Abstract
Introduction:
To evaluate the long-term efficacy and safety of 3 commercially available fixed combinations of prostaglandin analogs or a prostamide with timolol maleate in patients with primary open angle glaucoma or ocular hypertension.
Methods:
In this randomized, prospective, single-blind study, intraocular pressure (IOP) was measured after a 1-month washout period and pachymetry was performed before randomizing patients to latanoprost 50 μg/timolol 5 mg/1 mL (L/T), bimatoprost 300 μg/timolol 5 mg/1 mL (B/T), or travoprost 40 μg/timolol 5 mg/1 mL (T/T). IOP was measured monthly for 6 months and then at 12 months by an investigator blinded to the study drug. Adverse reactions were recorded.
Results:
128 cases were included in the study. The 3 treatment groups had similar baseline characteristics and comparable IOP. All 3 combinations decreased IOP by at least 6 mmHg and IOP remained below 21 mmHg throughout the study. At 12 months L/T achieved greater reduction in IOP than the other 2 fixed combinations, but the difference between L/T and B/T was not statistically significant. At 6 months, more B/T-treated patients reported red eye (P < 0.05 vs L/T and T/T). At 12 months, fewer adverse reactions were reported, with no cases of red eye reported for L/T (P = 0.03 vs B/T).
Conclusions:
All 3 combinations are effective at lowering IOP but at 12 months L/T and B/T were found to be more effective than T/T. Treatments were well tolerated after 12 months but L/T showed less hyperemia than B/T throughout the study (P < 0.05).
Introduction
The term glaucoma covers a group of chronic optical neuropathies in which ganglion cell damage is associated with a loss of visual field.Citation1 Many patients with ocular hypertension will develop glaucoma.Citation2–Citation4 As high intraocular pressure (IOP) is one of the most important risk factors for glaucoma,Citation1–Citation6 most treatments focus on reducing IOP. TrabeculectomyCitation7 and laser trabeculoplastyCitation8 have been the main, nonpharmacological therapeutic options, but these procedures have potential side effects.
As an alternative, a number of different drugs have become available over the last 3 decades; the β-blocker timolol was introduced in the late 1970s.Citation9 Prostaglandin analogs were introduced in the late 1990sCitation10 and have proved to be more effective at lowering IOP than β-blockers.Citation11,Citation12 More recently, fixed combinations of these agents have been approved: latanoprost with timolol maleate (latanoprost 50 μg and timolol 5 mg/1 mL) was approved in April 2002.Citation13–Citation15 In October 2006, 2 further combinations were approved: travoprost/timolol (travoprost 40 μg and timolol 5 mg/1 mL)Citation16 and bimatoprost/timolol (bimatoprost 300 μg and timolol 5 mg/1 mL).Citation17
Since few data are available comparing the effectiveness and safety of some of these combinations,Citation18,Citation19 and no study has compared the 3 available fixed combinations of prostaglandins and timolol, we conducted a 12-month randomized study to compare the hypotensor effect of these 3 combinations and their tolerability in terms of local events reported by patients with primary open angle glaucoma or ocular hypertension who had used more than 1 antiglaucoma drug previously.
Patients and method
Participants
Patients were eligible for participation in the study if they met the following inclusion criteria: age ≥18 years, primary open angle glaucoma or ocular hypertension (IOP ≥ 21 mmHg at baseline), and previously treated with at least 2 hypotensor drugs. Exclusion criteria were known contraindication to any of the study treatments, use of any medicine that might affect IOP, abnormal ocular condition or symptom preventing the patient from entering the study according to the investigator’s judgment, and pregnancy or lactancy. The sample was recruited from outpatients attending the “Centre d’Atenció Primaria Manso” in Barcelona, Spain. All participants signed the informed consent before any study procedures were conducted.
Patients were assigned to medical interventions at random once we tested that patients fulfilled all the selection criteria of the study. Random codes were obtained by means of a computerized algorithm after the study protocol and related documents were approved by the Scientific Research Committee of the site.
