Abstract
Purpose
Squamous metaplasia in dry eye disease (DED) manifesting as the loss of conjunctival goblet cells results in reduced mucin secretion and tear film instability. The objective of this study was to evaluate the efficacy of a polyethylene glycol–propylene glycol/hydroxypropyl-guar (PEG-PG/HP-guar) artificial tear formulation in reducing the squamous metaplasia in patients with DED using conjunctival impression cytology (CIC).
Methods
In this Phase IV, single-arm, open-label study, DED patients (aged ≥18 years) with a corneal staining sum score ≥3 and tear film break-up time (TFBUT) <7 s self-administered the PEG-PG/HP-guar artificial tears, 3 times a day for a period of 90 days. The primary end point was the change from baseline in goblet cell density (Nelson’s CIC grading score) over the treatment period. Other end points were change in the corneal and conjunctival staining scores, and TFBUT. Statistical evaluation was performed using a paired t-test.
Results
In total, 49 patients (n=98 eyes) completed the study. Compared with baseline, there was a significant reduction in the mean CIC scores (ie, improvement in goblet cell density) at Days 30, 60, and 90 (1.6±0.5 vs 1.2±0.5, 0.9±0.5, and 0.8±0.5; P<0.0001). At Day 90, 22% of eyes demonstrated squamous metaplasia Grade 0 (ie, normal epithelium). Similar improvements were observed in the corneal staining scores (5.7 vs 3.1, 1.1, and 0.5; P<0.0001), conjunctival staining scores (5.5 vs 3.6, 1.6, and 0.9; P<0.0001), and TFBUT (4.8 vs 5.8, 6.3, and 6.8 s; P<0.0001) at Days 30, 60, and 90, respectively.
Conclusion
In this study, treatment with PEG-PG/HP-guar artificial tears for 90 days decreased CIC score, reduced corneal and conjunctival staining, and increased TFBUT in patients with DED. These results suggest that PEG-PG/HP-guar artificial tears can improve the ocular surface health and reverse the changes induced by squamous metaplasia in DED.
Introduction
The epithelial cells of the cornea and conjunctiva are nonkeratinized, stratified squamous cells that secrete glycosylated glycoproteins called mucins.Citation1–Citation4 Mucins are essential to maintain tear film integrity; they form the glycocalyx that helps the aqueous layer to adhere to the hydrophobic cornea, acts as a lubricant, and forms a mucosal barrier.Citation1,Citation2,Citation4 Interspersed within the conjunctival epithelia are goblet cells, which are specialized epithelial cells. These are the only cells that secrete the gel-forming mucin MUC5AC, which plays an important role in ocular surface protection and prevents it from desiccation.Citation1,Citation2,Citation4 Morphological changes, loss of goblet cells, or alteration in their function thus have an adverse impact on the tear film and the ocular surface.Citation1–Citation4
Dry eye disease (DED) is an ocular surface disorder characterized by tear hyperosmolarity and instability, which triggers a self-perpetuating chain of events that leads to dryness, cytokine-mediated inflammation and infiltration, an altered lacrimal function, and ocular surface damage.Citation5,Citation6 In DED, damage to the cornea and conjunctiva is manifested as squamous metaplasia, a pathological condition characterized by a nonsecretory, keratinized epithelium and loss of goblet cells. In DED, the goblet cell loss results in mucin deficiency which causes further tear film instability.Citation4 A decrease in MUC5AC glycoprotein expression is observed as the dry eye condition progresses.Citation7 Further, the degree of squamous metaplasia has been shown to correlate with the severity of DED.Citation8
Systane® Ultra (Alcon Research Ltd, Fort Worth, TX, USA) is an artificial tear formulation containing the viscoelastic agent hydroxypropyl-guar (HP-guar), demulcents polyethylene glycol (PEG) and propylene glycol (PG), sorbitol, and borate preserved in Polyquad®.Citation9,Citation10 These PEG-PG/HP-guar lubricant eye drops are indicated for symptomatic relief and extended ocular surface protection in patients with DED. The efficacy and safety of PEG-PG/HP-guar artificial tears in patients with DED have been demonstrated in clinical studies.Citation9–Citation15 However, the effect of these lubricant eye drops on goblet cell density in DED has not been studied.
