Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Background
The purpose of this study was to evaluate the safety and efficacy of topography-guided ablation using the WaveLight 400 Hz excimer laser in laser-assisted in situ keratomileusis (LASIK) for hyperopia and/or hyperopic astigmatism.
Methods
We prospectively evaluated 208 consecutive LASIK cases for hyperopia with or without astigmatism using the topography-guided platform of the 400 Hz Eye-Q excimer system. The mean preoperative sphere value was +3.04 ± 1.75 (range 0.75–7.25) diopters (D) and the mean cylinder value was −1.24 ± 1.41 (−4.75–0) D. Flaps were created either with Intralase FS60 (AMO, Irvine, CA) or FS200 (Alcon, Fort Worth, TX) femtosecond lasers. Parameters evaluated included age, preoperative and postoperative refractive error, uncorrected distance visual acuity, corrected distance visual acuity, flap diameter and thickness, topographic changes, higher order aberration changes, and low contrast sensitivity. These measurements were repeated postoperatively at regular intervals for at least 24 months.
Results
Two hundred and two eyes were available for follow-up at 24 months. Uncorrected distance visual acuity improved from 5.5/10 to 9.2/10. At 24 (8–37) months, 75.5% of the eyes were in the ±0.50 D range and 94.4% were in the ±1.00 D range of the refractive goal. Postoperatively, the mean sphere value was −0.39 ± 0.3 and the cylinder value was −0.35 ± 0.25. Topographic evidence showed that ablation was made in the visual axis and not in the center of the cornea, thus correlating with the angle kappa. No significant complications were encountered in this small group of patients.
Conclusion
Hyperopic LASIK utilizing the topography-guided platform of the 400 Hz Eye-Q Allegretto excimer and a femtosecond laser flap appears to be safe and effective for correction of hyperopia and/or hyperopic astigmatism. The results are impressive for refractive error correction and stability and for improvement of both uncorrected and corrected distance visual acuity.
Introduction
The evolution of laser technology has enabled treatment of myopic, hyperopic, and astigmatic eyes to become more accurate. There have been several reports of hyperopic laser-assisted in situ keratomileusis (LASIK) in the past.Citation1–Citation11 We have previously reported on the use of standard wavefront-optimized excimer ablations in hyperopic LASIK with good resultsCitation12 and on the use of topography-guided excimer ablations.Citation13,Citation14 Hyperopic patients invariably have a significant angle kappa.Citation15 We have anecdotally observed clinically superior visual axis centration in hyperopes when topography-guided ablations were attempted. In this study, we evaluated the safety and efficacy of topography-guided LASIK for hyperopia with or without astigmatism.
Materials and methods
Two hundred and eight consecutive eyes from 156 patients underwent LASIK for hyperopia or hyperopic astigmatism. The inclusion criteria were hyperopia +0.25 D to +8.0 D, astigmatism up to −6.0 D, and spherical equivalent +6.0 D at most. Patients with previous corneal surgery, a history of herpetic eye disease, corneal dystrophy, corneal scarring, keratoconus, severe dry eye, or collagen or vascular disease were excluded.
Preoperative evaluation included uncorrected distance visual acuity, refraction (manifest, cycloplegic), best spectacle corrected visual acuity, slit-lamp examination including fundus evaluation, corneal tomographic imaging with Oculyzer Scheimpflug corneal tomography (Alcon, Fort Worth, TX), Topolyzer Placido-disc based topography (Alcon), ultrasound central corneal pachymetry with the Nidek US-1800 (Echoscan, Achi, Japan) and Wavefront analysis with the Allegretto-Wave Tscherning wavefront analyzer (WaveLight Laser Technologie, Erlangen, Germany) measured at a pupil size of 6.5 mm. Contrast sensitivities were also measured using the CSV-1000 (Vector Vision, Arcanum, OH). Our initial pilot experience with 30 eyes had led us to use a customized nomogram-adjustment algorithm for the ablation treatment plan, as shown in . This was used to compensate for the regression we had encountered in the earlier cases.