The algorithm produced a block of 9 codes for patients allowing a balanced distribution 1:1:1 of study subjects to the 3 medical interventions evaluated. Eyes from the same patients always received the same medical intervention.
In order to evaluate IOP reduction at 12 months with the 3 commercially available fixed combinations, it was estimated that 111 cases that met all inclusion criteria and none of the exclusion criteria should be included, 37 in each treatment arm.
A sample of 37 cases per treatment arm allowed the detection of a reduction ≥2 mmHg with a significance level of 95%, power of 80,0%, in bilateral contrast, assuming a common standard deviation of 4.
Interventions
After a 1-month washout period without any antiglaucoma drugs, the patients were randomized to 1 of the 3 combination treatments: latanoprost 50 μg and timolol 5 mg/1 mL (L/T); travoprost 40 μg and timolol 5 mg/1 mL (T/T); or bimatoprost 300 μg and timolol 5 mg/1 mL (B/T). These treatments were administered at night according to the labeling. Prior to treatment, baseline pachymetry was undertaken with the DHG 5100E pachymeter and IOP was measured by Goldman applanation tonometry.
Evaluations were carried out every month by the same masked observer until month 6 and then at month 12. The patients were aware of the treatment that they used. All the examinations were performed between 8:00 and 10:30 am. IOP was measured and patients were asked about side effects and adverse events. The primary efficacy variable of the study was mean change in IOP between baseline and month 12.
Statistical analyses
The primary efficacy variable was IOP reduction with the 3 commercially available fixed combinations.
Baseline patient characteristics are described using mean (SD) values for quantitative variables; absolute and relative frequency distributions were used for qualitative variables. The Kolmogorov–Smirnov test was used to test for normal distribution. Reductions in IOP in follow-up visits are expressed in absolute and relative changes versus the baseline value in each study group.
The changes from baseline in IOP were calculated using measurements of central tendency (mean) and dispersion (95% confidence interval [CI]). Groups were compared by analysis of variance (ANOVA) and t tests for independent samples.
Results
Of the initial group of 141 cases, 13 were excluded because of extremely high initial IOP (3 cases in the B/T group), very high pachymetry values (2 cases in T/T group and 2 in B/T group), poor control of IOP (2 cases in the T/T group), substantial discomfort reported by the patient (2 cases in the L/T group), and fear of adverse effects of timolol maleate (2 cases in the B/T group). Thus, 128 cases completed the 12 months of follow-up (44 in the T/T group, 42 in the B/T group, and 42 in the L/T group).
The mean age of the overall sample was 68 years (range, 39–88 years). The ANOVA test was 0.064. The mean ages of patients in the individual treatment groups were similar, ranging from 70.95 years in the T/T group to 65.74 years in the B/T group. Overall, 68% were women as a result of simple random sampling (50% in the T/T group, 69% in the B/T group, and 85% in the L/T group), and there were no statistically significant differences between groups. The most frequently used prior treatment was L/T and a combination of latanoprost and dorzolamide/timolol. The most common comorbidities were cataracts (72% in the T/T group, 55% in the B/T group, and 71% in the L/T group), hypertension (54% in the T/T group, 52% in the B/T group, and 42% in the L/T group), and dyslipidemia (46% in the T/T group, 23% in the B/T group, and 31% in the L/T group). The only other underlying diseases present in more than 20% of patients in a given group were diabetes (23% in the L/T group) and myopia (21% in the L/T group).
Intraocular pressure
The initial IOP, after 1 month without any hypotensive drugs (washout period), was between 26 and 28 mmHg (26.4 mmHg for T/T, 27.6 mmHg for L/T, and 28.0 mmHg for B/T) and showed no significant differences between groups (P = 0.22, ANOVA). After 1 month of treatment, IOP decreased below 21 mmHg for all combinations (), and remained below 21 mmHg throughout follow-up.
