Abstract
Purpose
The purpose of this study was to evaluate the safety of high-dose intravitreal triamcinolone acetonide (IVTA) as affordable low-cost alternative to anti-vascular endothelial growth factor (anti-vascular endothelial growth factor [anti-VEGF] agents) in lower-middle-income countries.
Patients and methods
This was a retrospective interventional non-comparative case series. The study recruited patients who received 20 mg IVTA for treating various retinal and optic nerve diseases over the past 5 years. Main outcome measure was assessment of complications secondary to high-dose IVTA. The crosstabs procedure was used to display the interaction between the variables tested. The ANOVA test was used to analyze the differences among group means.
Results
The study included 207 eyes of 168 patients. The main indication for high-dose IVTA were diabetic macular edema 64%, and macular edema secondary to retinal vein occlusion 19%. The mean follow-up period post-injection was 22 months. Mean number of injections was 1.3. Cataract developed in 54% of eyes. Glaucoma developed in 18.5% of eyes. Glaucoma surgery for intractable glaucoma attributed to high-dose IVTA was needed in 1% of eyes. Endophthalmitis and retinal detachment developed in one patient each.
Conclusion
High-dose IVTA is a safe and cost-effective alternative to anti-VEGF agents. Cataract formation and intraocular pressure rise do not pose major adverse effects when weighed against the risk of vision loss due to inability to afford anti-VEGF treatment.
Introduction
The use of the intravitreal route for delivering triamcinolone acetonide (IVTA) has the advantages of delivering high-dose of steroids directly to the site of action, thereby achieving high potency in reversing macular edema and in restoring the integrity of the blood–retina barrier while evading the systemic side effects of parenteral and oral use. However, the initial enthusiasm for using IVTA has been quenched by the waning effect of the drug over relatively short duration; hence, the need for repeated injections to maintain initial anatomical and functional success, and by the ensuing complications that included ocular hypertension even after single injection, and steroid-induced cataract that delayed stable visual recovery until after cataract removal.Citation1,Citation2
Anti-vascular endothelial growth factor (anti-VEGF) agents include drugs that antagonize the action of VEGF, namely bevacizumab and ranibizumab, and drugs that compete with VEGF for its receptor binding site, namely aflibercept.Citation1 The emergence of anti-VEGF agents for treating macular edema was corroborated by evidence from landmark studies that demonstrated the favorable anatomic and functional outcomes of these agents while evading the side effects of IVTA.Citation3–Citation12 Consequently, the past two decades witnessed steady decline of reliance on IVTA for treating macular edema in favor of newer agents including anti-VEGF agents that currently pose as the standard therapeutic regimen for macular edema, in addition to intravitreal dexamethasone (Ozurdex) and intravitreal fluocinolone acetonide (Iluvien) sustained-delivery inserts.Citation13–Citation15 Nowadays, IVTA is largely considered a second therapeutic line that should be reserved to patients with macular edema deemed refractory to anti-VEGF agents or in whom anti-VEGF agents were contraindicated.Citation16–Citation18
Similar to IVTA, anti-VEGF agents had to be administered repeatedly in order to maintain the initial therapeutic effect. The longevity of the disease course and the large number of injections required per patient per year prompted several studies that focused on the cost-effectiveness of these agents. Data compiled from these studies revealed alarming figures in terms of the economic burden placed on health care providers in high-income countries from which these studies originated.Citation19–Citation21 Accordingly, the cost of anti-VEGF agents in middle- and low-income countries renders any therapeutic plan for the management of macular edema unrealistic to a wide range of their population. Based on the abovementioned results, the current study evaluated the safety of high-dose IVTA as affordable low-cost alternative to anti-VEGF agents in Egypt as an example of lower-middle-income countries.Citation22
Patients and methods
Patients
This was a retrospective interventional non-comparative case series conducted in a private ophthalmic center, MAGRABI eye hospital, Tanta, Egypt. The study recruited patients who received high-dose IVTA for treating various retinal and optic nerve diseases over the past 5 years. Patients were required to have a minimum follow-up period of 6 months to be eligible for the study. Patients were enrolled regardless of whether they were treatment naïve at the time of receiving high-dose IVTA or if they had had other lines of therapy such as laser or anti-VEGF agents. Exclusion criteria were history of glaucoma or steroid responsiveness, use of any topical antiglaucoma agent, retinal or optic nerve disease caused by infectious microorganisms, and active infection involving the ocular adnexa or ocular surface. The main outcome measure was assessment of complications that might develop secondary to high-dose IVTA. Diagnosis of the retinal or optic nerve disease was based on biomicroscopic examination and ancillary tests including fundus fluorescein angiography and spectral-domain optical coherence tomography whenever applicable. Selection of patients for enrollment in the study and decision on the compatibility of their condition with high-dose IVTA treatment were undertaken by an experienced retina specialist (HG).
