with no differences between treatment groups at day 90 (P=0.775). Corneal staining, Schirmer’s test, TBUT, and survey results were comparable. Higher mean improvements in uncorrected visual acuity were observed in the CMC-HA group at all study visits, reaching statistical significance at day 30 (P=0.013). Both treatments were well tolerated.
Conclusion
CMC-HA-containing artificial tears relieved post-LASIK ocular dryness as well as CMC alone, and demonstrated incremental benefit in uncorrected vision, with a favorable safety profile. Results support use of CMC-HA eye drops to reduce signs and symptoms of ocular dryness post-LASIK.
Plain language summary
Why was the study done? Dry eye is common after laser-assisted in situ keratomileusis (LASIK) surgery. This study was conducted to investigate whether the addition of hyaluronic acid (HA) to a standard carboxymethylcellulose (CMC)-based artificial tear formulation further improves signs and symptoms of dry eye following LASIK.
What did the researchers do and find? Improvements in signs and symptoms of dry eye were comparable between the novel artificial tear containing CMC and HA and a standard CMC-only formulation after 90 days. Higher mean improvements in uncorrected visual acuity were observed in the CMC plus HA group at all study visits, reaching statistical significance at day 30.
What do the results mean? The presence of HA may enhance recovery of the ocular surface epithelium, and improve surface optics, with a resultant benefit in visual outcomes in comparison with the standard CMC eye drop.
Introduction
Laser-assisted in situ keratomileusis (LASIK) is a common ophthalmic surgical procedure. Dry eye is a frequent occurrence after LASIK, and in significant cases, a reason for referral to tertiary eye centers. Dry eye symptoms are reported by up to 95% of patients immediately post-LASIK and up to 60% of patients 1 month post-surgery.Citation1 Symptoms typically peak between 1 and 3 months, and chronic dry eye lasting at least 6 months after surgery has been reported in 10%–40% of patients.Citation2–Citation4 In an analysis of 143 United States Army personnel, dry eye was observed in 0.8% at 12 months post-LASIK.Citation5 Several factors may contribute to the development of dry eye post-LASIK including iatrogenic corneal nerve damage, conjunctival goblet cell loss caused by suction devices, postoperative inflammation that could exacerbate a preexisting dry eye condition, and tear dysfunction or disruption of tear distribution during blinking from corneal surface changes.Citation1
Similar to dry eye of other etiologies, artificial tears (preferably preservative-free) are typically the first-line treatment for post-LASIK dry eye. Carboxymethylcellulose (CMC)-based artificial tears have been shown to be more effective in controlling dry eye symptoms after LASIK when compared with saline,Citation6 or hydroxypropyl methylcellulose 0.3% and dextran 0.1% in bicarbonate buffer.Citation7 A preservative-free hyaluronic acid (HA) 0.15% eye drop (Hylabak® or Hyabak®, Thea Pharmaceuticals, Clermond-Ferrand, France) has also been shown to effectively reduce signs of dry eye post-LASIK.Citation8 With its intrinsic properties of water retention, viscoelasticity, and promotion of corneal epithelial wound healing,Citation9 HA increases viscosity, and hydrates and lubricates the ocular surface.
A multi-ingredient formulation containing CMC 0.5% and HA 0.1% (Refresh Optive Fusion®, Allergan plc, Dublin, Ireland) has been recently introduced to the dry eye armamentarium.Citation10,Citation11 The organic osmolytes, glycerin and erythritol, are also included in this eye drop to reduce the cellular stress level of the ocular surface.Citation12 In a population with a range of dry eye signs and symptoms, a large Phase III study found this preserved formulation to be noninferior to a standard preparation containing CMC alone in reducing signs and symptoms of dry eye.Citation13
In the current study, the safety and efficacy of a preservative-free, multi-ingredient CMC-HA formulation (Refresh Optive Fusion® Sensitive, Allergan plc; not commercially available in all countries), similar to the preserved formulation (Refresh Optive Fusion®, Allergan plc) but containing a third protective osmolyte, l-carnitine, was compared with preservative-free CMC 0.5% (Refresh Plus®, Allergan plc) in the management of ocular dryness following LASIK surgery.