Figure 1 Changes in intraocular pressure (IOP) from baseline.
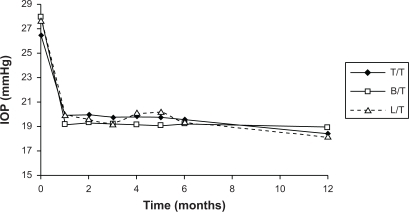
By month 12 significant IOP reductions were observed in all the 3 treatment groups. However, mean change from baseline was significantly greater with L/T and with B/T than with T/T; by month 12 the mean IOP reduction was 9.02 mmHg for patients treated with L/T, 8.56 mmHg for B/T-treated patients, and 6.61 mmHg for patients receiving T/T.
The mean IOP reductions throughout the period of the study are summarized in .
Table 1 Group comparisons for absolute decrease in intraocular Pressure during follow-up
The relative decrease in IOP at month 12 was 26.24% for T/T, 30.19% for B/T, and 32.35% for L/T.
Local adverse events
In all treatment groups, the most frequently reported adverse event at 6 months was red eye (22.7% [SD 0.4] in the T/T group, 45.2% [SD 0.5] in the B/T group, and 23.8% [SD 0.4] in the L/T group), and the differences between B/T and L/T and T/T were statistically significant (P = 0.027 vs T/T and P = 0.039 vs L/T). Dryness was reported in 22.7% (SD 0.4) of patients in the T/T group, 14.1% (SD 0.4) in the L/T group, and 9.5% (SD 0.3) in the B/T group; itching by 13.6% (SD 0.3) in the T/T group, 16.7% (SD 0.4) in the B/T group, and 14.3% (0.4) in the L/T group; and dark eye rings by 4.5% in the T/T group, 14.3% in the B/T group, and none in the L/T group. No other local adverse reaction was reported by more than 10% in a given treatment group ().
Figure 2 Reported symptoms at 6 and 12 months for the 3 fixed combinations: A) red eye; B) itching; C) dryness.
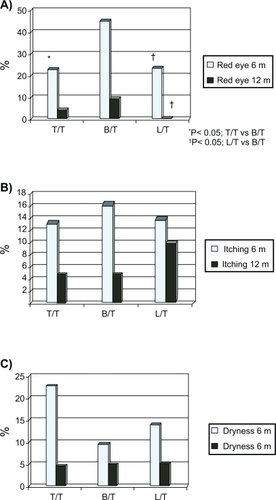
At 12 months, for all 3 combinations, the proportion of patients with such symptoms had decreased except for dark eye rings, which increased in the T/T group to 13.6% and in the L/T group to 15.0%. Of particular note was the decrease in red eye in the B/T group from 45.2% at 6 months to 9.5% (SD 0.3) at 1 year and the complete absence of red eye in the L/T group at 1 year. Despite this decrease, the differences between B/T and L/T remained statistically significant (P = 0.030).
