Abstract
Purpose:
Ketorolac 0.45% is a new formulation of topical ketorolac in which preservative (benzalkonium chloride, BAK) was removed and carboxymethylcellulose (CMC) was added to improve tolerability and reduce dosing frequency. This study compared the effects of ketorolac 0.45% on corneal wound healing to prior ketorolac formulations (0.4% and 0.5%), bromfenac 0.09%, and nepafenac 0.1%.
Methods:
Two parallel-group comparisons were performed in series. A 5-mm central epithelial wound was made in fresh porcine corneas. After 24 hours in minimum essential medium (MEM), corneas were incubated for 10 minutes with study drugs, Triton X-100 1% (positive control), or MEM (negative control), followed by 24 hours in MEM. The remaining wound area was stained, photographed, and quantified (pixels). Study 1 compared ketorolac 0.45% to ketorolac 0.4% and ketorolac 0.5%. Study 2 compared ketorolac 0.45% to bromfenac 0.09% and nepafenac 0.1%.
Results:
The mean (±SD) original wound area was 200,506 ± 4,363 pixels, which was reduced to 59,509 ± 4850 at 48 hours after exposure to Triton X-100 1%. In study 1, the mean remaining wound areas at 48 hours in pixels were 2969 ± 1633 with MEM, 586 ± 299 with ketorolac 0.45% (significantly reduced, P < 0.05 vs all other treatments), 10,228 ± 7541 with ketorolac 0.4%, and 50,674 ± 33,409 with ketorolac 0.5% (significantly enlarged, P < 0.05 vs MEM). In study 2, the mean remaining wound areas at 48 hours were 565 ± 1263 with MEM, 322 ± 229 with ketorolac 0.45% (significantly reduced, P < 0.01 vs bromfenac 0.09% and nepafenac 0.1%), 29,093 ± 14,295 with bromfenac 0.09% (significantly enlarged, P <0.01 vs MEM) and 47,322 ± 13,736 with nepafenac 0.1% (significantly enlarged, P < 0.01 vs MEM and vs bromfenac 0.09%).
Conclusion:
Corneas treated with ketorolac 0.45% healed as rapidly as those treated with MEM, likely secondary to addition of CMC and removal of BAK. In the ex vivo corneal organ culture model, ketorolac 0.45% had statistically less impact on corneal re-epithelialization than prior ketorolac formulations (0.4% and 0.5%), bromfenac 0.09%, and nepafenac 0.01%.
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDS) have anti-inflammatory, analgesic, and antipyretic properties.Citation1 NSAIDS primarily act as cyclooxygenase inhibitors and limit the production of endogenous prostaglandins in the arachidonic acid cascade.Citation2,Citation3 Topical NSAIDS are commonly used in ophthalmology to control postoperative inflammation, reduce pain following refractive surgery, inhibit intraoperative miosis, and treat allergic conjunctivitis.Citation4,Citation5 Commercially available topical NSAIDS may differ in the type and concentration of the active agent, concentration of preservative, pH, and/or additional surfactants.
Topical NSAIDS have been associated with corneal complications which include superficial punctate keratopathy, subepithelial infiltrates, epithelial defects, delayed epithelial healing, corneal anesthesia, ulceration, and perforation.Citation6–Citation12 While corneal epithelial toxicity has been attributed to both the active agents and the preservatives within topical formulations as well as pre-existing systemic conditions, keratitis, corneal ulceration, and perforation have all been reported in patients using preservative-free formulations.Citation8,Citation13,Citation14 Animal studies have suggested that the corneal toxicity of topical NSAIDS may result from an upregulation of matrix metalloproteinases (MMPs), endopeptidases involved in the remodeling of the corneal extracellular matrix.Citation15
As topical NSAIDs are commonly used in the perioperative setting, it is critical to develop formulations that do not inhibit epithelial healing. Topical ketorolac, first introduced in 1993, remains the most commonly used ophthalmic NSAID. Ophthalmic ketorolac 0.45% solution (Acuvail®, Allergan, Inc., Irvine, CA) was developed to maintain the efficacy of prior formulations of ketorolac while enhancing tolerability and reducing the dosing regimen. Changes in the new formulation, ketorolac 0.45%, include the removal of the preservative (BAK) and the surfactant (octoxynal 40) and the addition of carboxymethylcellulose (CMC) (), which prolongs drug retention on the eye, protects the ocular surface, and may also promote corneal re-epithelialization.Citation16,Citation17
Table 1 Compositions of the study ophthalmic NSAID solutions
A porcine corneal model of wound healing was developed which has previously been used to assess the role of growth factors in corneal re-epithelialization.Citation18,Citation19 Given the changes in the formulation of ketorolac, the present study was designed to compare the impact on wound healing of the new formulation of ketorolac 0.45% to prior generations of ketorolac (0.4% and 0.5%) as well as to bromfenac 0.09% and nepafenac 0.1%.