The current study was approved by the institutional review board of MAGRABI eye hospital in Egypt. The study adhered strictly to the tenets of the Declaration of Helsinki (2013 revision). All patients signed informed consent prior to enrollment. The consent included a statement explaining in detail the nature of high-dose IVTA injection procedure, the expected therapeutic outcome, and the possible complications of therapy.
Methods
High-dose IVTA preparation
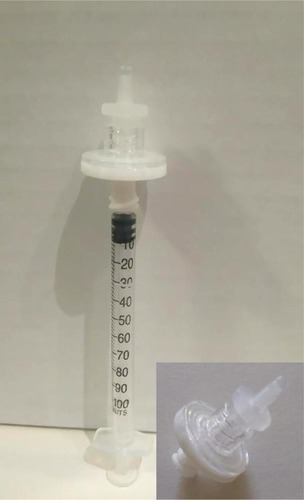
We adopted the filter technique described by Jonas et alCitation23 for preparing high-dose IVTA. The whole suspension from a commercial TA vial, containing 40 mg/mL was withdrawn into a 1 mL tuberculin syringe. The syringe was kept in an upright position, needle-up, for at least 30 minutes to allow the TA crystals to settle down occupying the lower 0.2 mL of the syringe. Subsequently, the supernatant vehicle occupying the upper 0.8 mL was slowly injected through a 0.22-µm pore size filter (ARCADOPHTA®, Toulouse, France; ). The filter allows passage of the vehicle while preventing passage of the crystals owing to their size. The crystals were diluted with ringer’s lactate solution, and again placed in an erect position for 10 minutes. The upper 0.8 mL was discarded through the filter, leaving the crystals in the lower 0.2 mL of the syringe. The whole procedure was repeated twice. Eventually, a 0.2 mL volume containing ~20 mg TA was injected into the vitreous cavity.
Figure 1 A 22-µm pore filter mounted on a 27-gauge 1 mL tuberculin syringe.
Abbreviations: IVTA, intravitreal triamcinolone acetonide; TA, triamcinolone acetonide.
Preoperative patient preparation
Patients were asked to use topical antibiotics q.i.d. for 2 days prior to high-dose IVTA injection. The injection procedure was done in the operating theater under complete sterile conditions. First, the periocular area was sterilized using povidone-iodine 10%, followed by the application of topical anesthetic agent, benoxinate hydrochloride 0.4%, twice at 5 minutes interval. Lid speculum was applied to separate the lashes from the site of injection. The ocular surface was prepared using povidone-iodine 5% drops applied in the conjunctival cul-de-sac. A cotton-tipped applicator soaked in anesthetic solution was pressed against the planned site of injection for 1 minute.
Injection technique
The site of injection was usually selected in the inferior temporal region for best exposure, and at a distance of 3.0, 3.5, or 4.0 mm from the limbus depending on whether the patient was aphakic, pseudophakic, or phakic, respectively. A 27-gauge tuberculin syringe was used for injection to avoid clogging of the TA crystals in the needle hub. The needle was advanced transconjunctivally toward the center of the vitreous cavity until the tip was visualized through the dilated pupil. High-dose IVTA was then slowly injected into the vitreous cavity. Routine anterior chamber (AC) paracentesis was done in all patients prior to retracting the needle to reduce the intraocular volume. A cotton tip applicator was pressed against the site of injection for 1 minute immediately after retracting the needle. These latter precautions were performed to safeguard against reflux of the drug or the vitreous through the injection site after retraction of the injection needle. In addition, AC paracentesis minimizes postoperative intraocular pressure (IOP) spikes. Topical antibiotics were used q.i.d. for 3 days after injection. During the postoperative period, IOP was checked at 1 day, 1 week, 1 month, and 3 monthly thereafter. Significant IOP rise was defined as increased IOP ≥5 mmHg from baseline or IOP >21 mmHg on two consecutive visits. Topical antiglaucoma drops were instated in case of significant IOP elevation.