Discussion
Although there are other risk factors associated with the development and progression of glaucoma besides IOP, the most widely studied and most important risk factor is IOP.Citation1,Citation3,Citation6 This is also a factor that can be readily modified by pharmacological intervention. Different studies associate high IOP with damage to the optic nerve and suggest that the onset of glaucoma is delayed and the severity reduced when IOP is reduced.Citation2,Citation4 It has been shown that 9.5% of patients with ocular hypertension (defined as IOP between 24 mmHg and 32 mmHg in one eye and between 21 mmHg and 32 mmHg in the other eye) are at risk of developing glaucoma, but this percentage falls to 4.4% if these patients are treated.Citation3 Although the target IOP suggested in the Advanced Glaucoma Intervention Study is 18 mmHg,Citation2 the European Glaucoma Society suggests that target IOP should be set so as not to damage the optic nerve.Citation20
IOP is determined largely by the balance between production of aqueous humor and outflow.Citation21 Most of the agents currently available act by increasing uveoscleral outflow of aqueous humor, as is the case of prostaglandin analogs,Citation22 by decreasing production of aqueous humor by the ciliary body, as is the case of β-blockers,Citation23 or both, as is the case of alpha2-adrenergic agonists such as brimonidine.Citation24 The clinical efficacy of such substances in lowering IOP has been shown in a number of studies. Nevertheless, the targets for IOP lowering are quite stringent and may not be reached using a single agent.Citation25 In this case, the idea of combining 2 agents is an attractive one, particularly if they work by different mechanisms as is the case of combinations of prostaglandins and β-blockers. Studies that compared combinations of latanoprost and timolol suggested that there was indeed an additive effect and lower IOP could be attained by such a strategy.Citation26 With the concept of combined therapy proven, it might also be expected that fixed combinations of these drugs, which can be applied in a single application, would improve the convenience of dosing and so improve adherence to treatment.Citation27 Although fixed combination treatments have been investigated,Citation13–Citation19 studies comparing the efficacy of the 3 prostaglandins fixed combinations are not available. Studies have however been conducted comparing fixed combination timolol/dorzolamide with a combination of timolol with unoprostone.Citation28 That study reported similar efficacy for the timolol/carbonic anhydrase inhibitor combination and the timolol/prostaglandin combination.
Our study aimed to assess the reduction in IOP with 3 commercially available fixed combinations for lowering IOP, namely L/T (latanoprost 50 μg and timolol 5 mg/1 mL), T/T (travoprost 40 μg and timolol 5 mg/1 mL), and B/T (bimatoprost 300 μg and timolol 5 mg/1 mL). The absolute decreases in IOP from baseline were similar to those reported in a study with the fixed combination of T/T (7–9 mmHg).Citation16 We also recorded local adverse reactions given that the tolerability is important for ensuring adherence to medication, particularly in the case of long-term therapy required to treat chronically elevated IOP.
The 3 combinations were good depressors of the IOP and all achieved substantial reductions in the IOP. However, at 6 months, there were differences in the symptoms reported: red eye, dark eye rings, and itching were reported more often in the B/T group whereas dryness was reported more frequently in the T/T group. After 1 year of follow-up, the overall rate of adverse reactions decreased. Thus red eye decreased in all groups, and in the L/T group, no patients reported this symptom.
These results from 1 year of follow-up support the long-term efficacy of these fixed combinations. The absolute decrease in IOP at 12 months was significantly larger for the L/T and B/T combinations than the T/T combination. For most symptoms, tolerance improved from 6 to 12 months. In particular, the number of patients with red eye decreased substantially to such an extent that, for the L/T fixed combination, no patients reported this condition. This suggests that these fixed combinations are well tolerated with the long-term use required in these patients.
Disclosure
The authors declare no conflicts of interest.
