Abstract
Visual function and retinal morphology were investigated to elucidate the influence of ischemia in patients with branch retinal vein occlusion (BRVO) and macular edema. In 41 consecutive patients with BRVO aged 68.9 ± 10.0 years (22 women and 19 men), the area of capillary nonperfusion was measured by fluorescein angiography. Retinal thickness and retinal volume were measured by optical coherence tomography, and mean retinal sensitivity was calculated for each of 9 macular subfields. Mean visual acuity and macular sensitivity within the central subfield were not significantly correlated with the nonperfused area. However, the macular sensitivity within the central 5 subfields and all 9 subfields showed significant negative correlations with the nonperfused area. Also, macular thickness and volume within all 9 subfields were significantly correlated with the nonperfused area. In conclusion, evaluation of both the fovea and the entire macular region may be important in patients with ischemic BRVO.
Introduction
Branch retinal vein occlusion (BRVO) is a common retinal vascular disease that often results in macular edema, which is the most frequent cause of visual impairment in these patients.Citation1,Citation2 Macular edema can develop at any time during the course of BRVO. Increased intravascular pressure and reduced blood flow in macular capillaries can lead to dysfunction of the endothelial blood–retinal barrier and to an increase of vascular permeability that results in macular edema.Citation3 Vascular endothelial growth factor (VEGF) has been suggested to play an important role in the pathogenesis of macular edema.Citation4–Citation6 Measurement of visual acuity and measurement of retinal thickness by optical coherence tomography (OCT) are widely considered to be useful for determining the treatment strategy in patients with BRVO. However, BRVO causes pathological changes (such as bleeding) that not only affect the fovea, but also the macular region and the peripheral retina, while visual acuity primarily reflects foveal function. Thus, it may be important to obtain a more detailed assessment of the vision of these patients.
Recently, microperimetry has been employed to assess visual function in BRVO patients with macular edema, and the retinal thickness and retinal sensitivity are reported to be related for the entire region of the macula affected by BRVO.Citation7,Citation8 We previously demonstrated by microperimetry that retinal thickness and retinal volume are more closely associated with retinal sensitivity than with BCVA in BRVO patients who have macular edema.Citation9 Parodi et alCitation10 reported that progressive loss of the perifoveal capillary network and consequent enlargement of the foveal avascular zone (FAZ) are related to a decrease of visual acuity in BRVO patients. On the other hand, FinkelsteinCitation11 reported that ischemic edema was associated with a good visual prognosis. Thus, ischemia may influence the visual acuity of BRVO patients with macular edema. However, the relation of ischemia to macular sensitivity in BRVO patients with macular edema remains unclear. Accordingly, we investigated both visual function (visual acuity and retinal sensitivity) and retinal morphology (retinal thickness and retinal volume) in BRVO patients with macular edema to elucidate the influence of ischemia.
Materials and methods
Patients
This study was performed in accordance with the Helsinki Declaration of 1975 (1983 revision), and was approved by the ethics committee of Tokyo Women’s Medical University. Forty-one consecutive patients with a mean age of 68.9 ± 10.0 years (22 women and 19 men) were included in this prospective uncontrolled study conducted in the Department of Ophthalmology of Tokyo Women’s Medical University (). Each patient had unilateral BRVO and the mean duration of the condition was 4.5 ± 2.8 months (range: 1–12 months). Patients were diagnosed as having hypertension if their systolic blood pressure was ≥140 mm Hg and diastolic blood pressure was >90 mm Hg, or if systolic pressure was ≥140 mm Hg at one examination and diastolic pressure was ≥90 mm Hg on a different day, or if the patient was already taking antihypertensive medication.Citation12 A diagnosis of hyperlipidemia was based on a total cholesterol ≥240 mg/dL, triglycerides ≥160 mg/dL, low-density lipoprotein cholesterol ≥130 mg/dL, or use of cholesterol-lowering medication.Citation12
Table 1 Clinical and demographic data of the BRVO patients
All patients had cystoid macular edema (≥250 μm on OCT) involving the foveal center. The exclusion criteria were 1) previous ocular surgery, 2) diabetes mellitus with diabetic retinopathy, 3) previous macular laser photocoagulation, 4) previous intravitreal injection of triamcinolone acetonide or anti-VEGF agents, 5) a history of ocular inflammation, 6) marked retinal hemorrhage (including macular bleeding involving the intrafoveal or subfoveal spaces), and 7) serous retinal detachment. Twenty-one patients had superior vein occlusion and 20 patients had inferior occlusion.