Methods
Two parallel-group comparisons were performed in series to evaluate epithelial wound closure in an ex vivo porcine corneal organ culture model that has been previously described.Citation18,Citation19 In the first experiment, epithelial debrided porcine corneas were exposed to different formulations of the ketorolac molecule, namely ketorolac 0.45% (Acuvail®, Allergan, Inc.), ketorolac 0.4% (Acular LS®; Allergan, Inc.), and ketorolac 0.5% (Acular®, Allergan, Inc.). In the second experiment, ketorolac 0.45% was compared to bromfenac 0.09% (Xibrom®, Ista Pharmaceuticals, Irvine, CA) and nepafenac 0.1% (Nevanac®, Alcon Laboratories, Fort Worth, TX).
In brief, porcine eyes were obtained from a local abattoir, transported to the laboratory on ice in a moist chamber, and processed for corneal culture on the same day. An epithelial wound was made by demarcating an area on the central cornea with a 5-mm trephine and then removing the epithelium within the circle with a small scalpel, leaving an intact basement membrane. Corneal-scleral rims, with approximately 4 mm of the limbal conjunctiva present, were excised and rinsed in sterilized phosphate-buffered saline. The excised corneas were placed epithelial-side down into a sterile cup. The endothelial corneal concavity was then filled with minimum essential medium (MEM) containing 1% agarose and 1 mg/mL rat tail tendon collagen maintained at 42°C. This mixture was allowed to gel. The cornea, along with its supporting gel, was inverted (endothelial side down) and then transferred to a 35-mm dish. The culture medium (∼2 mL) was added dropwise to the surface of central cornea until the limbal conjunctiva was covered, leaving the epithelium exposed to the air. The corneas were then cultured in a humidified 5% CO2 incubator at 37°C in MEM for 24 hours to ensure initial wound recovery.
After 24 hours, a 50-μL volume of MEM, Triton X-100 (1%; Dow Chemical Company, Midland, MI), or study drugs (ketorolac 0.45%, ketorolac 0.4%, ketorolac 0.5%, nepafenac 0.01%, or bromfenac 0.09%) was applied to the epithelial wound surface for 10 minutes. MEM alone was used as a negative control, and Triton X-100 was used as a positive control. Corneas were rinsed twice with 3 mL of phosphate-buffered saline and incubated in 2 mL of fresh MEM for an additional 24 hours prior to staining. The remaining wound area was then stained with Richardson staining solution and photographed.Citation20 The staining area was quantified (in pixels) using Adobe Photoshop® (Adobe Systems, Inc., San Jose, CA) as previously described. A total of five corneas was used in each treatment group.
Data were presented as the mean of remaining wound area ± standard deviation (SD). One way analysis of variance (ANOVA) with a Newman-Keuls posttest was used for statistical comparison among groups. A P-value of <0.05 was considered statistically significant.
Results
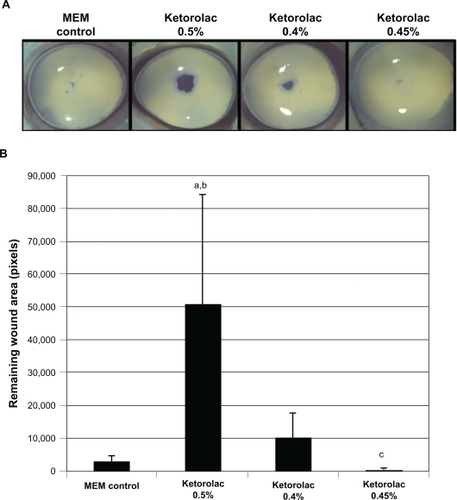
The mean (SD) original wound area induced by the 5 mm trephine was 200,506 ± 4363 pixels. At 48 hours, corneas treated with Triton X-100 1% had a mean (SD) remaining wound area of 59,509 ± 4850 pixels (). In study 1, the mean (SD) remaining wound areas at 48 hours following treatment with MEM control, ketorolac 0.45%, ketorolac 0.4%, and ketorolac 0.5% were 2969 ± 1633 pixels, 586 ± 299 pixels, 10,228 ± 7541 pixels, and 50,674 ± 33,409 pixels, respectively (). Corneas treated with ketorolac 0.45% had a significantly smaller mean remaining wound area than did corneas treated with MEM control, ketorolac 0.4%, and ketorolac 0.5% (P < 0.05). In contrast, the mean remaining wound area of corneas treated with ketorolac 0.5% was not statistically different than those treated with Triton X-100 1%.
