Abstract
Purpose
To report the outcomes of patients who underwent goniotomy with the Kahook Dual Blade (KDB) either as a standalone procedure or in combination with cataract extraction.
Patients and methods
This retrospective chart review included 111 eyes of 90 patients who underwent KDB goniotomy from January to November 2016 at Glaucoma Associates of Texas. KDB goniotomy was combined with cataract surgery in 100 eyes. The main outcome measures were postoperative intraocular pressure (IOP) and number of IOP lowering medications.
Results
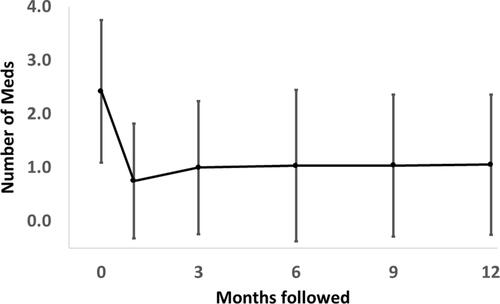
Preoperatively, mean IOP was 17.1 ± 4.7 mmHg (range 8–34 mmHg) and mean number of IOP lowering medications was 2.4 ± 1.3 (range 0–6). Postoperatively, mean IOP was 14.9 mmHg, 13.9 mmHg, 14.1 mmHg, 14.4 mmHg, and 14.7 mmHg at 1, 3, 6, 9, and 12 months follow-up, respectively (all p <0.004). Mean numbers of IOP lowering medications were 0.8, 1.0, 1.0, 1.0, and 1.6 at 1, 3, 6, 9, and 12 months follow-up, respectively (all p <0.001). The cumulative reoperation rates for uncontrolled IOP after KDB were 0%, 1.0%, 2.1%, and 4.6% at 3, 6, 9, and 12 months, respectively. Eyes with a preoperative IOP >21 mmHg were significantly more likely to undergo reoperation (p = 0.038, log-rank test). There were no serious complications at any time point in the follow-up period.
Conclusion
The Kahook Dual Blade results in a reduction in IOP and use of glaucoma medications after one year of follow-up. Further prospective studies are needed to fully characterize safety and efficacy.
Introduction
Glaucoma is a leading cause of irreversible blindness, with estimates projecting nearly 112 million people affected worldwide by the year 2040.Citation1 Elevated intraocular pressure (IOP) is a significant risk factor for disease progression. Controlling IOP is the mainstay of glaucoma therapy, and when medical therapy inadequately lowers IOP, surgical intervention is often indicated. While traditional glaucoma surgeries such as trabeculectomy and aqueous shunt implantation result in substantial IOP reductions, they are also associated with significant risks.Citation2,Citation3 Recently, minimally invasive glaucoma surgeries (MIGS) have gained in popularity due to their favorable safety profile, ability to be combined with cataract surgery, and the reduction or elimination of adjunctive glaucoma medications. This is especially relevant for patients who experience difficulty with medication adherence or those with significant ocular surface disease from topical glaucoma medications.
It is currently thought that a major component of IOP dysregulation is caused by obstruction of aqueous outflow at the level of the trabecular meshwork (TM),Citation4,Citation5 and by removing the TM, the natural outflow pathways may possibly be restored. This can be achieved in a variety of ways, and several of the recent MIGS procedures have targeted the TM by either bypassing or removing the tissue altogether. Goniotomy was first described by Otto Barkan in 1938Citation6 and has been used as a surgical treatment for pediatric glaucoma. However, traditional goniotomy has not had great long-term success in adults, which may be due, in part, to re-closure of the trabecular leaflets, incomplete removal of the TM, and/or scarring and membrane formation across the TM leaflets, leading to elevated IOP. A more thorough removal of the TM and inner wall of Schlemm’s canal may allow for patency of the opening to Schlemm’s canal and potentially result in a sustained reduction in IOP.
The Kahook Dual Blade (KDB, New World Medical, Rancho Cucamonga, CA) is a novel single-use ophthalmic knife with two parallel blades designed to remove a strip of TM tissue and the inner wall of Schlemm’s canal. The procedure can be performed alone or in combination with other procedures through a clear corneal incision. Preclinical studies have shown that the dual blade achieves a more complete removal of TM tissue without injuring surrounding tissues than either goniotomy with a microvitreoretinal blade or the trabectome.Citation7 There are, however, limited clinical studies evaluating the safety and efficacy of this treatment. The goal of the current study is to report the outcomes of patients undergoing KDB goniotomy, either as a standalone procedure or in combination with cataract surgery, with up to one year of follow-up.