Methods
Study design and subjects
This double-masked, randomized, parallel-group trial (ClinicalTrials.gov identifier: NCT01886690) was conducted between August 2013 and August 2014 at 13 Canadian centers and at one center in Australia in accordance with Good Clinical Practice guidelines. Independent ethics committee approval of the protocol was obtained from each site (Table S1). Adult candidates for bilateral LASIK surgery for myopia or hyperopia who provided prior written informed consent were enrolled. Key inclusion and exclusion criteria are summarized in .
Table 1 Key inclusion and exclusion criteria assessed at baseline (day 1)
Study visits and treatment
Visits were scheduled 1–30 days before LASIK surgery (screening), on the day of surgery (baseline/day 1), and on postsurgical days 2, 10, 30, 60, and 90. On day 1 after successful completion of LASIK, subjects were randomized 1:1 to receive preservative-free CMC-HA or CMC alone (), according to a scheme prepared by Allergan’s bio-statistics department. Treatment group assignment was also stratified by worse eye preoperative refractive error (manifest refraction spherical equivalent) into one of three categories: standard myope (−1.00 to −6.00 D), moderate myope (−6.125 to −8.00 D), or hyperope (+1.00 to +5.00 D).
Table 2 Study artificial tear formulationsTable Footnote*
Subjects administered one or two drops every hour while awake on days 1 and 2, and at least every 2 hours while awake from days 2 to 10. At the day 10, 30, and 60 study visits, investigators prescribed one of the following regimens based on the patient’s dry eye signs and symptoms: at least every 2 hours while awake; six to eight times per day; three to five times per day; or one or two times per day. Gatifloxacin 0.3% ophthalmic drops (Zymar®, Allergan plc) and prednisolone acetate 1.0% ophthalmic suspension (Pred Forte®, Allergan plc) were used post-surgery for ~10 days.
Outcome measures
The primary efficacy measure was the Ocular Surface Disease Index© (OSDI) score at post-LASIK day 90.Citation13,Citation14 Uncorrected distance visual acuity (UDVA) was a key secondary efficacy measure. A LogMAR chart at 3 m was used to measure UDVA, and the LogMAR line read was considered the last line that a subject could read three or more letters. Other secondary measures included corneal staining,Citation15 tear break-up time (TBUT) with sodium fluorescein, and Schirmer’s test (with anesthesia). All assessments were performed in each eye. Symptom surveys graded on visual analog scales of 0 (no symptom) to 100 (maximum symptom), and an acceptability questionnaire, were administered. Subjects reported average daily dosing frequency. Safety measures included adverse events (AEs), biomicroscopy findings, intraocular pressure (IOP), and corrected distance visual acuity (CDVA).
Data analysis
The per-protocol (PP) population (randomized subjects who had no significant protocol violations) was used for the primary efficacy analysis. The intent-to-treat (ITT) population (all randomized subjects) was used for supportive and sensitivity efficacy analyses. The safety population consisted of all treated subjects.
For the primary efficacy variable, OSDI questionnaire score at day 90, noninferiority of CMC-HA compared with CMC in the PP population was tested using an analysis of variance (ANOVA) model with treatment and stratification factors of preoperative refraction strata (standard myope, moderate myope, or hyperope) as fixed effects. Using the CI procedure, a prespecified margin of less than 4.7 units for the upper limit of the two-sided 95% CI based on treatment differences was set to consider the CMC-HA formulation noninferior to CMC.Citation16 Other analyses of OSDI score included comparisons at each scheduled visit, changes from baseline at all visits other than day 90, and comparison of subscale domain scores and subgroup analyses of OSDI scores for each preoperative refraction randomization stratum.