The aim of this study was to evaluate the efficacy of PEG-PG/HP-guar artificial tears in reducing squamous metaplasia in patients with DED, as assessed by conjunctival impression cytology (CIC).
Methods
This was a 3-month, prospective, Phase IV, single-center, open-label, single-arm study conducted from December 2009 to September 2010, at the Department of Ophthalmology, Medical Group Las Lomas, San Isidro, Buenos Aires, Argentina. Following screening (Day 0), eligible patients with DED were to instill 1 drop of the PEG-PG/HP-guar lubricant eye drops 3 times a day for a period of 90 days. Both eyes of patients were included. Patients had follow-up visits at Days 30, 60, and 90.
Patients were assigned a study number in numerical sequence. The test lubricant drops were provided by the designated study staff. All patients provided written informed consent before enrollment into the study. The study was approved by the Ethics Committee-Teaching and Research Committee of Medical Group Las Lomas and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practices. The study was registered with the National Administration of Drugs, Foods, and Medical Devices in compliance with the local regulatory requirements of Argentina.
Patients
Male and female patients aged ≥18 years who had active signs and symptoms of dry eye as determined by the study investigator, a sodium fluorescein corneal staining sum of ≥3 in either eye, a tear film break-up time (TFBUT) of <7 s, and a CIC of Grade I to Grade III were considered eligible to participate in the study.
Patients were ineligible if they had/met any of the following conditions: 1) had punctal occlusion of any type, 2) had any severe or serious ocular or other medical condition, 3) were hypersensitive to the test product or any excipients, 4) were unwilling to discontinue contact lens wear at least 7 days prior to the screening visit or during the study period, 5) had a history of ocular trauma in either eye within 3 months prior to the screening visit or any ocular surgery within 6 months prior to the screening visit or planned surgery during the study period, 6) had any ocular infection or a history of ocular herpes, 7) were using either β-blockers or any other drug with anticholinergic side effects or any other topical ocular medication, 8) were using any systemic medication that could contribute to dry eye (including, but not limited to, cold and allergy treatments, tricyclic antidepressants, and hormone replacement therapies) and had not been on a stable dose for at least 30 days prior to the screening visit or had any anticipated change in dosing regimen during the course of the study, or 9) participated in any other investigational clinical study within 30 days prior to the screening visit.
Study end points
The primary end point of the study was the change from baseline in goblet cell density (determined by CIC) over the 3-month treatment period. Exploratory end points were the change from baseline in 1) total corneal staining score, 2) total conjunctival staining score, 3) TFBUT at Days 30, 60, and 90, and 4) adverse events (AEs).
Assessments
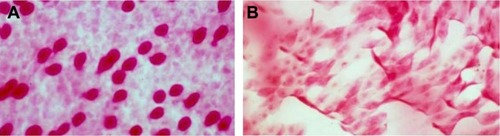
All efficacy assessments were performed at Day 0 (baseline) and at Days 30, 60, and 90 of the study. Impression cytology specimens were obtained from different areas of the conjunctiva of each patient. Briefly, after topical anesthesia Propacaína (Anestalcon, Alcon Laboratories, Inc., Fort Worth, Texas, USA) in the inferior sac, semicircular filters (polyvinylidene fluoride filter, ~15 mm diameter, 22 µm pore; EMD Millipore, Billerica, MA, USA) were applied to the inferior tarsal and bulbar conjunctiva for ~10 s. The filters were peeled off and immersed in absolute ethanol for fixation. The specimens were rehydrated in 70% ethyl alcohol and placed successively in periodic acid-Schiff (PAS) reagent, sodium metabisulfite, Gill’s hematoxylin, and Scott’s tap water. The specimens were rinsed with 95% alcohol and absolute alcohol. The filter papers were made transparent with xylene, mounted on glass slides, and examined under a conventional light microscope (400× magnification). The specimens were graded using the Nelson grading system (). The point-counting technique was used for morphometric analysis, where PAS-positive areas were counted across 15 random microscopic high-power fields on a 100-point and 50-line grid on a video system coupled to the microscope. For each subject, an average of the 15 fields was taken. All the specimens were analyzed by the same investigator who was masked to the origin of the samples. Sample CIC images showing epithelial and goblet cell morphology in normal and severe dry eye are shown in .