Table 1 Nomogram adjustment used for amount of sphere to be corrected
The operative technique was as follows. A disposable patient interface was used for each eye treated with femtosecond laser for flap creation. A drop of proparacaine 1% (Alcaine, Alcon) was instilled into the patient’s eye just before the procedure. The eyelids were painted with povidone iodine antiseptic 10% (Betadine, Purdue Pharma LP, Stamford, CT),) and the eye lashes were isolated with sterile plastic drapes (Tegaderm, 3M Health Care, St Paul, MN). The femtosecond laser was used to cut the corneal flaps in all cases. The flaps were cut to expose a stromal bed of at least 9.0 mm in diameter and 135 μm in depth, which was sufficiently large for a hyperopic (large diameter) ablation and its accompanying blend zone. Intraoperative flap diameter was measured with a surgical caliper. Ultrasound pachymetry measurement of the residual stromal bed was done with the same corneal pachymeter that had been used preoperatively. This value was subtracted from the preoperative corneal thickness, and the difference was taken as the corneal flap thickness (subtraction pachymetry). The 400 Hz Eye-Q excimer was used to perform ablations in all cases using the topography-guided platform. Images were taken from the Oculyzer (Scheimpflug-based corneal tomography).
Patients were examined half an hour postoperatively to check for any flap irregularities or complications.
Postoperatively parameters evaluated were uncorrected and corrected distance visual acuity, cornea tomography and topography, manifest refraction, wavefront changes, and contrast sensitivity. These examinations were repeated during follow-up at day 1, week 1, and months 1, 3, and 6. Evaluations were carried out every 6 months thereafter. All surgeries were performed by a single surgeon (AJK) in an outpatient refractive surgery center in Athens, Greece. Measurements and data gathering were undertaken by the surgeon and his associate staff.
Results
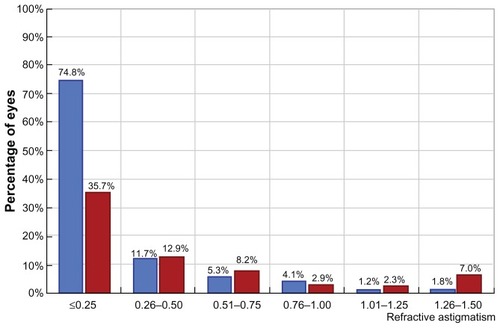
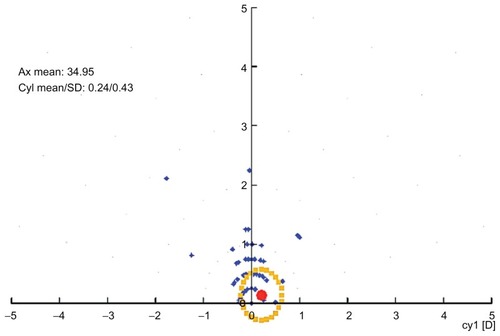
Two hundred and two eyes were available for evaluation at two years; three patients were lost to follow-up and five eyes received enhancement procedures prior to completion of our evaluation. The mean age was 40.4 ± 11.8 (range 19–62) years. Males comprised 54% of the cases and females comprised 46%. The mean preoperative sphere value was +3.04 ± 1.75 (range +0. 75 to +7.25) D, and the mean cylinder value was -1.24 ± 1.41 (range from −4.75 to 0) D. Uncorrected distance visual acuity improved from 5.5/10 to 8.2/10. At 24 (8–37) months, 75.5% of the eyes were in the ±0.50 D range of the refractive goal and 94.4% were in the ±1.00 D range. Mean preoperative sphere values were +3.04 ± 1.75 D and the mean preoperative cylinder value was −1.24 ± 1.41; the respective postoperative values were −0.39 ± 0.3 D and −0.35 ± 0.25. There was an increase in best spectacle-corrected visual acuity from 9.1/10 preoperatively to 9.5/10 postoperatively. shows a comparison of preoperative corrected distance visual acuity in blue and postoperative uncorrected distance visual acuity in red, indicating obvious improvement. shows the preoperative cylinder distribution in red and the postoperative cylinder distribution in blue. is a vectogram of preoperative cylinder distribution in blue dots (distance on the y axis represents amount and on the x axis represents degrees, with the yellow dots representing the postoperative cylinder vectogram).
Figure 1 Flap-making report generated by the FS200 femtosecond laser.
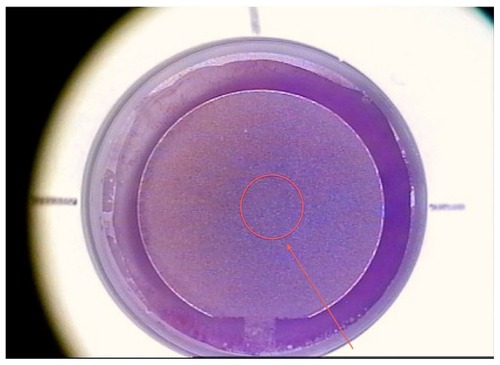
Figure 2 Concept of topography-guided ablation.