UDVA and CDVA were recorded and analyzed in letters read. For example, 20/50 equivalent was reported as 35 letters, and 20/20 plus two letters as 57 letters. Change from baseline in corneal staining was analyzed using the Wilcoxon rank-sum and Wilcoxon signed-rank tests. Schirmer’s test, TBUT, UDVA, symptoms and acceptability surveys, product usage (daily dosing frequency), as well as IOP and CDVA safety measures were analyzed by the same ANOVA model as the primary efficacy analysis. Significance level for all analyses was P<0.05.
A sample size of 91 subjects per treatment group (182 total subjects) was planned to provide 90% power to determine noninferiority in OSDI score at day 90, based on a one-sided type I error rate of 0.025 and the assumptions of no treatment difference and a common SD of 9.7.
Results
Subject disposition and baseline characteristics
Of the 207 subjects screened, 148 subjects were enrolled and randomized (75 subjects, CMC-HA group; 73 subjects, CMC group) and 146 (98.6%) completed the study. Among the randomized subjects, 126 had no significant protocol violations and 125 (99.2%) completed the study. Since the number of patients was less than initially planned, the power for the primary efficacy analysis was 83.4% rather than 90% as intended.
Baseline characteristics were similar between the two groups; mean (SD) age was 33.3 (8.9) years (range, 20–56 years) and 90.5% of subjects were Caucasian. Corneal topography and aberrometry measurements were similar between treatment groups at baseline. History of ophthalmic conditions reported in more than two subjects included blepharitis (seven [4.7%]), corneal scar (four [2.7%]), corneal abrasion (three [2.0%]), dry eye (three [2.0%]), and pinguecula (three [2.0%]).
Efficacy evaluation
Primary efficacy variable
In the PP population, mean (SD) OSDI scores were 3.9 (4.6) in the CMC-HA group and 4.1 (5.0) in the CMC group at baseline. OSDI scores increased post-LASIK surgery, peaking at day 10, then steadily decreased at subsequent follow-up visits (). At day 90, mean (SD) OSDI scores were 7.0 (8.5) in the CMC-HA group and 6.6 (7.1) in the CMC group. At baseline and day 90, mean OSDI scores were within the normal range (0–12) for both treatment groups. At day 90, the difference in OSDI scores was 0.4 (95% CI, −2.4, 3.2; P=0.775); the upper limit of the 95% CI was below the prespecified clinical margin of 4.7 units, demonstrating that CMC-HA provided equivalent relief of symptoms and was statistically noninferior to CMC.
Figure 1 Mean overall Ocular Surface Disease Index© (OSDI) scores at study visits.
Abbreviations: CMC, carboxymethylcellulose 0.5%; CMC-HA, carboxymethylcellulose 0.5%, hyaluronic acid 0.1%.
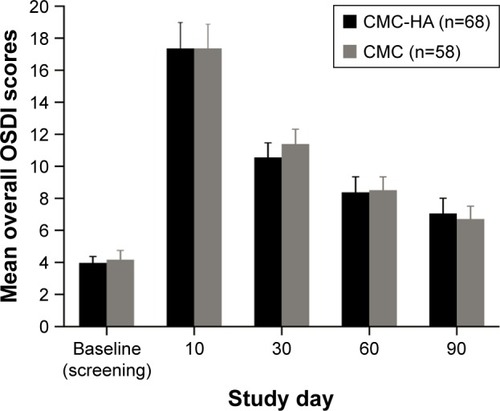
There were no significant differences in the mean (SD) change from baseline in OSDI score at day 90 between the treatment groups (3.1 [9.1] for CMC-HA and 2.5 [8.1] for CMC), and no between-group differences in other analyses of OSDI scores.
Results of preoperative refraction subgroups
Manifest refraction spherical equivalent was similar at baseline between the two treatment groups (−3.72 [range, −7.88 to 3.00] CMC-HA; −3.79 [range, −8.00 to 4.75] CMC). Of all enrolled subjects, at baseline, 119 were stratified as standard myopes, 23 as moderate myopes, and six as hyperopes. Similar to the overall population, subgroup analyses based on baseline refractive strata demonstrated no significant differences in OSDI scores between CMC-HA and CMC treatment groups. Within the CMC group, hyperopes (n=3) had significantly greater changes from baseline in OSDI scores at days 60 and 90 than standard myopes (n=59; P<0.001 and P=0.025, respectively) and moderate myopes (n=11; P=0.013 and P=0.032, respectively), but this effect was not observed in the CMC-HA group ().