Fundus findings
As baseline screening, patients underwent ophthalmoscopy and biomicroscopic examination using a slit-lamp with a fundus contact lens. They also underwent standard fundus color photography and fluorescein angiography, which was performed with a Topcon TRC-50EX fundus camera, an image-net system (Tokyo Optical Co. Ltd., Japan), and a preset lens with a slit-lamp.
A masked grader independently assessed ischemic retinal vascular occlusion on the fluorescein angiograms by measuring the ischemic area of the retina with the public domain Scion Image program, as reported previously.Citation4–Citation6 On digital photographs of the fundus, the optic disc was outlined with a cursor, then its area was measured, as was also done for the nonperfused area of the retina. Then the nonperfused area was divided by the disc area to calculate the severity of retinal ischemia. The average nonperfused area was 34.7 ± 34.8 disc areas, with a range of 0 to 117 disc areas.
In addition, retinal sensitivity was investigated by microperimetry, and retinal thickness and retinal volume were measured by OCT.
Measurement of BCVA
Each patient underwent measurement of best-corrected visual acuity (BCVA) with an SC-2000 System chart (Nidek, Gamagori, Japan). BCVA was measured in decimal units on a Landolt chart by the orthopticists. The chart brightness was set at 80 to 320 cd/m2, and chart contrast was more than 74%. The results were converted to the logarithm of the minimum angle of resolution scale (logMAR).
Optical coherence tomography
OCT was performed with an instrument from Zeiss-Humphrey Ophthalmic Systems (Zeiss Stratus OCT3, Carl Zeiss Meditec, Dublin, CA) to measure the foveal thickness. At each visit, all patients underwent Stratus OCT examination in the vertical cross-section with the instrument centered on the fovea and in the fast macular thickness mode. On these views, retinal thickness was defined as the distance between the inner surface of the neurosensory retina and the retinal pigment epithelium. Foveal thickness was calculated as the average retinal thickness within a circle of 500 μm radius centered on the fovea. A retinal thickness map and retinal volume map were obtained by scanning 6 × 6 mm (20° × 20°) areas of the macular region, which was divided into 9 subfields: 1) fovea, 2) superior inner macula, 3) nasal inner macula, 4) inferior inner macula, 5) temporal inner macula, 6) superior outer macula, 7) nasal outer macula, 8) inferior outer macula, and 9) temporal outer macula.Citation9 The diameters of the central, inner, and outer circles were 1, 3, and 6 mm, respectively. In each region, measurement of retinal thickness and volume was automatically performed by computer software. The mean macular thickness at the one subfield (fovea) covering the central 1 × 1 mm (4° × 4°), at 5 subfields (fovea, superior inner, nasal inner, inferior inner, and temporal inner) covering the central 3 × 3 mm (10° × 10°), and at all 9 subfields covering the entire central 6 × 6 mm (20° × 20°) were thus determined.
Retinal volume was calculated as follows. A central macular thickness map measuring 6.00 mm in diameter was generated. The circular map was subdivided into 9 quadrants. The middle and inner circle diameters were 3.00 mm and 1.00 mm, respectively. The mean retinal thickness was calculated for each of the 9 quadrants from the obtained radial scans. Multiplying the mean retinal thickness by the area of the quadrant generated the volume for each of the 9 quadrants. The total macular volume at the 1 subfield (fovea) covering the central 1 × 1 mm (4° × 4°), at 5 subfields (fovea, superior inner, nasal inner, inferior inner, and temporal inner) covering the central 3 × 3 mm (10° × 10°), and at all 9 subfields covering the entire central 6 × 6 mm (20° × 20°) were thus determined as the sum of the quadrant volume.