Table 1 Nelson’s grading system for impression cytology
Figure 1 Representative PAS-and H and E-stained images at 400× magnification showing (A) a normal number and morphology of goblet cells (Grade 0) (B) squamous metaplasia (Grade 3).
For TFBUT, 2% sodium fluorescein (1–5 µL) was instilled onto the bulbar conjunctiva. The subject was instructed to blink several times, without squeezing, to distribute the fluo-rescein. Thereafter, the subject was asked to stare straight ahead without blinking, and the examiner observed the eye through a set slit-lamp (10× magnification) using cobalt blue light; a Wratten #12 yellow filter was used to enhance observation of the tear film over the entire cornea.
TFBUT measurements preceded the corneal staining. The degree of corneal fluorescein staining was measured with a slit-lamp, for each of the 5 regions of the cornea (central, nasal, temporal, superior, and inferior) on a scale ranging from 0 (no staining) to 3 (severe staining). The scores of the 5 areas were summed to obtain the total score for each eye, resulting in a scale from 0 (no staining in any area) to 15 (severe staining in all the areas).
For conjunctival staining, 5 µL of Lissamine Green was instilled in each eye, and the staining was examined with a slit-lamp for each of the 6 regions (proximal superior, proximal inferior, and distal regions of both temporal and nasal conjunctiva). Each region was graded on a scale of 0 (none) to 3 (severe). The scores of the 6 areas of the conjunctiva were summed for each eye, resulting in a scale from 0 (no staining in any region) to 18 (severe staining in all regions).
Data on AEs, self-reported or observed, were collected at each visit and recorded in an AE form. For each AE, the severity (mild, moderate, or severe) and the causality were determined by the investigator.
Statistics
Statistical analyses were performed using SAS software. A sample size of 50 patients was planned to be enrolled. The differences in mean values between the baseline and subsequent visits following treatment were estimated within ±0.40 standard deviation (SD) using a 95% 2-sided confidence interval, based on repeated analysis of variance.
For all outcomes, paired t-tests were conducted to compare baseline vs outcomes at Days 30, 60, and 90.
Results
Of the 50 patients enrolled, 49 (n=98 eyes) completed the study at Day 90. One patient discontinued the study due to a nonserious AE (intolerance to the test formulation). The patients were aged between 21 and 70 years; 90% (n=45) of patients were female.
Primary end point
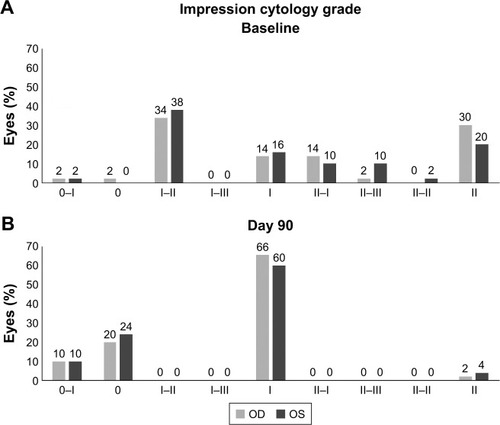
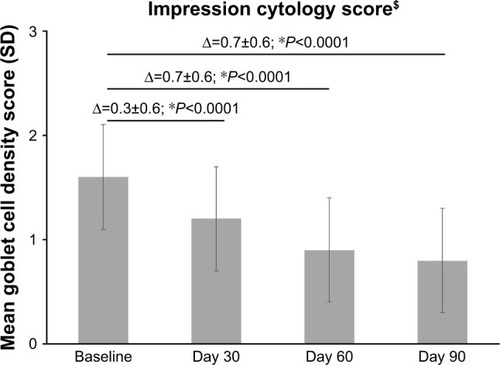
There was a significant improvement in CIC score at all follow-up time points compared to baseline (P<0.0001; ). The mean±SD cytology score decreased from 1.6±0.5 units at baseline (range, 0–3) to 1.2±0.5 units at Day 30, 0.9±0.5 units at Day 60, and 0.8±0.5 units (range, 0–2) at Day 90 ().
Figure 2 Mean conjunctival impression cytology scores (ie, mean goblet cell density) at baseline and Days 30, 60, and 90 after treatment with PEG-PG/HP-guar artificial tears.
Abbreviations: PEG-PG/HP-guar, polyethylene glycol–propylene glycol/hydroxypropyl-guar; SD, standard deviation.