Table 3 OSDI scores at baseline (screening visit) and follow-up visits based on preoperative refractive error stratification (ITT population)
Secondary efficacy variables
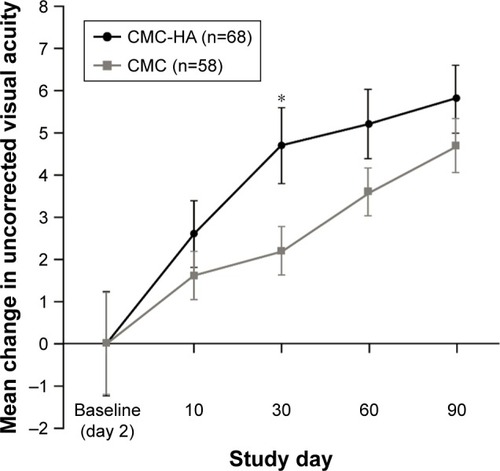
Mean (SD) UDVA on day 2 post-LASIK surgery was 51.1] (10.0) letters for the CMC-HA group and 53.1 (9.5) letters for the CMC group (PP population). Following day 2, visual acuity increased at all follow-up visits after LASIK in both treatment groups (P≤0.047), with higher mean improvements from day 2 in the CMC-HA group, and a statistically significant difference was observed between groups at day 30 (P=0.013) (). At day 90, the mean (SD) change from day 2 in visual acuity was 5.8 (5.2) letters in the CMC-HA group and 4.7 (6.0) letters in the CMC group (P=0.285).
Figure 2 Change in mean uncorrected visual acuity in the worse eye post-LASIK surgery.
Abbreviations: CMC, carboxymethylcellulose 0.5%; CMC-HA, carboxymethylcellulose 0.5%, hyaluronic acid 0.1%; LASIK, laser-assisted in situ keratomileusis.
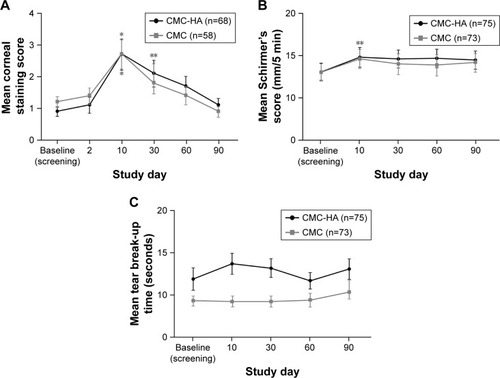
In both treatment groups, corneal fluorescein staining increased (worsened) after LASIK from a mean (SD) score of 0.9 (1.3) for the CMC-HA group and 1.2 (1.3) for the CMC group at baseline and peaked at day 10, before progressively decreasing (improving) up to day 90 (). Schirmer’s scores generally increased in both CMC-HA and CMC groups, but there were no significant differences compared with baseline at day 90 (). Mean (SD) TBUT was higher in the CMC-HA group compared with the CMC group at baseline (11.9 [11.7] vs 9.3 [5.1] seconds; P=0.084) and at each follow-up study visit; no significant differences were observed at any follow-up visit up to day 90 compared with baseline in either treatment group ().
Figure 3 Mean (A) corneal staining, (B) Schirmer’s test score, and (C) tear break-up time at study visits.
Abbreviations: CMC, carboxymethylcellulose 0.5%; CMC-HA, carboxymethylcellulose 0.5%, hyaluronic acid 0.1%.