Functional mapping by microperimetry
Microperimetry was performed with the MP-1 microperimeter (Nidek, Gamagori, Japan) using an infrared fundus camera with a liquid crystal display controlled by dedicated software. The MP-1 performs microperimetry and assesses fixation independently with an automated eye tracking system that provides real-time compensation for eye movements and therefore allows presentation of a stimulus at precisely the predefined retinal location. That is, if the reference area moves, the stimulus is also automatically moved, while the stimulus is not delivered if the reference area cannot be detected. The retinal sensitivity threshold can be measured easily because the strength of the stimulus is altered automatically and progressively during microperimetry. Color fundus photographs can also be acquired and the findings can be registered either automatically or manually along with the infrared image. At the end of testing, microperimetry data can also be superimposed on the digital fundus photograph. Thus, microperimetry is performed while observing a target set on the fundus, so the target is precisely located and testing is reliable even in patients who do not have stable fixation.
Each patient underwent fundus-monitored microperimetry with the MP-1 system (Nidek, Gamagori, Japan). Its software performs automatic tracking of fundus movements and evaluates every frame acquired for fundus shift in the x and y directions relative to a reference frame obtained with an infrared camera at the start of the examination. Microperimetry settings were identical for all examinations, and Goldmann III stimuli were presented in random order according to a 4-2-1 double staircase strategy. The stimulus intensity ranged from 0 to 20 decibels (dB) (0 dB corresponded to the strongest signal intensity of 127 cd/m2) in 1-dB steps, and the duration of each stimulus was 200 milliseconds. The target was varied in size according to the patient’s visual acuity. Retinal sensitivity maps were obtained by using the macula 20° program of the MP–1. Mean retinal sensitivity was calculated from the sensitivity in each of the 9 subfields on the retinal map generated by OCT.Citation9 The mean macular sensitivity was determined for 5 locations covering the central 4° field, 29 locations covering the central 10° field (5 subfields: fovea, superior inner, nasal inner, inferior inner, and temporal inner), and 57 locations covering the entire central 20° field (all 9 subfields).
Statistical analysis
All analyses were performed with SAS System 9.1 software (SAS Institute Inc., Cary, NC). Results are presented as the mean ± SD or frequency. To examine the relationship between visual acuity (logMAR) or retinal sensitivity and the severity of retinal ischemia, as well as the relationship between retinal thickness or retinal volume and the severity of retinal ischemia, Pearson’s correlation coefficients were calculated. Two-tailed P values of less than 0.05 were considered to indicate statistical significance.
Results
BCVA ranged from 20/200 to 20/25 (median: 20/60), with the mean logMAR value being 0.55 ± 0.38 (range: 0 to 1.2) (). shows the mean macular sensitivity, macular thickness, and macular volume within the central 4°, 10°, and 20° fields.
Table 2 Functional and anatomic measurements in the BRVO patients with macular edema
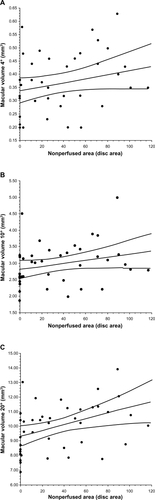
Visual acuity (logMAR) was not significantly correlated with the nonperfused area (ρ = 0.16, P = 0.317) (). Macular sensitivity within the 10° and 20° fields showed significant negative correlations with the nonperfused area (ρ = −0.35, P = 0.021, and ρ = −0.36, P = 0.020, respectively), but the macular sensitivity within the central 4° field was not significantly correlated with the nonperfused area (ρ =−0.25, P = 0.108) (). Although macular thickness within the 20° field was significantly correlated with the nonperfused area (ρ = 0.33, P = 0.037), the macular thickness within the central 4° or 10° fields was not significantly correlated with the nonperfused area (ρ = 0.28, P = 0.071, and ρ = 0.27, P = 0.092, respectively) (). Likewise, macular volume within the 20° field was significantly correlated with the nonperfused area (ρ = 0.36, P = 0.019), but macular volume within the central 4° or 10° fields was not (ρ = 0.29, P = 0.062, and ρ = 0.26, P = 0.103, respectively) ().