Materials and Methods
A retrospective chart review was performed for all patients who underwent a KDB goniotomy at Glaucoma Associates of Texas from January to November 2016. The study protocol was reviewed by the Sterling IRB ethics board, which granted a waiver of consent for this retrospective analysis of existing health records. The study followed the tenets of the Declaration of Helsinki, and patient data were collected and maintained with confidentiality.
Surgical Procedure
The Kahook dual blade is a single-use ophthalmic knife that is inserted into the eye via a clear corneal incision. The distal end of the stainless steel body consists of a pointed tip that pierces the TM and facilitates entry of the blade into Schlemm’s canal. A ramp proximal to the tip lifts and stretches the TM to theoretically enable smooth cutting of the TM with the parallel dual blades as the device is moved circumferentially along the canal, exposing the outer wall of Schlemm’s canal. The strip of TM can often be removed from the eye with intraocular forceps or aspirated from the eye.
Dual blade goniotomy was performed either as an isolated procedure or in combination with cataract surgery by seven glaucoma-trained surgeons (MRB, DGG, MEE, RLF, OS, DSG, HLK). For the dual blade procedure, a clear corneal incision was made in the temporal cornea, and the anterior chamber was filled with a cohesive viscoelastic (sodium hyaluronate). The head was then rotated 30 to 45 degrees away from the surgeon, and the microscope tilted 30 to 45 degrees toward the surgeon. A direct goniolens was then used to visualize the nasal angle structures. Additional viscoelastic was injected into the eye if needed to improve visualization. The dual blade was then inserted into the anterior chamber, and the tip of the blade used to pierce the TM so that the footplate of the blade could be seated within Schlemm’s canal. The device was then advanced along Schlemm’s canal in the direction of the pointed tip, either in the clockwise or counterclockwise direction. After adequate removal of the TM, the blade was rotated 180 degrees and inserted back into Schlemm’s canal at the point of original insertion, and advanced in the opposite direction. Following treatment, the back wall of Schlemm’s canal could be visualized as an opaque white line. The dual blade was then removed from the anterior chamber, and the free-floating strip of TM was removed with microsurgical forceps when present and possible. The head and microscope were then rotated back to their original position and the viscoelastic and any blood reflux was irrigated out of the eye. When this procedure was combined with cataract surgery, the goniotomy was usually performed prior to cataract surgery; however, this varied based on surgeon and patient.
The patients were followed postoperatively as per the surgeon’s discretion. Typically, patients were seen at postoperative day 1, week 1, and with subsequent visits determined based on the degree of inflammation and/or other concomitant procedures performed. Patients were typically placed on a topical fourth-generation fluoroquinolone antibiotic and topical steroid, which was tapered according to the surgeon’s clinical judgment. The need for reinstating glaucoma medications and the choice of medication used was also at the discretion of the surgeon.
Statistical Analysis
Interval level data were summarized with means and standard deviations and di- or polychotomous data were summarized with proportions. Means were compared between follow-up times with the paired t-test and between groups with the two-sample t-test. Cumulative proportions of eyes reoperated were calculated using Kaplan–Meier methods and were compared between groups with the log-rank test. Eyes included in the Kaplan–Meier plots which had not undergone reoperation were censored at the time of their last follow-up. Eyes having undergone prior reoperation were censored from the intraocular pressure summary statistics for each visit.
Results
Characteristics of Study Patients
A total of 111 eyes of 90 patients underwent surgery. The mean age was 70 years (range 23 to 94 years). The number of male patients was 48 (53%) and the majority of patients were Caucasian (n = 52, 58%) (See for baseline characteristics). Primary open-angle glaucoma was the most common diagnosis (n = 66, 60%). The severity of glaucoma varied from mild to severe with an average mean deviation on visual field testing of −6.4 ± 5.8 dB (range: −24.5 to 0.4 dB). Surgery was combined with cataract extraction in 100 (90%) eyes. Follow-up ranged from 1 to 15 months. The median (interquartile range) of follow-up was 12 (IQR 3.6) months. Overall, 72 of 111 eyes were evaluated at 12 months. The remaining 39 eyes were not seen at 12 months due to follow-up time less than 12 months at the time of data collection (n=34), the need for additional glaucoma surgery (n=4), or loss to follow-up (n=1).