Patient surveys revealed that burning/stinging, grittiness/foreign body sensation, dryness, difficult/uncomfortable vision, and overall ocular pain/discomfort scores worsened from baseline, peaking at day 2 following LASIK in both treatment groups. Ocular symptom scores gradually improved at follow-up visits in both CMC-HA and CMC groups with no significant differences observed from baseline at day 90, except for dryness in both treatment groups (). Assessment of the short- and long-term acceptability of study eye drops showed no differences between CMC-HA and CMC in the average response to each of the six items assessed (P>0.188).
Table 4 Severity of ocular symptoms at baseline (screening visit) and follow-up study visits after LASIK (ITT population)
The use of study eye drops declined over time in both treatment groups. At day 10 the mean (SD) number of times the study product was used per day over the previous week was 8.4 (2.8) in the CMC-HA group and 9.0 (3.3) in the CMC group, and at day 90 product usage decreased to 3.6 (2.4) and 3.9 (2.3) times per day, respectively. Patient-reported drop use was consistent with dosage assignment by the investigator, which shifted to lower levels of dosing later after LASIK surgery.
Safety evaluation
Significantly fewer subjects in the CMC-HA group (16 [21.3%]) compared with the CMC group (27 [37.0%]) reported experiencing AEs of any causality (P=0.036). In addition, fewer subjects reported ocular AEs in the CMC-HA group (six [8.0%]) than in the CMC group (12 [16.4%]); the most common ocular AEs were blepharitis reported in seven subjects (one [1.3%] with CMC-HA, six [8.2%] with CMC) and punctate keratitis reported in six subjects (three [4.0%] with CMC-HA, three [4.1%] with CMC). The incidence of treatment-related AEs was low in both groups (one [1.3%] dysgeusia with CMC-HA, one [1.4%] blepharitis with CMC), and no subjects discontinued the study because of AEs. Clinically significant (more than one severity grade increase [worsening] from baseline) biomicroscopy findings were equally reported in the CMC-HA (34 [45.3%]) and CMC (38 [52.1%]) groups; such findings are typical in post-LASIK patients.
Baseline IOP was similar between groups: mean (SD) 15.4 (2.5) mmHg for the CMC-HA group and 15.2 (2.8) mmHg for the CMC group. Both treatment groups had significant decreases in measured IOP by day 90 (mean change from baseline, −2.6 mmHg for both treatment groups; P<0.001 for within-group differences). This result was expected because of corneal thinning from the LASIK procedure.
At baseline, CDVA was similar between treatment groups, and no differences were observed in the change from baseline in the total number of letters read at all follow-up visits (P≥0.139 for between-group comparisons). Significant within-group improvements in CDVA from baseline were recorded in both CMC-HA (1.8 [3.2] letters; P<0.001) and CMC (1.2 [4.0] letters; P=0.013) groups by day 90.
Discussion
LASIK is considered to disrupt the lacrimal functional unit that maintains normal tear production and lubrication, through severing of corneal nerves and direct ocular surface effects during the procedure.Citation17 Approximately 50% of subjects have dry eye signs and symptoms as early as 1 week after LASIK.Citation18 Albietz et alCitation19 reported diminished myopic outcomes in chronic dry eye patients more frequently than in normal patients. Moreover, visual regression after LASIK was related to chronic dry eye, highlighting the importance of adequate postoperative dry eye management.Citation19 Post-LASIK dry eye symptoms are typically managed with artificial tears. Punctal plug occlusion, topical cyclosporine A, topical steroids, autologous serum tears, and scleral lenses may be used to control more severe dry eye following LASIK.Citation1 This study looked at the efficacy and safety of a preservative-free, multi-ingredient CMC-HA formulation. The CMC-only formula was selected as the comparator since it is considered a standard lubricating eye drop post-LASIK.
OSDI scores increased following LASIK surgery in both CMC-HA and CMC treatment groups, peaking at day 10, then progressively improving to normal levels (overall score <13).Citation14 Other studies have reported similar significant increases in OSDI scores after femtosecond laser-assisted LASIK surgery,Citation20,Citation21 which can remain elevated up to 6 months.Citation20 In this study, the new eye drop formulation containing CMC and HA met the primary efficacy endpoint and was noninferior to a formulation of CMC alone for change in OSDI score from baseline at day 90.