Figure 1 Correlation between best-corrected visual acuity (BCVA) converted to the logarithm of the minimum angle of resolution scale (logMAR) and the nonperfused area. The visual acuity (logMAR) was not significantly correlated with the nonperfused area in BRVO patients with macular edema (ρ = 0.16, P = 0.317).
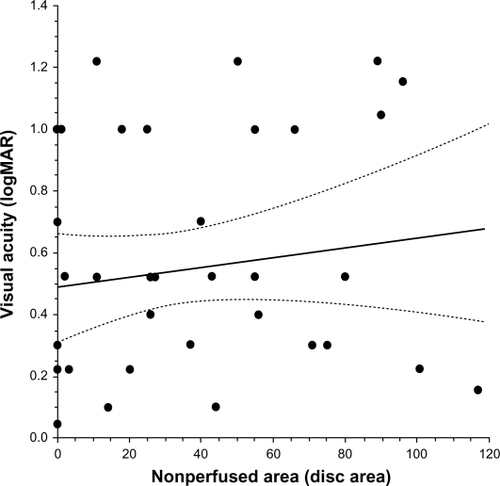
Figure 2 Correlation between macular sensitivity and the nonperfused area in BRVO patients with macular edema. A) Macular sensitivity within the central 4° was not significantly correlated with the nonperfused area (ρ = −0.25, P = 0.108), but macular sensitivity within the (B) 10° field and (C) the 20° field showed significant negative correlations with the nonperfused area (ρ = −0.35, P = 0.021, and ρ = −0.36, P = 0.020, respectively).
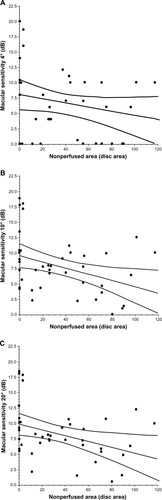
Figure 3 Correlation between macular thickness and the nonperfused area in BRVO patients with macular edema. The macular thickness within the central (A) 4° field or (B) the 10° field was not significantly correlated with the nonperfused area (ρ = 0.28, P = 0.071, and ρ = 0.27, P = 0.092, respectively), but the macular thickness within the 20° field (C) showed a significant correlation with the nonperfused area (ρ = 0.33, P = 0.037).
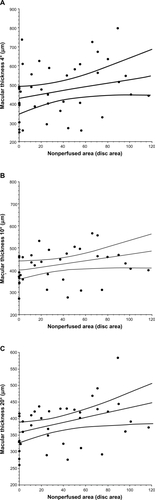
Figure 4 Correlation between macular volume and the nonperfused area in BRVO patients with macular edema. The macular volume within the central (A) 4° field or (B) the 10° field was not significantly correlated with the nonperfused area (ρ = 0.29, P = 0.062, and ρ = 0.26, P = 0.103, respectively). However, the macular volume within the 20° field (C) was significantly correlated with the nonperfused area (ρ = 0.36, P = 0.019).