References
- American Academy of OphthalmologyGlaucomaBasic and Clinical Science Course2007–2008San Francisco, CA, USA
- The Advanced Glaucoma Intervention Study (AGIS)The relationship between control of intraocular pressure and visual field deteriorationAm J Ophthalmol200013042944011024415
- KassMAHeuerDKHigginbothamEJThe Ocular Hypertension Treatment Study: a randomized trail determines that tropical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucomaArch Ophthalmol200212070171312049574
- HeijlALeskeMCBengtssonBReduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma TrialArch Ophthalmol20021201268127912365904
- Collaborative Normal-Tension Glaucoma Study GroupThe effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucomaAm J Ophthalmol19981264985059780094
- LeskeMCHeijlAHusseinMEarly Manifest Glaucoma Trial Group: Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trialArch Ophthalmol2003121485612523884
- MillsKBTrabeculectomy: a retrospective long-term follow-up of 444 casesBr J Ophthalmol1981657907957326225
- ThomasJVSimmonsRJBelcherCD3rdArgon laser trabeculoplasty in the presurgical glaucoma patientOphthalmology1982891871977088501
- HeelRCBrogdenRNSpeightTMAveryGSTimolol: a review of its therapeutic efficacy in the topical treatment of glaucomaDrugs1979173855369807
- AlexanderCLMillerSJAbelSRProstaglandin analog treatment of glaucoma and ocular hypertensionAnn Pharmacother20023650451111895065
- AlmAStjernschantzJEffects on intraocular pressure and side effects of 0.005% latanoprost applied once daily, evening or morning. A comparison with timolol. Scandinavian Latanoprost Study GroupOphthalmology1995102174317529098273
- WatsonPGLatanoprost. Two years’ experience of its use in the United Kingdom. Latanoprost Study GroupOphthalmology199810582879442782
- LarssonLIEffect on intraocular pressure during 24 hours after repeated administration of the fixed combination of latanoprost 0.005% and timolol 0.5% in patients with ocular hypertensionJ Glaucoma20011010911411316092
- LarssonLIThe effect on diurnal intraocular pressure of the fixed combination of latanoprost 0.005% and timolol 0.5% in patients with ocular hypertensionActa Ophthalmol Scand20017912512811284748
- PfeifferNEuropean Latanoprost Fixed Combination Study GroupA comparison of the fixed combination of latanoprost and timolol with its individual componentsGraefes Arch Clin Exp Ophthalmol200224089389912486510
- SchumanJSKatzGJLewisRAEfficacy and safety of a fixed combination of travoprost 0.004%/timolol 0.5% ophthalmic solution once daily for open-angle glaucoma or ocular hypertensionAm J Ophthalmol200514024225016086946
- HommerAGanfort Investigators Group IA double-masked, randomized, parallel comparison of a fixed combination of bimatoprost 0.03%/timolol 0.5% with non-fixed combination use in patients with glaucoma or ocular hypertensionEur J Ophthalmol200717536217294383
- MartinezASanchezMA comparison of the safety and intraocular pressure lowering of bimatoprost/timolol fixed combination versus latanoprost/timolol fixed combination in patients with open-angle glaucomaCurr Med Res Opin2007231025103217519068
- MartinezASanchezMBimatoprost/timolol fixed combination vs latanoprost/timolol fixed combination in open-angle glaucoma patientsEye (Lond)20092381081818535605
- BonomiLMarchiniGMarraffaVascular risk factors for primary open angle glaucoma: the Egna-Neumarkt StudyOphthalmology20001071287129310889099
- SeilerTWollensakJThe resistance of the trabecular meshwork to aqueous humor outflowGraefes Arch Clin Exp Ophthalmol198522388914007511
- TorisCBCamrasCBYablonskiMEEffects of PhXA41, a new prostaglandin F2 alpha analog, on aqueous humor dynamics in human eyesOphthalmology1993100129713048371915
- CoakesRLBrubakerRFThe mechanism of timolol in lowering intraocular pressure. In the normal eyeArch Ophthalmol19789620452048363105
- TorisCBGleasonMLCamrasCBYablonskiMEEffects of brimonidine on aqueous humor dynamics in human eyesArch Ophthalmol1995113151415177487618
- UusitaloRJPalkamaALong-term evaluation of timololActa Ophthalmol (Copenh)1989675735812686343
- RuloAHGreveELHoyngPFAdditive effect of latanoprost, a prostaglandin F2 alpha analogue, and timolol in patients with elevated intraocular pressureBr J Ophthalmol1994788999027819171
- PatelSCSpaethGLCompliance in patients prescribed eyedrops for glaucomaOphthalmic Surg1995262332367651690
- DayDGSchacknowPNWandMTimolol 0.5%/dorzolamide 2% fixed combination vs timolol maleate 0.5% and unoprostone 0.15% given twice daily to patients with primary open-angle glaucoma or ocular hypertensionAm J Ophthalmol200313513814312566015