At baseline (), 6.1% of eyes (n=6/98) were classified as having abnormal CIC Grades II–III, 25.5% of eyes (n=25/98) as Grade II, 36.7% of eyes (n=36/98) as Grades I–II, and 15.3% (n=15/98) of eyes as having Grade I severity. By Day 90, the severity of squamous metaplasia reduced in most eyes; 22% of eyes (n=22/98) had improved to Grade 0 (ie, normal morphology), whereas 64% of eyes (n=63/98) were classified as Grade I; only 3 eyes were classified to have Grade II severity (). No change in grade was observed for 8.2% of eyes (n=8/98) during the study.
Exploratory end points
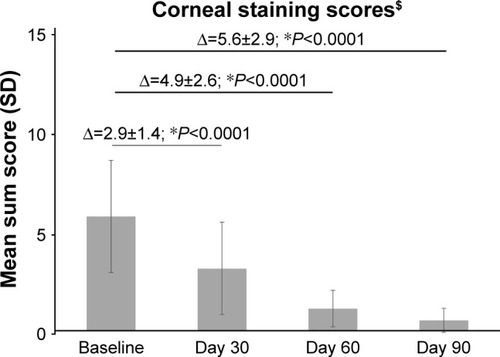
The cornea and conjunctival staining scores and TFBUT improved over the duration of the study. As shown in , there was a marked decrease in the corneal staining sum score from baseline to Days 30, 60, and 90 (P<0.0001). The mean±SD corneal staining score improved from 5.7±2.8 units (range, 3–11) at baseline to 3.1±2.3 units at Day 30 (range, 0–8), 1.1±0.9 units at Day 60 (range, 0–3), and 0.5±0.6 units (range, 0–2) at Day 90. All eyes demonstrated a total staining sum score <3, with 59% of eyes (n=58/98) having a staining sum score of 0 at Day 90.
Figure 4 The mean corneal staining sum score at baseline and Days 30, 60, and 90 after treatment with PEG-PG/HP-guar artificial tears.
Abbreviations: PEG-PG/HP-guar, polyethylene glycol–propylene glycol/hydroxypropyl-guar; SD, standard deviation.
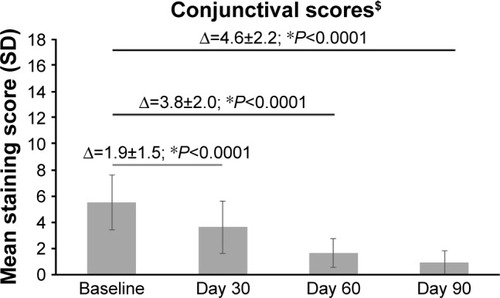
Compared with baseline, there was a notable decrease in the mean±SD conjunctival staining score at all follow-up visits (P<0.0001; ). The mean conjunctival staining scores improved from 5.5±2.1 units (range, 3–11) at baseline to 3.6±2.0 units at Day 30 (range, 0–9), 1.6±1.1 units at Day 60 (range, 0–5), and 0.9±0.9 units (range, 0–3) at Day 90.
Figure 5 The mean conjunctival staining score at baseline and Days 30, 60, and 90 after treatment with PEG-PG/HP-guar artificial tears.
Abbreviations: PEG-PG/HP-guar, polyethylene glycol–propylene glycol/hydroxypropyl-guar; SD, standard deviation.
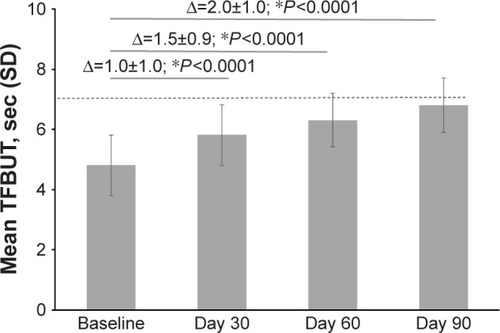
At the baseline, the mean±SD TFBUT was 4.8±1.0 s (range, 3–6), and increased to 5.8±1.0 s (range, 3–8) at Day 30 and 6.3±0.9 s (range, 4–8) at Day 60, ending at 6.8±0.9 s (range, 5–9) at Day 90 (P<0.0001 vs baseline at all time points; ). At Day 90, almost 61% of patients reached a TFBUT of ≥7 s vs none at baseline.