Table 1 Baseline Characteristics
Effect of KDB on Postoperative IOP and Pressure Lowering Medication Use
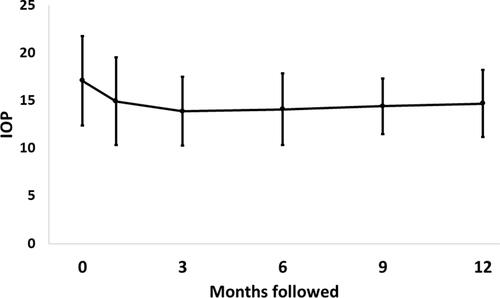
Mean IOP decreased from 17.1 ± 4.7 mmHg at baseline to 14.9 ± 4.6 mmHg, 13.9 ± 3.6 mmHg, 14.1 ± 3.8 mmHg, 14.4 ± 2.9 mmHg, and 14.7 ± 3.5 mmHg at 1, 3, 6, 9, and 12 months follow-up, respectively (all p <0.004) (). The efficacy of KBD on IOP lowering by follow-up visit and preoperative IOP are detailed in . From postoperative month 1 through month 12, the percentage of eyes with IOP lowered by 20% of the preoperative measurement ranged from 32% to 42%. The higher the preoperative IOP, the greater the IOP lowering effect of KDB on these eyes. In eyes with a preoperative IOP >21 mmHg, mean IOP decreased by 8.6 ± 4.7 mmHg at 12 months, compared to eyes with a preoperative IOP <14 mmHg, which demonstrated a 1.8 ± 3.5 mmHg reduction in IOP at 12 months. As expected, the percentage of eyes with a 20% lowering increased with preoperative pressure.
Table 2 KDB Efficacy on IOP and Medication Use by Preoperative IOP Levels and Follow-Up Visit
Figure 1 Intraocular pressure (IOP, mmHg) at baseline and at follow-up. There is an early drop in IOP that remains relatively stable during the first 12 months of follow-up. (Error bar = 1 standard deviation).
Medication use also decreased after KDB goniotomy. Mean medication use preoperatively and at follow-up are displayed in . The average number of medications used preoperatively was 2.4 ± 1.3 (range 0 to 6), and 18 patients were also using oral medications to control IOP. Medication use decreased to 0.8 ± 1.1, 1.0 ± 1.2, 1.0 ± 1.4, 1.0 ± 1.3, and 1.1 ± 1.3 at 1, 3, 6, 9, and 12 months follow-up, respectively (all p <0.001). details medication use following KDB goniotomy by follow-up visit and preoperative number of medications used. From postoperative month 1 through month 12, the percentage of eyes with a reduction in the number of medications required ranged from 75% to 90%. Similar to IOP, the percentage of eyes with postoperative medication reduction increased with the number of medications used preoperatively. At all follow-up visits, more than 80% of the eyes experienced either a 20% lowering of IOP or reduction in the number of medications.
Table 3 KDB Efficacy on IOP and Medication Use by Preoperative Number of Medications and Follow-Up Visit
Reoperation for IOP Control
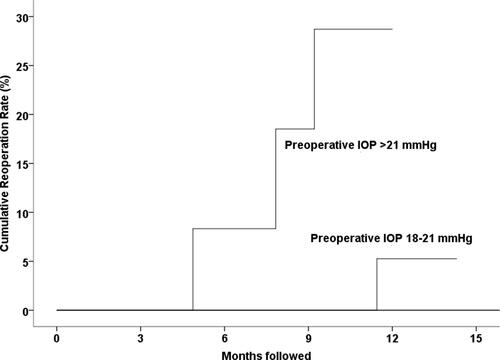
Four eyes receiving KBD underwent reoperation to surgically lower IOP. Cumulative reoperation rates were 0%, 1.0%, 2.1%, and 4.6% at 3, 6, 9, and 12 months, respectively. displays Kaplan–Meier cumulative failure rates by preoperative IOP group. Eyes with preoperative IOP >21 mmHg were statistically significantly more likely to undergo reoperation than those with an IOP between 18 mmHg and 21 mmHg (p = 0.038, log-rank test) and those with a preoperative IOP <18 mmHg (p = 0.005). At 12 months of follow-up, 28.7% of the eyes with a preoperative IOP >21 mmHg, 5.3% of the eyes with a preoperative IOP 18–21 mmHg, and zero percent of eyes with a preoperative IOP <18 mmHg required reoperation to lower IOP.