Figure 3 Comparison of preoperative corrected distance visual acuity in blue and postoperative uncorrected distance visual acuity in red, showing obvious improvement.
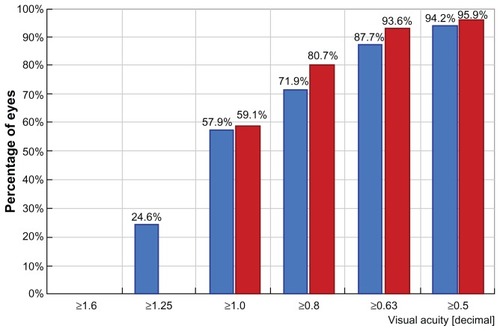
Figure 5 Vectogram of preoperative cylinder distribution in blue dots.
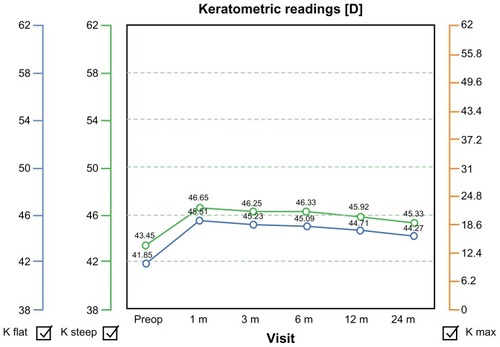
shows the predictability graph, with red dots showing overcorrection and blue dots showing undercorrection; the green dots are within ±0.5 D. This graph shows a slight tendency for overcorrection of higher refractive errors, which was our preoperative aim in order to compensate for any long-term regression effect. is the safety graph, with lines gained and lost, indicating a good safety profile with this technique and an impressive 46.6% of cases gaining at least one line of acuity postoperatively. Flap diameter was 8.9 ± 0.2 mm and flap thickness was 135 ± 8 μm, calculated by ultrasonic subtraction pachymetry. The keratometry readings showed an initial decrease within the first month, then demonstrated a progressive slow decline over the first 2 years, suggesting a predictable long-term regression of the initial hyperopic effect ().
Figure 6 Predictability graph with red dots showing overcorrection and blue dots showing undercorrection, with green dots within ±0.5 diopters.
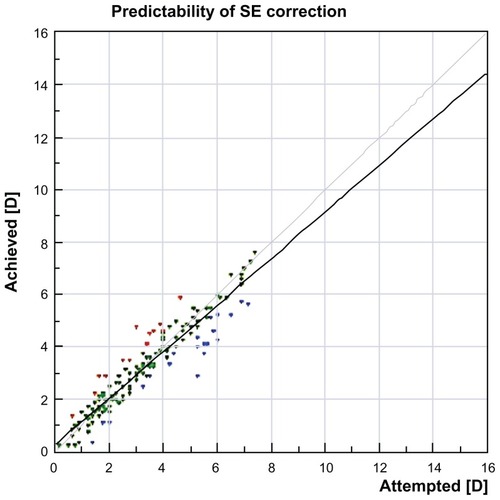
Figure 7 Safety graph with lines gained and lost, showing a good safety profile with this technique and an impressive 46.6% of cases gaining at least one line of acuity postoperatively.
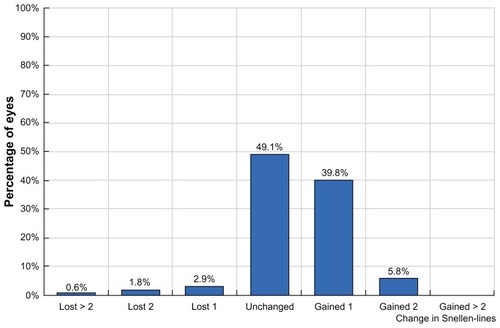
Figure 8 Keratometry readings showed an initial increase within the first month, indicative of effective hyperopic correction, but demonstrated a progressive slow decline over the first 2 years postoperatively, suggesting long-term regression of the initial hyperopic effect.
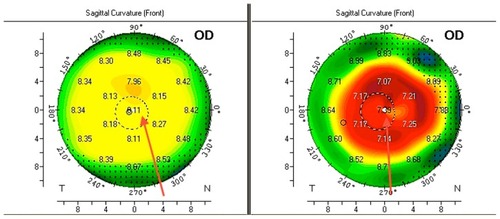
shows the results of preoperative and postoperative Scheimpflug-generated tomography. The red arrows point to the visual axis and line of sight. It is obvious that when one evaluates centration of the hyperopic ablation achieved in this hyperopic eye, the optical zone appears decentered in reference to the pupillary image (dotted circle). It is nevertheless centered on the visual axis of this eye with a significant angle kappa. The root mean square of higher-order aberrations increased by 15% from a preoperative value of 0.2–0.0 μm to a postoperative value of 0.23 μm at 12 months of follow-up. There was no epithelial downgrowth or any other significant complications noted in this small group. Low contrast sensitivity score results at 12 cycles/degree (column C on the chart) improved from an average preoperative value of 6.4 to 6.8.