Statistical analysis
Crosstabs (XCitation2)
The crosstabs procedure forms two-way and multiway tables and provides a variety of tests and measures of association for two-way tables. The structure of the table and whether categories are ordered determine what test or measure to use. Crosstabs statistics and measures of association are computed for two-way tables only. If you specify a row, a column, and a layer factor (control variable), the crosstabs procedure forms one panel of associated statistics and measures for each value of the layer factor (or a combination of values for two or more control variables). For example, if “gender” is a layer factor for a table of “married” (yes, no) against “life” (is life exciting, routine, or dull), the results for a two-way table for the females are computed separately from those for the males and printed as panels following one another.
“One-way ANOVA” (F-test)
It is a procedure used for testing the differences among the means of two or more treatments. It was noted that if means of subgroups are greatly different, the variance of the combined groups is much larger than the variance of the separate groups. The analysis of variance format for the analysis of differences in means is based on this fact. It is used in circumstances when it is desirable to study several variables. The significance of the measured data was considered as follows: not significant (NS), when P>0.05; significant (S), when P<0.05; highly significant (HS), when P<0.01, where “P” is the probability (reflect of null hypothesis).
Results
Study population
Baseline patient characteristics
The study included 207 eyes of 168 patients (116 men and 52 women), with a mean age of 58 years (range: 16–94 years; SD 13). Mean preoperative IOP was 14.07 mmHg (range: 8–20 mmHg; SD 2.5). Phakic eyes constituted 159 eyes (77%) of the study population. The indications for high-dose IVTA were diabetic macular edema (DME) which was detected in 132 eyes (64%), macular edema secondary to retinal vein occlusion (RVO) which was detected in 39 eyes (19%), optic nerve disease which was detected in 14 eyes (7%). This latter group included anterior ischemic optic neuropathy (AION), papillitis, and diabetic papillopathy, which were detected in eight, three, and three eyes, respectively. Other indications were uveitis, which was detected in nine eyes (4.3%), macular edema secondary to vitreomacular traction (VMT), which was detected in six eyes (3%), Irvine– Gass syndrome which was detected in five eyes (2.4%), and interferon-related retinopathy, which was detected in two eyes (1%) (). The mean follow-up period post-injection was 22 months (range: 6–60 months; SD 16.4). Mean number of injections was 1.3 (range: 1–6; SD 0.6).
Table 1 Baseline patient characteristics
Postoperative complications
In the postoperative period, one patient (0.5%) developed retinal detachment and another patient developed endophthalmitis. The main postoperative complication in the remaining 205 eyes was cataract formation or progression of pre-existing cataract, which was detected in 86 (54%) out of 159 phakic eyes included in the study, and that eventually needed cataract surgery. The second main complication was increased IOP post-injection, which was detected in 90 eyes (44%). The IOP returned to normal baseline value in 52 eyes using single topical antiglaucoma medication, which was discontinued after 1–2 weeks. The remaining 38 eyes (18.5%) developed persistent rise of IOP and needed to continue on antiglaucoma drop(s) to normalize the IOP. Five patients (2.4%) developed unresponsive glaucoma to maximum antiglaucoma treatment. One of these patients (0.5%) had neovascular glaucoma (NVG) and two patients (1%) had panuveitis with posterior synechiae and peripheral anterior synechiae (PAS) formation. Four patients had filtering surgery, and one patient needed cyclophotocoagulation ( and ).
Table 2 Postoperative complications
Table 3 Stratification of patients with increased IOP following high-dose IVTA injection
Correlation between high-dose IVTA injections and postoperative complications
Effect of high-dose IVTA on cataract formation
Increased frequency of high-dose IVTA injection was associated with increased risk for cataract formation. The risk increased steadily up to four injections (P=0.003), afterward, the risk leveled up for more than four injections ().