Figure 1 Representative images of corneas with original wound and those following treatment with Triton X-100 1%.
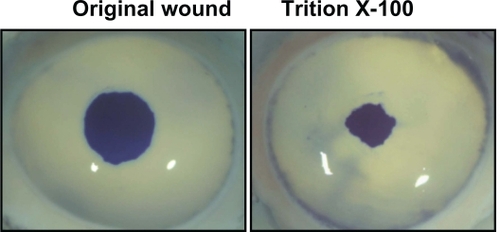
Figure 2 Remaining epithelial wound area in corneas treated with various ophthalmic solutions of ketorolac. Representative images of corneas treated with ketorolac 0.5%, 0.4%, and 0.45% A) and corresponding intensity of the wound area staining B) are shown.
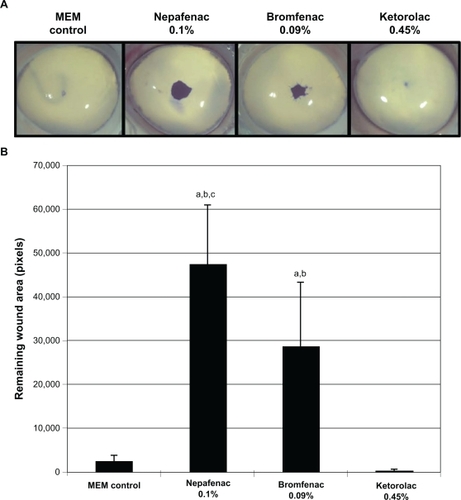
In study 2, corneas treated with MEM control, ketorolac 0.45%, bromfenac 0.09%, and nepafenac 0.1% had mean (SD) remaining wound areas at 48 hours of 565 ± 1263 pixels, 322 ± 229 pixels, 29,093 ± 14,295 pixels, and 47,322 ± 13,736 pixels, respectively (). The remaining wound areas were significantly smaller in corneas treated with ketorolac 0.45% as compared to those treated with bromfenac 0.09% or nepafenac 0.1% (P < 0.01). Corneas treated with nepafenac 0.1% had a significantly larger mean remaining wound area than those treated with bromfenac 0.09% (P < 0.01). The mean remaining wound area of corneas treated with nepafenac 0.1% was not statistically different from that of corneas treated with Triton X-100 1%.
Figure 3 Remaining epithelial wound area in corneas treated with nepafenac 0.1%, bromfenac 0.09%, and ketorolac 0.45%. Representative images of corneas treated with nepafenac 0.1%, bromfenac 0.09%, and ketorolac 0.45% A) and corresponding intensity of the remaining wound area B) are shown.
Discussion
The use of topical NSAIDs for the treatment of postoperative pain and inflammation has increased in recent years, and NSAIDs have now become an integral part of the perioperative regimen.Citation5 There remain, however, concerns about the impact of NSAIDs on corneal re-epithelialization following ocular surgery.Citation14,Citation21 Given the significance of reestablishing a normal epithelial barrier, it is important to select a perioperative NSAID that does not interfere with epithelial wound healing.