Among the refractive status groups (standard myope, moderate myope, hyperope), post hoc analyses revealed larger increases in OSDI scores from baseline to days 60 and 90 among the hyperopic eyes compared with standard and moderate myopic eyes in the CMC group, but no difference in the CMC-HA group. Although this finding must be interpreted with caution because of the small number of subjects in the hyperope subgroup, it is consistent with the greater degree of peripheral ablation for hyperopic treatment that is linked to post-LASIK dry eye.Citation3,Citation18 The cornea is steepened during hyperopic treatment with more tear instability resulting over the central cornea.Citation22,Citation23 The HA polymer and other ingredients in the CMC-HA formulation may provide improved benefit to the post-operative corneal surface than the CMC-only formula. Additional studies of hyperopic LASIK patients and those at higher risk of dry eye are warranted to further delineate the beneficial role of CMC-HA eye drops.
Corneal staining also worsened after LASIK and was followed by improvements over time, consistent with overall recovery of the corneal surface. Although there was a marginally higher mean TBUT in the CMC-HA group compared to the CMC group at baseline, this difference was not statistically significant. Schirmer’s scores and TBUT generally increased following surgery, and were not significantly different from baseline or between groups by day 90. Clinical signs of dry eye have been reported as early as 1 week after LASIK surgery, and compromised tear function can persist for a month; signs can remain for 6 months to 1 year.Citation2,Citation3,Citation5,Citation24 There were no significant differences between the CMC-HA and CMC treatment groups in any of these measures.
Importantly, in the context of post-LASIK care, UDVA improved more rapidly following surgery in the CMC-HA group compared with CMC, with higher mean improvements observed in the CMC-HA group at all study visits, reaching statistical significance at day 30 (P=0.013). This observation may reflect a greater rate of corneal surface epithelial healing combined with synergistic effects of CMC and HA such as increased drop viscosity, better ocular retention, tear film stabilization, and activation of the CD-44 receptor by HACitation25–Citation28 leading to improved surface optics. Combination CMC and HA has been shown to reduce goblet cell loss in a mouse model of dry eye,Citation10 a mechanism that may contribute to the positive outcomes observed in this study, as goblet cell loss is associated with dryness post-LASIK.Citation23,Citation29–Citation31 The safety profile of CMC-HA was also incrementally improved compared with CMC, with fewer total AEs of any causality, ocular AEs, and biomicroscopy findings.
With a greater understanding of the roles of tears, tear film osmolarity, and ocular surface inflammation in dry eye disease,Citation32–Citation34 new artificial tear formulations have enhanced interactions with ocular surface cells by preventing loss of cell volume, cellular stress and inflammation, and by eliminating damaging preservatives. Artificial tear formulations consisting of CMC or HA alone have been shown to be effective in reducing signs and symptoms of post-LASIK dry eye.Citation6–Citation8 A combination of diquafosol tetrasodium and HA has also been reported to potentially benefit vision and improve dry eye symptoms after LASIK.Citation35 The benefit of osmoprotectants in lubricant drops used to treat dry eye post-LASIK has also been demonstrated, with a significant improvement in symptoms reported following treatment with HA and adjuvant trehalose compared with HA alone.Citation36
The current study is the only one to our knowledge, that looks at the combination of CMC and HA in post-LASIK dry eye. The novel artificial tear containing CMC and HA, as well as glycerin, l-carnitine, and erythritol as organic osmolytes, was well tolerated by subjects post-LASIK surgery, and its overall performance suggests that the presence of HA may enhance recovery of the ocular surface epithelium, improve surface optics, and further promote visual recovery in comparison with the standard CMC eye drop. This formulation should be considered a suitable treatment option for management of post-LASIK dry eye.