Table 4 Relative contribution (%) of number of high-dose IVTA injections to the development of complications
Effect of high-dose IVTA on glaucoma development and initiation of antiglaucoma treatment
Statistical analysis revealed that high-dose IVTA injection was directly proportional to increased incidence of glaucoma development (P=0.046). Moreover, increased number of IVTA injections was a significant contributing factor in the initiation of topical antiglaucoma agents needed to control the IOP (P=0.02, 0.01, and 0.01) for one, two, and three anti-glaucoma agents, respectively, and for the need for glaucoma surgery (P=0.01) ().
Correlation between the primary pathology and the development of glaucoma, the need for antiglaucoma agents and glaucoma surgery
Statistical analysis revealed high index of association between certain retinal and optic nerve diseases, namely diabetic retinopathy (including DME and diabetic papillopathy), VMT-related macular edema, Irvine–Gass syndrome, AION and retinal vein occlusion, and the development of glaucoma after high-dose IVTA administration (P=0.03). Similarly, a high index of association was noted between these pathologies and increased dependency on antiglaucoma agents (P=0.01, 0.001, and 0.05 for one, two, and three antiglaucoma agents, respectively) and for the need for glaucoma surgery, (P<0.001) ().
Table 5 Relative contribution (%) of the primary pathology to the development of complications
Discussion
The efficacy of anti-VEGF regimens for treating different retinal diseases is overshadowed by great dismay regarding their cost, not to mention disease entities as wet age-related macular degeneration (AMD), DME, and macular edema secondary to retinal vein occlusion. These specific pathologies mandate enrolling the patients in lengthy treatment protocols that include loading dose followed by pro re nata or treat-and-extend (TER) regimens.Citation24–Citation29 For instance, according to these regimens, the median number of injections needed in patients with DME was up to 9–11 injections in the first year,Citation30 and 17 injections over 5 years period.Citation31 In terms of anti-VEGF treatment cost, a study by Gupta et alCitation26 published in 2010 found that the mean cost of using ranibizumab for neovascular AMD as per the TER regimen per patient at 1 year was USD 16,114.52 and the mean cost per patient between years 1 and 2 was USD 13,971.44 as compared to the mean cost of a patient treated with ranibizumab over 1 year in a MARINA/ANCHOR and PrONTO study protocol, which equals to USD 28,314.16 and USD 15,880.07, respectively. Another study by Mitchell et alCitation21 in 2012 reported that the incremental cost of ranibizumab for DME was ₤4,191 for 0.17 quality-adjusted life-year (QALY), and ₤ 24,028 incremental cost-effectiveness ratio (ICER) for a simulated model of 15-year time span based on 1-year follow-up data from RESTORE trial. A more recent study by Ross et alCitation19 on the use of anti-VEGF agents in DME found that the ICERs of aflibercept and ranibizumab compared with bevacizumab for 624 participants during 1 year were USD 1,110,000 per QALY and USD 1,730,000 per QALY, respectively. The projected 10-year ICERs using mathematical modeling were USD 349,000 per QALY and USD 603,000 per QALY, respectively. The authors concluded that the treatment costs of aflibercept and ranibizumab would need to decrease by 69% and 80%, respectively, to reach a cost-effectiveness threshold of USD 100,000 per QALY compared with bevacizumab during a 10-year horizon. In comparison with the USA and the UK as representatives of high-income economies, the current study cited Egypt as representative of lower-middle-income economies, in which the nominal gross domestic product (GDP) per capita is ~USD 3,685.Citation22,Citation32 In that country, single ranibizumab injection 0.5 mg is available at the government-owned health insurance organization, at a subsidized cost of ÊGP 1,387 (USD 78.6),Citation33 which means that the cost of treatment when employing the abovementioned regimens could reach approximately EGP 15.257 (USD 864.2) in 1 year and EGP 23,579 (USD 1,336) in 5 years per treated eye, exclusive of physician fees and ancillary tests. Nevertheless, in non-insured private practice, patients are required to pay EGP 4,000 (USD 227) for single ranibizumab injection 0.5 mg; accordingly, the cost of treatment could reach EGP 44,000 (USD 2,493) in one year and EGP 68,000 (USD 3,852) in 5 years per treated eye, exclusive of physician fees and ancillary tests. The fore-mentioned GDP per capita in Egypt makes these figures highly unrealistic for a sizeable portion of the country population and mandates urgent demand for a cheaper alternative with parallel efficacy to anti-VEGF agents and acceptable safety profile.