Figure 6 Mean TFBUT at baseline and Days 30, 60, and 90 after treatment with PEG-PG/HP-guar artificial tears.
Abbreviations: PEG-PG/HP-guar, polyethylene glycol–propylene glycol/hydroxypropyl-guar; SD, standard deviation; TFBUT, tear film break-up time.
One patient discontinued the study due to a nonserious AE (intolerance to the test formulation). No other AEs were reported in the study.
Discussion
This study demonstrated that the use of PEG-PG/HP-guar-based artificial tears can help in reversing the changes induced by squamous metaplasia in DED. After 3 months of treatment, a 50% decrease in the squamous metaplasia score (ie, an increase in goblet cell density) was observed compared with baseline, the majority of eyes (~92%) demonstrated an improvement in the Nelson’s impression cytology grade, and 22% of eyes reached Grade 0 (ie, normal epithelial morphology and >500 goblet cells/mm2).
Similar improvements were observed in other signs of DED. There was an approximate 92% and 74% reduction in the mean corneal staining sum score and conjunctival staining sum score from baseline by Day 90, respectively. An increase of 2 s was observed in the mean TFBUT at Day 90. Both corneal and conjunctival stainings are indicative of a compromised ocular surface, and patients with DED have a faster TFBUT than normal individuals due to an unstable tear film. These results show that PEG-PG/HP-guar artificial tears improve ocular surface health and enhance the tear film stability in DED, which is in agreement with the outcomes reported with HP-guar-containing artificial tears in the literature.Citation9–Citation19
The observed therapeutic effect with PEG-PG/HP-guar lubricant eye drops is most likely due to the unique gelling ability of HP-guar in situ. When exposed to tear film pH, HP-guar together with borate ions forms a soft gel-like cross-linked matrix on the innermost mucin layer, allowing longer retention of the demulcents PEG and PG on the ocular surface.Citation9,Citation10,Citation16 This in turn results in longer-lasting hydration and lubrication on the ocular surface and provides a conducive environment for healing and renewal of the damaged goblet and epithelial cells.Citation9,Citation10,Citation16 Alternatively, a significant reduction in the expression of inflammatory marker human leukocyte antigen-DR has been observed in DED patients with regular use of PEG-PG/HP-guar eye drops.Citation20,Citation21 Squamous metaplasia and loss of goblet cells in DED are considered to be the consequences of inflammation, and reversal of the epithelial damage that is observed with PEG-PG/HP-guar thus could also be due to a decrease in the inflammatory response.
Other artificial formulations, such as those containing carboxymethylcellulose and sodium hyaluronate, have also been shown to improve impression cytology grades and reduce squamous metaplasia in keratoconjunctivitis sicca and DED, respectively.Citation22,Citation23 Artificial tears are the first choice of treatment and are used in the management of all forms of DED to provide ocular comfort. Overall, these findings suggest that the effect of some artificial tears may extend beyond symptomatic relief and can be beneficial in restoring the ocular surface homeostasis in DED.
This study did not have a specific safety end point; only self-reported or observed AEs were to be recorded. One patient discontinued the study due to an AE; no other AEs were reported. However, the safety of PEG-PG/HP-guar lubricant eye drops has been evaluated in other studies.Citation9,Citation10,Citation12–Citation14 These lubricant eye drops are well tolerated with no changes in visual acuity, and no abnormalities reported on slit-lamp examination.Citation9,Citation10,Citation12–Citation14
The absence of a control/comparator group, the open-label design, and a lack of a statistical hypothesis are some of the limitations of this study. In addition, the study did not assess symptomatic improvement in patients which would have been a useful correlation for the change in signs of DED translating into visible improvement in patient’s quality of life. Also, though not due to the study criteria, none of the patients in the study had impression cytology ≥Grade 3; data from these patients would have provided more supportive evidence of the effect of PEG-PG/HP-guar artificial tears in reversing ocular surface damage/squamous metaplasia even in severe DED. The inclusion of such patients should therefore be considered in future studies. Further, an analysis by age groups would be useful to understand if any difference is observed in the treatment efficacy due to aging effects.
Conclusion
The PEG-PG/HP-guar artificial tear formulation has a beneficial effect on the tear film stability and ocular health. The PEG-PG/HP-guar artificial tears, used thrice daily for 90 days, reduced squamous metaplasia, in addition to improving TFBUT and corneal staining in patients with DED. Further clinical studies are required to confirm the present findings.