Acknowledgments
This study was sponsored by Allergan plc, Dublin, Ireland. Writing and editorial assistance was provided to the authors by Kakuri M Omari, PhD, of Evidence Scientific Solutions, Philadelphia, PA, USA, and was funded by Allergan plc. Neither honoraria nor payments were made for authorship. The authors would like to thank the patients who participated in this study and acknowledge the investigators who contributed to the study conduct. The investigators included: Canada: Eser Adiguzel, Montreal, QC; Michael Bense, St John’s, NL; W Bruce Jackson, Ottawa, ON; Jeffrey Chambers, Kelowna, BC; Clara Chan, Toronto, ON; Sameh Fanous, Saint-Laurent, QC; Sheldon Herzig, Toronto, ON; Chris Jackman, St John’s, NL; Hugh Jellie, Waterloo, ON; Joseph King, Edmonton, AB; Christoph Kranemann, Toronto, ON; Andrew Taylor, Niagara Falls, ON; Avi Wallerstein, Montreal, QC; James Wiens, Winnipeg, MB. Australia: Patrick Versace, Bondi Junction, NSW.
Supplementary material
Table S1 Ethics committees providing study protocol approval
Disclosure
Avi Wallerstein received research funding for the conduct of the study. W Bruce Jackson received research funding for the conduct of the study and has served as a consultant to Allergan plc. Amir Moezzi received research funding from Allergan plc for the conduct of the study. Peter A Simmons was an employee of Allergan plc, Irvine, CA, USA at the time the study was conducted and has patents licensed to Allergan plc relevant to the reported study. Hugh Lin was an employee of Allergan plc, Irvine, CA, USA at the time the study was conducted and is currently a medical director at Genentech, a company that does operate in the ophthalmology space. Jeffrey Chambers reports no conflicts of interest in this work.
References
- RaoofDPinedaRDry eye after laser in-situ keratomileusisSemin Ophthalmol2014295–635836225325861
- SolomonKDHolzerMPSandovalHPRefractive surgery survey 2001J Cataract Refract Surg200228234635511821220
- ShojaMRBesharatiMRDry eye after LASIK for myopia: incidence and risk factorsEur J Ophthalmol20071711617294376
- ChaoCGolebiowskiBStapletonFThe role of corneal innervation in LASIK-induced neuropathic dry eyeOcul Surf2014121324524439045
- BowerKSSiaRKRyanDSMinesMJDarttDAChronic dry eye in photorefractive keratectomy and laser in situ keratomileusis: manifestations, incidence, and predictive factorsJ Cataract Refract Surg201541122624263426796443
- LentonLMAlbietzJMEffect of carmellose-based artificial tears on the ocular surface in eyes after laser in situ keratomileusisJ Refract Surg1999152 SupplS227S23110202728
- AlbietzJMLentonLMMcLennanSGEarlMLA comparison of the effect of refresh plus and bion tears on dry eye symptoms and ocular surface health in myopic LASIK patientsCLAO J20022829610012054380
- AstakhovYSAstakhovSYLisochkinaABAssessment of dry eye signs and symptoms and ocular tolerance of a preservative-free lacrimal substitute (Hylabak®) versus a preserved lacrimal substitute (Systane®) used for 3 months in patients after LASIKClin Ophthalmol201372289229724353401
- GomesJAAmankwahRPowell-RichardsADuaHSSodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitroBr J Ophthalmol200488682182515148219
- SheYLiJXiaoBEvaluation of a novel artificial tear in the prevention and treatment of dry eye in an animal modelJ Ocul Pharmacol Ther201531952553026322539
- SimmonsPAVehigeJGInvestigating the potential benefits of a new artificial tear formulation combining two polymersClin Ophthalmol2017111637164228979093
- CorralesRMLuoLHChangEYPflugfelderSCEffects of osmoprotectants on hyperosmolar stress in cultured human corneal epithelial cellsCornea200827557457918520508