The efficacy of IVTA in treating various retinal disorders is time-honored and consolidated by compelling evidence from several studies.Citation34–Citation37 The therapeutic effect of IVTA lasted for 4–6 months when using 4 mg dose and up to 9 months for high-dose IVTA (20–25 mg). Afterward, more injections were needed to maintain the initial favorable therapeutic effect.Citation16,Citation38–Citation40 The current study evaluated the safety of high-dose IVTA (20 mg) in treating macular edema secondary to retinal vascular diseases and in suppressing inflammation in various retinal and optic nerve disorders. The reason for using high dosage of IVTA in our study was to achieve longevity and potency of the therapeutic effect compared to 4 mg dose. In our study, the mean duration of follow-up was 22 months. During that period, the mean number of injections was 1.3, with 22% of our patients needing more than two injections to maintain the initial favorable therapeutic outcome. The main complication attributed to high-dose IVTA encountered in the current study was cataract formation or progression of pre-existing cataract, which affected 54% of the study population. All eyes with cataract formation had cataract surgery within the following 6 months. Cataractogenesis was directly proportional to increased frequency of high-dose IVTA injection (P=0.003). The second most common complication was IOP spikes that affected 44% of the study population. Of this cohort, 18.5% developed established glaucoma and had to rely on topical antiglaucoma treatment through the end of the follow-up period. Five patients (2.4%) required glaucoma surgery due to intractable glaucoma. In three of these five patients, intractable glaucoma was more likely attributed to their disease entity rather than to high-dose IVTA. One patient developed NVG due to relentless neovascularization secondary to diabetic retinopathy. The other two patients had panuveitis and developed posterior synechiae and PAS with consequent compromised aqueous outflow. Accordingly, we could argue that the incidence of glaucoma surgery due to intractable glaucoma attributed to high-dose IVTA in the current study was 1%. In the present study, statistical analysis revealed that high-dose IVTA injection was directly proportional to the incidence of glaucoma among the study population (P=0.046). Similarly, increased number of IVTA injections correlated to increased number of topical antiglaucoma agents or glaucoma surgery needed to control the IOP. In accordance with our results, cataractogenesis and IOP rise were the cardinal complications associated with IVTA according to previous studies. In two reports by the Diabetic Retinopathy Clinical Research (DRCR) network,Citation3,Citation41 the use of IVTA 4 mg was associated with 40%–50% incidence of glaucoma, and 51%–59% incidence of cataract. Glaucoma surgery was needed in 1%–1.6% of cases. In the SCORE study, 41% of eyes treated with 4 mg IVTA required IOP-lowering medication during the 12-month study duration and 1.4% of the same cohort required glaucoma surgery between 12 months and 24 months, whereas 35% of eyes treated with 4 mg IVTA developed cataract.Citation42 In a study by Gillies et alCitation16 using IVTA 4 mg for refractory DME, the incidence of significant IOP rise (≥5 mmHg) was 68%, antiglaucoma medication was initiated in 44% of cases, glaucoma surgery was required in 6% of cases, and the incidence of cataract formation was 54%. The follow-up period was 2 years. Five-year extension of the same study reported 79% incidence of significant IOP rise, initiation of glaucoma therapy in 56% of cases, the need for glaucoma surgery in 3% of cases, and 71% incidence of cataract formation.Citation17 The above data demonstrated that the use of high-dose IVTA in our study did not inflict additional safety hazards in terms of incidence of cataract, significant IOP rise, or the need for glaucoma medication or surgery compared to the 4 mg dose. This notion is further corroborated by studies on high-dose IVTA usage and that reported its safety profile in comparison with lower dose formula.Citation23,Citation43–Citation47