Figure 3 Kaplan–Meier cumulative failure rates by preoperative intraocular pressure (IOP) group. Eyes with preoperative IOP >21 mmHg were statistically significantly more likely to undergo reoperation than those with IOP between 18 mmHg and 21 mmHg (p = 0.038, log-rank test) and those with preoperative IOP <18 mmHg (p = 0.005).
Complications
Complications (apart from reoperation to control IOP) were assessed on 115 eyes of 91 patients and were found infrequently. A single intraoperative complication of a cyclodialysis cleft was repaired during the time of surgery and was not seen postoperatively. The most common postoperative complication was hyphema, which occurred in 4 patients within the first postoperative week and resolved by postoperative month 1 in all but one patient. This patient underwent complex cataract extraction, goniosynechiolysis, and KDB and was also on chronic anticoagulant therapy; hyphema was no longer noted after 3 months.
Discussion
The current study demonstrates that goniotomy with the KDB, either alone or in combination with cataract extraction, results in a reasonable reduction in IOP and glaucoma medication use in various types and stages of glaucoma. After one year of follow-up, among those eyes not reoperated, IOP decreased by a mean of 1.6 mmHg (10% from baseline) and medication use also decreased by an average of 1.2 (53% from baseline), with 75% of the patients having at least one or more reduction in medication. The severity of glaucoma included in this study ranged from mild to severe, and surgery effectively lowered IOP in both open- and closed-angle glaucomas. The safety profile of this procedure was also favorable, with the most common complication being self-limited hyphema. This study also indicates that while the most robust IOP reduction was achieved in those with higher IOPs, even in eyes with well-controlled IOP, medication use was decreased.
The TM is a logical target for MIGS procedures because it is the area of greatest resistance to aqueous outflow and it is easy to access. Goniotomy by means of direct incision with a microvitreoretinal blade, ablation with the trabectome, or removal of a strip of TM with the KDB aims to re-establish and enhance flow through the conventional outflow pathway. Unlike traditional goniotomy, which commonly results in leaflets of residual TM especially in adults, the KDB removes a complete strip of TM and may thus result in improved IOP control.
The results of this study seem to be comparable to those of the trabectome. Bussel et al evaluated the outcomes of trabectome following failed trabeculectomy after one year of follow-up.Citation8 After standalone trabectome, mean IOP decreased from 23.7 mmHg at baseline to 16.2 mmHg (28% reduction, p <0.01) and mean medications decreased from 2.8 to 2.0 (p <0.01). Combined phacoemulsification-trabectome resulted in an IOP decrease from 20.0 mmHg to 15.6 mmHg (19% reduction, p = 0.11), while medications decreased from 2.5 to 1.6 (p = 0.24). Okeke et al reported similar outcomes in phakic patients from the Trabectome Study Group undergoing trabectome alone or in combination with cataract surgery.Citation9 After 12 months of follow-up, the IOP for the trabectome only group was reduced from 21.0 mmHg to 15.8 mmHg (p <0.01) and 26.5 mmHg to 16.3 mmHg (p <0.01) in the combined surgery group. Additionally, both groups decreased medication use by one at 12 months. Ahuja et al evaluated outcomes of trabectome in open-angle glaucomas using two different success criteria.Citation10 The first required a postoperative IOP ≤21 mmHg or ≥20% reduction in IOP, and a second stricter criterion required an IOP ≤18 mmHg and ≥20% reduction in IOP. Success rates with the stricter criterion were low at 22% after 24 months, suggesting that trabectome is more appropriate for individuals who do not require a very low IOP. The authors have not seen this level of failure with the KDB, but longer-term studies are required. A recent meta-analysis found that on average, trabectome lowers IOP by approximately 31% to a final IOP near 15 mmHg while decreasing the number of medications by less than one, with a low rate of serious complications.Citation11
Studies evaluating the safety and efficacy of the Kahook dual blade are limited. The earliest clinical study by Greenwood et al reported the six-month outcomes of patients who underwent combined cataract extraction and KDB goniotomy.Citation12 A total of 71 eyes were included in the study, with the majority of patients diagnosed with primary open-angle glaucoma (70%). Mean IOP decreased from 17.4 mmHg at baseline to 12.8 mmHg (p <0.001), and glaucoma medication use also decreased from 1.6 to 0.9 (p = 0.005). Intraoperative blood reflux was the most commonly observed adverse event (39.4%). Surgeons reported that in 98% of the cases, the use of the KDB was straightforward and efficient.