Acknowledgments
This study was funded by Alcon Research Ltd. (now Novartis Pharmaceutical Corporation), Fort Worth, TX, USA. Medical writing support for this paper was provided by Shivani Vadapalli (Novartis Healthcare Pvt. Ltd., Hyderabad, India).
Disclosure
The authors report no conflicts of interest in this work.
References
- HodgesRRDarttDATear film mucins: front line defenders of the ocular surface; comparison with airway and gastrointestinal tract mucinsExp Eye Res2013117627823954166
- StephensDNMcNamaraNAAltered mucin and glycoprotein expression in dry eye diseaseOptom Vis Sci201592993193826267059
- MaCauleyHAGuaschGThree cheers for the goblet cell: maintaining homeostasis in mucosal epitheliaTrends Mol Med201521849250326144290
- GipsonIKGoblet cells of the conjunctiva: a review of recent findingsProg Retin Eye Res201654496327091323
- BronAJde PaivaCSChauhanSKTFOS DEWS II pathophysiology reportOcul Surf201715343851028736340
- De PaivaCSVillarrealALCorralesRMDry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-γInvest Ophthalmol Vis Sci20074862553256017525184
- ZhangJYanXLiHAnalysis of the correlations of mucins, inflammatory markers, and clinical tests in dry eyeCornea201332792893223538614
- MurubeJRivasLBiopsy of the conjunctiva in dry eye patients establishes a correlation between squamous metaplasia and dry eye clinical severityEur J Ophthalmol200313324625612747645
- SpringsCNovel hydroxypropyl-guar gellable lubricant eye drops for treatment of dry eyeAdv Ther2010271068169020803266
- BenelliUSystane lubricant eye drops in the management of ocular drynessClin Ophthalmol2011578379021750611
- TorkildsenGThe effects of lubricant eye drops on visual function as measured by the inter-blink interval visual acuity decay testClin Ophthalmol2009350150619789659
- DavittWFBlooensteinMMartinAChristensenMTMartinAEEfficacy in patients with dry eye after treatment with a new lubricant eye drop formulationJ Ocul Pharmacol Ther201026434735320653478
- KadingDA two-week clinical evaluation of the safety of Systane Ultra in contact lens-wearing patientsClin Ophthalmol20104273220169046
- McDonaldMSchachetJLLievensCWKernJRSystane® Ultra lubricant eye drops for treatment of contact lens-related drynessEye Contact Lens201440210611024552755
- WaduthantriSYongSSTanCHHtoonHMTongLLubricant with gelling agent in treating dry eye in adult Chinese patientsOptom Vis Sci201289111647165323069726
- UbelsJLClousingDPVan HaitsmaTAPre-clinical investigation of the efficacy of an artificial tear solution containing hydroxypropyl-guar as a gelling agentCurr Eye Res200428643744415512952
- ChristensenMTCohenSRinehartJClinical evaluation of an HP-guar gellable lubricant eye drop for the relief of dryness of the eyeCurr Eye Res2004281556214704914
- HartsteinIKhwargSPrzydrygaJAn open-label evaluation of HP-Guar gellable lubricant eye drops for the improvement of dry eye signs and symptoms in a moderate dry eye adult populationCurr Med Res Opin200521225526015801996
- Cervan-LopezISaenz-Frances-San-BaldomeroFBenitez-Del-CastilloJMReduction of corneal permeability in patients treated with HP-Guar: a fluorometric studyArch Soc Esp Oftalmol20068132733216804776
- FernandezKBEpsteinSPRaynorGSModulation of HLA-DR in dry eye patients following 30 days of treatment with a lubricant eyedrop solutionClin Ophthalmol201591137114526170605
- SánchezMAArriola-VillalobosPTorralbo-JiménezPThe effect of preservative-free HP-Guar on dry eye after phacoemulsification: a flow cytometric studyEye (Lond)20102481331133720300126
- GreneBRLankstonPMordauntJHarroldMGwonAJonesRUnpreserved carboxymethylcellulose artificial tears evaluated in patients with keratoconjunctivitis SiccaCornea19921142943011424648
- AragonaPPapaVMicaliASantoconoMMilazzoGLong term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eyeBr J Opthalmol2001862181184