The current study detected statistically significant correlation between the primary pathology and the incidence of significant IOP rise in patients treated with high-dose IVTA, the dependency on antiglaucoma medication, and the need for glaucoma surgery. This suggests that rise of IOP in those patients postinjection was not attributed solely to high-dose IVTA but also to the molecular mechanisms involved in the pathogenesis of these diseases that might have rendered those patients more prone to significant IOP rise over time. Accordingly, these disease categories entail a high-risk patient profile that is more prone to develop significant IOP rise post high-dose IVTA and that warrants close monitoring of the IOP levels and dose spacing determined by IOP levels to guard against IOP spikes and progression to glaucoma. This observation is corroborated by recent studies that established a cause–effect relationship between the presence of DM, RVO, proliferative vitreoretinopathy, and ocular inflammation and the development of glaucoma.Citation48–Citation52 Limitations of the current study included lack of concurrent comparison group comprising patients treated with anti-VEGF agents to compare the cost of the drug and the quality of life including ocular safety, treatment outcome, and follow-up dropouts. Another important limitation is the retrospective design of the study that allowed recruitment of patients with wide-range of pathologies and with different disease stages of the same pathology, and regardless of whether they were treatment naïve or were previously treated. This could be a source of bias at the time of statistical analysis. An important consideration related to IVTA dose used in the current study was that we employed a filter technique in preparing 20 mg TA for intravitreal injection. Although we concur that non-filter methods of preparing IVTA dose,Citation53,Citation54 particularly centrifugation yield more accurate dosage by evading possible dilution of TA crystals when using the filter technique, we could argue that centrifugation technique might not be practical in every day practice due to high machinery cost and relatively sophisticated methodology. Moreover, in our study, we have been able to visualize the bolus of IVTA persisting in the vitreous cavity for at least 3 months, with the majority of our patients (88%) maintaining the initial favorable therapeutic outcome with a single injection over ~2 years mean follow-up period, which indicates the high dose delivered.
Conclusion
High-dose IVTA is a safe and cost-effective alternative to anti-VEGF agents. Cataract formation and IOP rise secondary to high-dose IVTA do not pose major adverse effects when weighed against the risk of vision loss due to cost-related noncompliance with anti-VEGF agents. Single or repeated injections of high-dose IVTA do not entail additional risk compared to that of the lower dose formula (4 mg) reported in literature. Patients presenting with primary pathology that poses high-risk for IOP spikes should be identified prior to initiating treatment.
Data sharing statement
The statistical data used to support the findings of this study are included within the article. The data collected from history taking and clinical examination of patients recruited in the current study are confidential. Access to these data is restricted by Magrabi Eye Hospital, Tanta, Egypt, in accordance with the hospital’s patients’ data protection policy. Data are available for researchers who meet the criteria for access to confidential data through contacting the hospital’s medical director, Professor Hammouda Ghoraba.
Contact details: Professor Hammouda Ghoraba, Medical Director, Magrabi Eye Hospital, 107 El-Gaish Street, Tanta Qism 2, Tanta, Gharbia Governorate, Egypt. Email: [email protected].
Acknowledgments
The study was conducted at Magrabi Eye hospital, Tanta, Egypt.
Disclosure
The manuscript discusses the use of high-dose IVTA as alternative to anti-VEGF agents. Currently, high-dose IVTA is used as an off-label ocular therapeutic agent that is not approved by the US Food and Drug Administration. The authors report no conflicts of interest in this work.