In this ex vivo corneal organ culture model, the corneal epithelial wounds healed as rapidly with the ketorolac 0.45% formulation as with the MEM control. Significant differences in the rate of wound healing were found between both prior ketorolac formulations (ketorolac 0.5% and ketorolac 0.4%) and the new ketorolac 0.45% formulation, in which BAK was removed and CMC was added. In addition, significant differences in the rate of wound healing were observed when either bromfenac 0.09% or nepafenac 0.1% were compared to ketorolac 0.45%. Of note, the remaining wound areas at 48 hours following treatment with ketorolac 0.5% and nepafenac 0.1% were not statistically different from those after treatment with the positive control, Triton X-100 1%, a detergent known to jeopardize cell viability by disrupting cell membrane integrity.Citation22
The mechanism by which ophthalmic NSAID solutions delay wound healing is not precisely known. However, it has been shown in animal models that ophthalmic NSAID solutions may increase the expression of MMP-1 (collagenase-1), MMP-2 (gelatinase A), and MMP-8 (collagenase-2), which, in turn, may impair corneal wound healing by destroying newly deposited extracellular matrix, interrupting epithelial cell interaction, and disrupting epithelial basement membrane.Citation15 The preservative BAK included in most ophthalmic NSAID solutions may also contribute to the delay in epithelial wound healing by inducing inflammation, increasing transepithelial permeability, and decreasing epithelial vitality.Citation23–Citation25
Both the removal of the preservative as well as the addition of CMC in the ketorolac 0.45% formulation likely play a role in the similar impact on epithelial wound healing between ketorolac 0.45% and the MEM control. Despite a higher concentration of active agent relative to ketorolac 0.4%, ketorolac 0.45% has significantly less effect on epithelial wound healing. CMC has been shown to stimulate epithelial wound healing in animal models as well as in in vitro human corneal epithelial cells culture models.Citation16,Citation26 Though the exact mechanism has yet to be determined, CMC has been shown to stimulate epithelial cell migration by binding to matrix proteins and facilitating epithelial cell attachments.Citation16 The results of the current study also demonstrate the role of the active ingredients in each topical NSAID, as significant differences were observed in the wound healing effect of bromfenac 0.09% and nepafenac 0.1% despite equivalent concentrations of BAK.
There are a limited number of prospective clinical studies comparing the effects of topical NSAIDs on corneal re-epithelialization, and most reports in the literature are based on retrospective case series. Durrie et al reported in a double-masked, randomized prospective study of eyes post-photorefractive keratectomy that ketorolac 0.4% and nepafenac 0.1% treated eyes achieved complete re-epithelialization significantly faster than bromfenac 0.09% treated eyes, though these results may have been affected by thrice daily treatment in each study arm and daily contact lens removal.Citation6 A prospective, double-masked study in which one eye received ketorolac 0.4% and the fellow eye received nepafenac 0.1% following epi-laser in situ keratomileusis (LASIK) was halted after only 14 eyes of 7 patients due to differences in wound healing (5.7 ± 1.1 days with ketorolac 0.4% vs 7.9 ± 2.1 days with nepafenac) and statistically significantly greater mean haze scores at week 2 and month 1 in eyes treated with nepafenac.Citation12 In contrast, there was no difference in the rate of corneal epithelial wound healing between the eyes treated with nepafenac 0.1% and ketorolac 0.4% once the NSAIDs were instilled after placement of the bandage contact lens.Citation27
The ex vivo model of corneal wound healing used in this study demonstrates that the addition of CMC and the removal of BAK in the new formulation of ketorolac 0.45% allows for more rapid corneal epithelial wound healing than prior ketorolac formulations, nepafenac 0.1%, and bromfenac 0.09%. Limitations of the current study include the lack of treatment arms involving CMC or vehicle alone and the inability to fully assess the structural integrity of the healed corneal wounds. Ultimately, animal and in vitro models do not necessarily take into account differences in exposure times on the ocular surface or clinical dosing regimens. Additional clinical studies directly comparing the effects of topical NSAIDs on wound healing are warranted to corroborate the results of the present study in porcine eyes.
Disclosure
This study was funded by Allergan, Inc. Dr McDermott is a consultant to Allergan, Inc. Ms Villanueva and Drs Schiffman and Hollander are employees of Allergan, Inc. Editorial assistance in the preparation of this manuscript was provided by Hadi Moini, PhD, of Pacific Communications, a wholly owned subsidiary of Allergan, Inc.