- SimmonsPALiuHCarlisle-WilcoxCVehigeJGEfficacy and safety of two new formulations of artificial tears in subjects with dry eye disease: a 3-month, multicenter, active-controlled, randomized trialClin Ophthalmol2015966567525931807
- SchiffmanRMChristiansonMDJacobsenGHirschJDReisBLReliability and validity of the Ocular Surface Disease IndexArch Ophthalmol2000118561562110815152
- ChenJJRaoKPflugfelderSCCorneal epithelial opacity in dysfunctional tear syndromeAm J Ophthalmol2009148337638219541283
- MillerKLWaltJGMinkDRMinimal clinically important difference for the Ocular Surface Disease IndexArch Ophthalmol201012819410120065224
- NettuneGRPflugfelderSCPost-LASIK tear dysfunction and dysesthesiaOcul Surf20108313514520712970
- De PaivaCSChenZKochDDThe incidence and risk factors for developing dry eye after myopic LASIKAm J Ophthalmol2006141343844516490488
- AlbietzJMLentonLMMcLennanSGChronic dry eye and regression after laser in situ keratomileusis for myopiaJ Cataract Refract Surg200430367568415050267
- HuangJCSunCCChangCKMaDHLinYFEffect of hinge position on corneal sensation and dry eye parameters after femtosecond laser-assisted LASIKJ Refract Surg201228962563122947290
- LiMZhaoJShenYComparison of dry eye and corneal sensitivity between small incision lenticule extraction and femtosecond LASIK for myopiaPLoS One2013810e7779724204971
- LindstromRLHardtenDRHoutmanDMSix-month results of hyperopic and astigmatic LASIK in eyes with primary and secondary hyperopiaTrans Am Ophthalmol Soc19999724126010703127
- AlbietzJMLentonLMMcLennanSGEffect of laser in situ keratomileusis for hyperopia on tear film and ocular surfaceJ Refract Surg200218211312311934197
- BattatLMacriADursunDPflugfelderSCEffects of laser in situ keratomileusis on tear production, clearance, and the ocular surfaceOphthalmology200110871230123511425680
- PeachRJHollenbaughDStamenkovicIAruffoAIdentification of hyaluronic acid binding sites in the extracellular domain of CD44J Cell Biol199312212572648314845
- MackayCRTerpeHJStauderRMarstonWLStarkHGünthertUExpression and modulation of CD44 variant isoforms in humansJ Cell Biol19941241–271827507492
- BrignoleFPisellaPJDupasBBaeyensVBaudouinCEfficacy and safety of 0.18% sodium hyaluronate in patients with moderate dry eye syndrome and superficial keratitisGraefes Arch Clin Exp Ophthalmol2005243653153815965673
- LernerLESchwartzDMHwangDGHowesELSternRHyaluronan and CD44 in the human cornea and limbal conjunctivaExp Eye Res19986744814849820796
- AlbietzJMMcLennanSGLentonLMOcular surface management of photorefractive keratectomy and laser in situ keratomileusisJ Refract Surg200319663664414640428
- RodriguezAERodriguez-PratsJLHamdiIMGalalAAwadallaMAlioJLComparison of goblet cell density after femtosecond laser and mechanical microkeratome in LASIKInvest Ophthalmol Vis Sci20074862570257517525186
- RyanDSBowerKSSiaRKGoblet cell response after photorefractive keratectomy and laser in situ keratomileusisJ Cataract Refract Surg20164281181118927531295
- BaudouinCAragonaPMessmerEMRole of hyperosmolarity in the pathogenesis and management of dry eye disease: proceedings of the OCEAN group meetingOcul Surf201311424625824112228
- PflugfelderSCCorralesRMde PaivaCST helper cytokines in dry eye diseaseExp Eye Res201311711812524012834
- StevensonWChauhanSKDanaRDry eye disease: an immune-mediated ocular surface disorderArch Ophthalmol201213019010022232476
- TodaIIdeTFukumotoTIchihashiYTsubotaKCombination therapy with diquafosol tetrasodium and sodium hyaluronate in patients with dry eye after laser in situ keratomileusisAm J Ophthalmol20141573616.e1622.e124528935
- Mateo OrobiaAJCasas PascualPCristobal BescosJAEffects of 3% trehalose as an adjuvant treatment after LASIKClin Ophthalmol20171134735328243058