References
- VirgiliGParravanoMEvansJRGordonILucenteforteEAnti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysisCochrane Database Syst R20176CD007419
- Diabetic Retinopathy Guidelines GroupDiabetic Retinopathy GuidelinesLondonThe Royal College of Ophthalmologists20121147
- Diabetic Retinopathy Clinical Research NetworkElmanMJAielloLPRandomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edemaOphthalmology201011761064.e351077.e3520427088
- MichaelidesMKainesAHamiltonRDA prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2Ophthalmology201011761078.e21086.e220416952
- MassinPBandelloFGarwegJGSafety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II studyDiabetes Care201033112399240520980427
- NguyenQDShahSMKhwajaAAREAD-2 Study GroupTwo-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) studyOphthalmology2010117112146215120855114
- Schmidt-ErfurthULangGEHolzFGRESTORE Extension Study GroupThree-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension studyOphthalmology201412151045105324491642
- The RISE and RIDE research groupLong-term outcomes of ranibizumab therapy for diabetic macular edema: The 36-month results from two phase III trials: RISE and RIDEOphthalmology20132013202223706949
- WellsJAGlassmanARAyalaARDiabetic Retinopathy Clinical Research NetworkAflibercept, bevacizumab, or ranibizumab for diabetic macular edema: Two-year results from a comparative effectiveness randomized clinical trialOphthalmology201612361351135926935357
- DoDVSchmidt-ErfurthUGonzalezVHThe DA VINCI Study: phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edemaOphthalmology201111891819182621546089
- DoDVNguyenQDBoyerDda Vinci Study GroupOne-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edemaOphthalmology201211981658166522537617
- BrownDMSchmidt-ErfurthUDoDVIntravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studiesOphthalmology2015122102044205226198808
- HallerJABandelloFBelfortROzurdex Geneva Study GroupRandomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusionOphthalmology201011761134.e31146.e320417567
- CallananDGGuptaSBoyerDSOzurdex PLACID Study GroupDexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edemaOphthalmology201312091843185123706947
- CampochiaroPABrownDMPearsonAFAME Study GroupLong-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edemaOphthalmology20111184626.e2635.e221459216
- GilliesMCSutterFKSimpsonJMLarssonJAliHZhuMIntravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trialOphthalmology200611391533153816828501
- GilliesMCSimpsonJMGastonCFive-year results of a randomized trial with open-label extension of triamcinolone acetonide for refractory diabetic macular edemaOphthalmology2009116112182218719796823
- YilmazTWeaverCDGallagherMJIntravitreal triamcinolone acetonide injection for treatment of refractory diabetic macular edema: a systematic reviewOphthalmology2009116590291119410949
- RossELHuttonDWSteinJDDiabetic Retinopathy, Clinical Research NetworkCost-effectiveness of Aflibercept, Bevacizumab, and Ranibizumab for Diabetic Macular Edema Treatment: Analysis From the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness TrialJAMA Ophthalmol2016134888889627280850
- RégnierSAMalcolmWHaigJXueWCost-effectiveness of ranibizumab versus aflibercept in the treatment of visual impairment due to diabetic macular edema: a UK healthcare perspectiveClinicoecon Outcomes Res2015723524725999748
- MitchellPAnnemansLGallagherMCost-effectiveness of ranibizumab in treatment of diabetic macular oedema (DME) causing visual impairment: evidence from the RESTORE trialBr J Ophthalmol201296568869322399690
- World Bank Country and Lending Groups – World Bank Data Help Desk https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groupsAccessed November 8, 2018
- JonasJBHaylerJKPanda-JonasSIntravitreal injection of crystalline cortisone as adjunctive treatment of proliferative vitreoretinopathyBr J Ophthalmol20008491064106710966969
- FungAELalwaniGARosenfeldPJAn optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degenerationAm J Ophthalmol2007143456658317386270
- SpaideRRanibizumab according to need: a treatment for age-related macular degenerationAm J Ophthalmol2007143467968017386275
- GuptaOPShienbaumGPatelAHFecarottaCKaiserRSRegilloCDA treat and extend regimen using ranibizumab for neovascular age-related macular degeneration clinical and economic impactOphthalmology2010117112134214020591490
- WeckerTEhlkenCBühlerAFive-year visual acuity outcomes and injection patterns in patients with pro-re-nata treatments for AMD, DME, RVO and myopic CNVBr J Ophthalmol2017101335335927215744