References
- PayanDGKatzungBGNonsteroidal anti-inflammatory drugs; Nonopioid analgesics; drugs used in goutKatzungBGBasic and Clinical Pharmacology6th edLondon, UKPrentice Hall International1995536559
- KoayPThe emerging roles of topical non-steroidal anti-inflammatory agents in ophthalmologyBr J Ophthalmol19968054804858695573
- WarnerTDMitchellJACyclooxygenases: new forms, new inhibitors, and lessons from the clinicFASEB J200418779080415117884
- KimSJFlachAJJampolLMNonsteroidal anti-inflammatory drugs in ophthalmologySurv Ophthalmol201055210813320159228
- O’BrienTPEmerging guidelines for use of NSAID therapy to optimize cataract surgery patient care [Erratum in: Curr Med Res Opin. 2005;21(9):1431–1432]Curr Med Res Opin20052171131113716004683
- DurrieDSKennardMGBoghossianAJEffects of nonsteroidal ophthalmic drops on epithelial healing and pain in patients undergoing bilateral photorefractive keratectomy (PRK)Adv Ther20072461278128518165210
- GillsJPVoltaren associated with medication keratitisJ Cataract Refract Surg19942011108133470
- GuideraACLuchsJIUdellIJKeratitis, ulceration, and perforation associated with topical nonsteroidal anti-inflammatory drugsOphthalmology2001108593694411320025
- ProbstLEMachatJJCorneal subepithelial infiltrates following photorefractive keratectomyJ Cataract Refract Surg19962232818778354
- SherNAKruegerRRTealPJansRGEdmisonDRole of topical corticosteroids and nonsteroidal anti-inflammatory drugs in the etiology of stromal infiltrates after excimer photorefractive keratectomyJ Refract Corneal Surg19941055875887530111
- ShimazakiJSaitoHYangHYTodaIFujishimaHTsubotaKPersistent epithelial defect following penetrating keratoplasty: an adverse effect of diclofenac eyedropsCornea19951466236278575187
- TrattlerWMcDonaldMDouble-masked comparison of ketorolac tromethamine 0.4% versus nepafenac sodium 0.1% for postoperative healing rates and pain control in eyes undergoing surface ablationCornea200726666566917592313
- FlachATopically applied nonsteroidal anti-inflammatory drugs and corneal problems: an interim review and commentOphthalmology200010771224122610889089
- GaynesBIOnyekwulujeATopical ophthalmic NSAIDs: a discussion with focus on nepafenac ophthalmic suspensionClin Ophthalmol20082235536819668727
- ReviglioVERanaTSLiQJAshrafMFDalyMKO’BrienTPEffects of topical nonsteroidal anti-inflammatory drugs on the expression of matrix metalloproteinases in the corneaJ Cataract Refract Surg200329598999712781288
- GarrettQSimmonsPAXuSCarboxymethylcellulose binds to human corneal epithelial cells and is a modulator of corneal epithelial wound healingInvest Ophthalmol Vis Sci20074841559156717389485
- SasakiHYamamuraKMukaiTPharmacokinetic prediction of the ocular absorption of an instilled drug with ophthalmic viscous vehicleBiol Pharm Bull200023111352135611085365
- XuKPDingYLingJDongZYuFSWound-induced HB-EGF ectodomain shedding and EGFR activation in corneal epithelial cellsInvest Ophthalmol Vis Sci200445381382014985295
- XuKPLiYLjubimovAVYuFSHigh glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healingDiabetes20095851077108519188434
- RichardsonKCJarettLFinkeEHEmbedding in epoxy resins for ultrathin sectioning in electron microscopyStain Technol19603531332313741297
- FlachAJCorneal melts associated with topically applied nonsteroidal anti-inflammatory drugsTrans Am Ophthalmol Soc20019920521011797308
- VennstromLBysellCBjorkelundHLundqvistHAnderssonKReal-time viability assay based on 51Cr retention in adherent cellsBiotechniques200844223724018330352
- ChaSHLeeJSOumBSKimCDCorneal epithelial cellular dysfunction from benzalkonium chloride (BAC) in vitroClin Experiment Ophthalmol200432218018415068436
- EpsteinSPChenDAsbellPAEvaluation of biomarkers of inflammation in response to benzalkonium chloride on corneal and conjunctival epithelial cellsJ Ocul Pharmacol Ther200925541542419857103
- KusanoMUematsuMKumagamiTSasakiHKitaokaTEvaluation of acute corneal barrier change induced by topically applied preservatives using corneal transepithelial electric resistance in vivoCornea2010291808519770721
- GarrettQXuSSimmonsPACarboxymethyl cellulose stimulates rabbit corneal epithelial wound healingCurr Eye Res200833756757318600489
- DonnenfeldEDHollandEJDurrieDSRaizmanMBDouble-masked study of the effects of nepafenac 0.1% and ketorolac 0.4% on corneal epithelial wound healing and pain after photorefractive keratectomyAdv Ther200724485286217901034