More recently, Sieck et al reported the outcomes of 197 eyes that underwent combined cataract extraction and KDB goniotomy or KDB as a standalone procedure.Citation13 Success was defined as an IOP reduction of at least 20% from baseline and/or a reduction of at least one glaucoma medication. Unlike the patients in the current study, all patients were started on pilocarpine (1%) solution 4 times daily during the first postoperative month, in addition to the standard topical antibiotic and steroid regimen. After 12 months, the success rate was 71.8% in the combined group and 68.8% in the KDB alone group. Success was also stratified according to severity of glaucoma, with KDB being more effective in those with less severe disease (success rate of 77.1% in mild, 68.1% in moderate, 60.0% in severe, and 77.8% in indeterminate glaucoma). Similar to our study, complications were mild, with hyphema (17.3%) being the most common. Four patients required additional surgery for uncontrolled IOP within the first postoperative month.
Our study highlights a key factor in evaluating MIGS outcomes. While traditional glaucoma surgeries such as trabeculectomy and tube shunts are typically reserved for more advanced cases, most MIGS procedures are used in mild stages of disease with the aim of moderately lowering IOP as well as decreasing the dependence on glaucoma medications. The current study comprised patients at various stages of glaucoma, including those with advanced disease. Grover et al previously demonstrated that proportions of success following gonioscopy-assisted transluminal trabeculotomy (GATT) in primary open-angle glaucoma patients was heavily dependent upon stage of disease, with high proportions of failure occurring in eyes with a mean deviation worse than −15 dB. Alternatively, relatively high proportions of success were reported in eyes with a mean deviation better than −15 dB.Citation14 The effect of KDB goniotomy in patients with severe or refractory glaucoma was recently reported by Salinas et al.Citation15 In this retrospective study, 53 eyes with severe or refractory glaucoma underwent KDB goniotomy as a standalone procedure. Twelve eyes had already undergone previous glaucoma surgery. Mean preoperative IOP was 18.4 mmHg and mean number of IOP lowering medications was 2.6. After 6 months of follow-up, 57.7% of the eyes achieved an IOP reduction of ≥20%, and the proportion of patients with a decrease of at least 1 medication was 63.3%. Overall, there was a 24% reduction in IOP and 36% reduction in number of medications. Unlike the findings from Grover et al, disease stage as measured by baseline mean deviation, pattern standard deviation, and retinal nerve fiber layer thickness were not significantly associated with failure.
Traditional definitions of success, as used in the Tube Versus Trabeculectomy study, Primary Tube Versus Trabeculectomy study, Ahmed Baerveldt Comparison study, and Ahmed Versus Baerveldt study, which required IOP lowering of greater than or equal to 20% and an IOP of less than 21 mmHg, do not seem pertinent when discussing MIGS in patients with mild/moderate stages of glaucoma. By these standards, the proportion of success from KBD goniotomy is relatively low. However, if one considers KDB goniotomy as a dual-purpose procedure that achieves a modest IOP reduction in conjunction with decreasing glaucoma medication burden, one can see from our study that these goals are accomplished at relatively reasonable rates.
This study adds to the growing body of literature evaluating the use of the KDB and supports the mounting evidence supporting canal-based procedures. The limitations of the study include its retrospective design, the variable amounts of treated TM that were not controlled for, and variations in the postoperative management. This study is also not powered to assess the effect of stand-alone KDB on IOP and medication reduction, as the majority of cases were combined with cataract surgery. As follow-up continues to grow, we will be able to better determine the long-term effects on IOP and medication use. In the future, a prospective, randomized control trial comparing KDB alone versus KDB combined with cataract surgery is needed to verify the results of this study.