Discussion
With regard to the sensitivity of the techniques that we employed, we previously reported that macular sensitivity was correlated with both macular thickness and volume in all 9 subfields of patients with BRVO and macular edema.Citation9 Such findings suggest that our techniques are sensitive for detecting central local deficits in patients with BRVO and macular edema. However, the present study demonstrated that the mean visual acuity (logMAR) and macular sensitivity within the central 4° field were not significantly correlated with the nonperfused area in BRVO patients with macular edema. It has been reported that progressive loss of the perifoveal capillary network with enlargement of the FAZ is related to a decrease of visual acuity in BRVO patients.Citation10 Therefore, foveal function (including visual acuity) may depend more on the extent of damage to the perifoveal capillary network than on the nonperfused area after BRVO, although why foveal function reflects the perifoveal capillary network rather than the nonperfused area is still unclear. Tyrberg et alCitation13 reported a significant correlation between an increase of FAZ diameter and an increase in the implicit time of the innermost concentric rings and of the third concentric ring in the first order kernel on multifocal electroretinography (mfERG), while an increase of the total area (FAZ and perifoveal intercapillary areas) was correlated with an increase in the implicit time in the same areas on the mfERG. This suggests that a larger nonperfused area is associated with greater alteration of neuronal function in the macular region due to ischemia. Therefore, it is possible that the mean visual acuity (logMAR) and macular sensitivity within the central 4° field were not significantly correlated with the nonperfused area in BRVO patients with macular edema because alterations of neuronal function due to ischemia were minor due to the small size of the nonperfused area. Furthermore, Tyrberg et alCitation13 concluded that alterations of neuronal function in the macular region due to ischemia might precede the deterioration of visual acuity because the ischemic area was correlated with prolongation of the implicit time regardless of whether visual acuity was preserved (0.6 or better). Thus, deterioration of visual acuity might occur after alterations of neuronal function due to ischemia. On the other hand, macular sensitivity within the 10° and 20° fields showed significant negative correlations with the nonperfused area. Because photoreceptor cells are damaged by retinal ischemia, functional retinal impairment may become more severe as the ischemic region increases. Thus, evaluation of visual function not only for the fovea but also the entire macular region may be important in patients with ischemic BRVO.
We also found that the macular thickness and volume within the 20° field were significantly correlated with the nonperfused area. Interestingly, macular sensitivity and macular thickness/volume within the 20° field showed parallel changes in the present study. We recently reported that increased intraocular levels of VEGF and sICAM-1 or a decreased level of PEDF are associated with increased vascular permeability and the severity of retinal ischemia in BRVO patients.Citation14,Citation15 Thus, when the severity of ischemia increases, changes in these factors result in an increase of macular edema. As macular edema increases, ischemia also worsens, with the result that macular function may be impaired. This hypothesis is supported by previous reports that the retinal thickness at the central fovea influences visual function in BRVO patients.Citation16–Citation19 In addition, tissue expansion due to edema could stretch the capillary network and lead to transient capillary closure because of the high tissue fluid pressure in patients with ischemic macular edema.Citation11 Progressive capillary occlusion may contribute to ischemic macular edema, resulting in the functional impairment of photoreceptor cells. Taken together, these findings suggest that functional and mechanical damage to photoreceptor cells due to ischemic macular edema might reduce macular sensitivity.
One limitation of the present study is that the macular thickness/volume, macular sensitivity, and visual acuity may change due to the natural course of the disease after the onset of BRVO. Because this was a cross-sectional study, a longitudinal investigation would be needed to clarify the relations between macular thickness/volume, macular sensitivity, and visual acuity in BRVO patients. Second, FinkelsteinCitation11 reported that ischemic edema was associated with a good visual prognosis. Because our study was cross-sectional, it did not provide data on the response to treatment or the prognosis of the patients, such as whether those with or without ischemia had any particular outcomes. Third, because it has been reported that diffuse disorganization of the outer photoreceptor layer beneath the fovea often results in poor visual acuity even after complete resolution of macular edema,Citation20 it is also possible that such diffuse disorganization of the outer photoreceptor layer could have influenced visual function in our subjects. However, we could not assess the relationship of the outer photoreceptor layer to the visual prognosis because detection of the junction between the inner and outer layers was difficult with our OCT equipment. Accordingly, the prognosis of patients with BRVO and macular edema needs to be investigated in more detail in the future.