- PayneJFWykoffCCClarkWLTREX-DME Study GroupRandomized Trial of Treat and Extend Ranibizumab with and without Navigated Laser for Diabetic Macular Edema: TREX-DME 1 Year OutcomesOphthalmology20171241748127836430
- SugimotoMIchioANunomeTKondoMTwo year result of intravitreal bevacizumab for diabetic macular edema using treat and extend protocolMedicine (Baltimore)20179616e640628422832
- Diabetic Retinopathy Clinical Research NetworkWellsJAGlassmanARAflibercept, bevacizumab, or ranibizumab for diabetic macular edemaN Engl J Med2015372131193120325692915
- ElmanMJAyalaABresslerNMDiabetic Retinopathy Clinical Research NetworkIntravitreal Ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial resultsOphthalmology2015122237538125439614
- International Monetary Fund 2016 Available from: http://en.wikipedia.org/wiki/List_of_countries_by_GDP_(nominal)_per_capitaAccessed October 25, 2017
- Central Administration of Pharmaceutical Affairs (CAPA)Egyptian Drug Authority (EDA) – Ministry of Health and Population of Egypt Available from: http://www.eda.mohp.gov.egAccessed October 25, 2017
- BeerPMBakriSJSinghRJLiuWPetersGB3rdMillerMIntraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injectionOphthalmology2003110468168612689886
- MachemerRSugitaGTanoYTreatment of intraocular pro-liferations with intravitreal steroidsTrans Am Ophthalmol Soc197977171180545825
- DanisRPCiullaTAPrattLMAnlikerWIntravitreal triamcinolone acetonide in exudative age-related macular degenerationRetina200020324425010872928
- MartidisADukerJSGreenbergPBIntravitreal triamcinolone for refractory diabetic macular edemaOphthalmology2002109592092711986098
- JonasJBIntravitreal triamcinolone acetonide for treatment of intraocular oedematous and neovascular diseasesActa Ophthalmol Scand200583664566316396641
- KurzPASuhlerEBFlaxelCJInjectable intraocular corticosteroidsBeckerMDavisJSurgical Management of Inflammatory Eye DiseaseBerlin, HeidelbergSpringer2008116
- PeymanGAMoshfeghiDMIntravitreal triamcinolone acetonideRetina200424348849015187684
- Diabetic Retinopathy Clinical Research NetworkA randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edemaOphthalmology200811591447145918662829
- ScottIUIpMSVanveldhuisenPCSCORE Study Research GroupA randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular Edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6Arch Ophthalmol200912791115112819752420
- JonasJBKreissigISöfkerADegenringRFIntravitreal injection of triamcinolone for diffuse diabetic macular edemaArch Ophthalmol20031211576112523885
- JonasJBKreissigIDegenringRFIntravitreal triamcinolone acetonide as treatment of macular edema in central retinal vein occlusionGraefes Arch Clin Exp Ophthalmol2002240978278312271378
- JonasJBKreissigIHuggerPSauderGPanda-JonasSDegenringRIntravitreal triamcinolone acetonide for exudative age related macular degenerationBr J Ophthalmol200387446246812642311
- JonasJBDegenringRFKreissigIFriedemannTAkkoyunIExudative age-related macular degeneration treated by intravitreal triamcinolone acetonide. A prospective comparative nonrandomized studyEye (Lond)200519216317015218517
- SpandauUHDerseMSchmitz-ValckenbergPPapoulisCJonasJBDosage dependency of intravitreal triamcinolone acetonide as treatment for diabetic macular oedemaBr J Ophthalmol2005898999100316024853
- European glaucoma societyTerminology and guidelines for glaucoma 4th edition – chapter 2: Classification and terminology Supported by the EGS Foundation: Part 1: Foreword; Introduction; Glossary; Chapter 2 Classification and TerminologyBr J Ophthalmol201710157312728424171
- LiFWenXZhangHFanXNovel insights into the role of long noncoding RNA in ocular diseasesInt J Mol Sci201617447827043545
- ZhaoYXChenXWDiabetes and risk of glaucoma: systematic review and a Meta-analysis of prospective cohort studiesInt J Ophthalmol20171091430143528944204
- ChamABansalMBandaHKSecondary glaucoma in CAPN5-associated neovascular inflammatory vitreoretinopathyClin Ophthalmol2016101187119727390515
- ParkHYLJeonSLeeMYParkCKGlaucoma progression in the unaffected fellow eye of glaucoma patients who developed unilateral branch retinal vein occlusionAm J Ophthalmol201717519420027793602
- García-ArumíJBoixaderaAGiraltJComparison of different techniques for purification of triamcinolone acetonide suspension for intravitreal useBr J Ophthalmol20058991112111416113361
- Rodriguez-ColemanHYuanPKimHIntravitreal injection of triamcinolone for diffuse macular edemaArch Ophthalmol200412271085108615249388