The findings of this study are promising and demonstrate that goniotomy with the Kahook dual blade in combination with cataract surgery decreases IOP and glaucoma medication use up to one year after surgery. This study adds to our understanding of the safety and efficacy of KDB goniotomy for the treatment of glaucoma. Further prospective studies are needed to better characterize the long-term safety and efficacy of dual blade goniotomy, both as a standalone procedure or combined with other surgical procedures.
Précis
Goniotomy with the Kahook Dual Blade can be performed alone or in combination with cataract surgery and results in a decrease in intraocular pressure and glaucoma medication use.
Disclosure
DSG (consultant and speaker for New World Medical) and OS (consultant to New World Medical). The authors report no other conflicts of interest in this work.
References
- Tham Y, Li X, Wong T, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi:10.1016/j.ophtha.2014.05.013
- Gedde SJ, Schiffman JC, Feuer WJ, et al. Three-year follow-up of the tube versus trabeculectomy study. Am J Ophthalmol. 2009;148:670–684. doi:10.1016/j.ajo.2009.06.018
- Budenz DL, Feuer WJ, Barton K, et al. Postoperative complications in the Ahmed Baerveldt comparison study during five years of follow-up. Am J Ophthalmol. 2016;163(75–82):e73. doi:10.1016/j.ajo.2015.11.023
- Grant WM. Clinical measurements of aqueous outflow. Am J Ophthalmol. 1951;34:1603–1605.
- Tamm ER. The trabecular meshwork outflow pathways: structural and functional aspects. Exp Eye Res. 2009;88:648–655. doi:10.1016/j.exer.2009.02.007
- Barkan O. Techniques of goniotomy. Arch Ophthalmol. 1938;19:217–221. doi:10.1001/archopht.1938.00850140059006
- Seibold LK, Soohoo JR, Ammar DA, et al. Preclinical investigation of ab interno trabeculectomy using a novel dual-blade device. Am J Ophthalmol. 2013;155(524–529):e522. doi:10.1016/j.ajo.2012.09.023
- Bussel II, Kaplowitz K, Schuman JS, et al. Outcomes of ab interno trabeculectomy with the trabectome by degree of angle opening. Br J Ophthalmol. 2015;99:914–919. doi:10.1136/bjophthalmol-2014-305577
- Okeke CO, Miller-Ellis E, Rojas M, et al. Trabectome success factors. Medicine (Baltimore). 2017;96:e7061. doi:10.1097/MD.0000000000007061
- Ahuja Y, Ma Khin Pyi S, Malihi M, et al. Clinical results of ab interno trabeculotomy using the trabectome for open-angle glaucoma: the Mayo Clinic series in Rochester, Minnesota. Am J Ophthalmol. 2013;156(927–935):e922. doi:10.1016/j.ajo.2013.06.001
- Kaplowitz K, Bussel II, Honkanen R, et al. Review and meta-analysis of ab-interno trabeculectomy outcomes. Br J Ophthalmol. 2016;100:594–600. doi:10.1136/bjophthalmol-2015-307131
- Greenwood MD, Seibold LK, Radcliffe NM, et al. Goniotomy with a single-use dual blade: short-term results. J Cataract Refract Surg. 2017;43:1197–1201. doi:10.1016/j.jcrs.2017.06.046
- Sieck EG, Epstein RS, Kennedy JB, et al. Outcomes of Kahook dual blade goniotomy with and without phacoemulsification cataract extraction. Ophthalmol Glaucoma. 2018;1:75–81. doi:10.1016/j.ogla.2018.06.006
- Grover DS, Smith O, Fellman RL, et al. Gonioscopy-assisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy: 24 months follow-up. J Glaucoma. 2018;27:393–401. doi:10.1097/IJG.0000000000000956
- Salinas L, Chaudhary A, Berdahl JP. et al. Goniotomy using the Kahook dual blade in severe and refractory glaucoma: six month outcomes. J Glaucoma;2018. 1. doi:10.1097/IJG.0000000000001019