In conclusion, we found that mean visual acuity (logMAR) and the macular sensitivity within the central 4° field were not significantly correlated with the nonperfused area in BRVO patients with macular edema, while the macular sensitivity within the 10° and 20° fields showed significant negative correlations with the nonperfused area. The macular thickness and volume within the 20° field was also significantly correlated with the nonperfused area. These findings suggest that evaluation not only of the fovea but also the entire macular region may be important in patients with ischemic BRVO and macular edema.
Disclosure
The authors have received no financial support. No conflicting relationship exists for any author.
References
- MichelsRGGassJDThe natural course of retinal branch vein obstructionTrans Am Acad Ophthalmol Otolaryngol1974782166177
- GutmanFAZegarraHThe natural course of temporal retinal branch vein occlusionTrans Am Acad Ophthalmol Otolaryngol1974782178192
- NomaHFunatsuHSakataKMacular microcirculation and macular oedema in branch retinal vein occlusionBr J Ophthalmol200993563063319208676
- NomaHFunatsuHYamasakiMPathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6Am J Ophthalmol2005140225626116086947
- NomaHMinamotoAFunatsuHIntravitreal levels of vascular endothelial growth factor and interleukin-6 are correlated with macular edema in branch retinal vein occlusionGraefes Arch Clin Exp Ophthalmol2006244330931516133018
- NomaHFunatsuHYamasakiMAqueous humour levels of cytokines are correlated to vitreous levels and severity of macular oedema in branch retinal vein occlusionEye2008221424816826241
- YamaikeNKitaMTsujikawaAMiyamotoKYoshimuraNPerimetric sensitivity with the micro perimeter 1 and retinal thickness in patients with branch retinal vein occlusionAm J Ophthalmol2007143234234417258527
- YamaikeNTsujikawaASakamotoARetinal sensitivity after intravitreal injection of bevacizumab for the treatment of macular edema secondary to retinal vein occlusionRetina200929675776719289982
- NomaHFunatsuHMimuraTHarinoSShimadaKFunctional-morphological correlates in patients with branch retinal vein occlusion and macular edemaRetinaIn press.
- ParodiMBVisintinFDella RupePRavalicoGFoveal avascular zone in macular branch retinal vein occlusionInt Ophthalmol199519125288537192
- FinkelsteinDIschemic macular edema. Recognition and favorable natural history in branch vein occlusionArch Ophthalmol199211010142714341417544
- KirchhoffACDrumMLZhangJXHypertension and hyperlipidemia management in patients treated at community health centersJ Clin Outcomes Manag200815312513119412346
- TyrbergMPonjavicVLovestam-AdrianMMultifocal electroretinogram (mfERG) in patients with diabetes mellitus and an enlarged foveal avascular zone (FAZ)Doc Ophthalmol2008117318518918324430
- NomaHFunatsuHMimuraTHarinoSEguchiSHoriSPigment epithelium-derived factor and vascular endothelial growth factor in branch retinal vein occlusion with macular edemaGraefes Arch Clin Exp Ophthalmol2010248111559156520714746
- NomaHFunatsuHMimuraTShimadaKIncrease of aqueous inflammatory factors in macular edema with branch retinal vein occlusion: a case control studyJ Inflamm (Lond)201074420738886
- SuzumaKKitaMYamanaTQuantitative assessment of macular edema with retinal vein occlusionAm J Ophthalmol199812634094169744374
- ImasawaMIijimaHMorimotoTPerimetric sensitivity and retinal thickness in eyes with macular edema resulting from branch retinal vein occlusionAm J Ophthalmol20011311556011162980
- YamaguchiYOtaniTKishiSSerous macular detachment in branch retinal vein occlusionRetina20062691029103317151490
- TsujikawaASakamotoAOtaMSerous retinal detachment associated with retinal vein occlusionAm J Ophthalmol20101492291301.e520103055
- OtaMTsujikawaAMurakamiTFoveal photoreceptor layer in eyes with persistent cystoid macular edema associated with branch retinal vein occlusionAm J Ophthalmol2008145227328018045566