Figure 9 Preoperative and postoperative Scheimpflug-generated tomography.
Abbreviation: OD, right eye.
Discussion
We have previously shown that a similar Allegretto excimer platform (200 Hz, wavefront-optimized excimer laser platform) can be safe and effective in hyperopic LASIK.Citation12 We have also shown that hyperopes have a significant angle kappa, and centering their laser correction on the pupil may be a mistake, suggesting that customized approaches to compensate for the angle kappa may be required in hyperopes.Citation15 In this study, we used a 400 Eye-Q topography-guided platform and a femtosecond laser for flap creation, and found that they can be superior in terms of safety and efficacy when performing hyperopic and hyperopic astigmatic corrections.
Intricacies of topography-guided hyperopic LASIK that we have encountered include the need for wider flap diameters and the difficulty of centering ablation within the stromal area exposed by the femtosecond laser flap. We aimed for slight (1–1.5 mm) nasal decentration, and slight (0.5 mm) superior decentration of the flaps in order to avoid flap edge and/or hinge placement within the ablation zone ( and ). We also calculated the shortest flap diameter to be at least 9 mm in order to achieve adequate ablation ().
Some authors have expressed concern about inducing higher order aberrations and astigmatism with hyperopic LASIK ablation.Citation4,Citation5 No significant wavefront changes occurred in our group. This may be in part attributable to use of the femtosecond laser instead of microkeratome flaps, as has been reported in the past.Citation16 More than half of our patients gained at least one line of corrected distance visual acuity.
At 24 months of follow-up, the mean refraction spherical equivalent was ±0.50 D of the intended postoperative refraction in over 80% of cases, which is comparable with or even better than the published results achieved using similar systems for correction of hyperopic refractive errors.Citation1–Citation11,Citation17 This efficacy also extended to the treatment of mixed hyperopic and astigmatic refractive errors which we theorize may be in part attributable to positive cylinder conversion and treatment of astigmatism on the steep meridian as well as addressing the angle kappa. We consider it inadvisable to perform hyperopic ablations in the center of the pupil, because this will invariably decenter the actual ablation in regard to the visual axis and line of sight, potentially inducing astigmatism. This principle leaves the central optical zone in these procedures untreated by the excimer because both the hyperopia and the cylinder are treated in a theoretical peripheral ring of 6.5–9.5 mm from the center of the visual axis. However, the keratometric regression noted over the average two-year follow-up period suggests that there is an intrinsic biomechanical mechanism in hyperopic LASIK, ie, “expansion” of the ablated mid peripheral cornea that has been ablated, resulting in progressive flattening of the steepened central cornea. We are currently using adjunctive collagen cross-linking techniques as a means of reducing and/or eliminating this effect.
Conclusion
Hyperopic and mixed astigmatism LASIK utilizing topography-guided 400 Hz Eye-Q excimer laser as well as the Intralase FS-60 and Alcon FS200 femtosecond lasers appear to be safe and very effective for correction of hyperopia and hyperopic astigmatism. The postoperative results at one and two years are impressive for correction of hyperopic and astigmatic refractive error and improvement in both corrected and uncorrected distance visual acuity, with minimal regression and need for enhancement.
Disclosure
This study was supported by an unrestricted fund from Alcon Laboratories, Fort Worth, TX.
References
- SalzJJStevensCALADARVision LASIK Hyperopia Study GroupLASIK correction of spherical hyperopia, hyperopic astigmatism, and mixed astigmatism with the LADARVision excimer laser systemOphthalmology200210991647165612208711
- LianJYeWZhouDWangKLaser in situ keratomileusis for correction of hyperopia and hyperopic astigmatism with the Technolas 117CJ Refract Surg200218443543812160152
- CaronesFVigoLScandolaELaser in situ keratomileusis for hyperopia and hyperopic and mixed astigmatism with LADARVision using 7 to 10-mm ablation diametersJ Refract Surg200319554855414518743
- El-AghaMSBowmanRWCavanaghDMcCulleyJPComparison of photorefractive keratectomy and laser in situ keratomileusis for the treatment of compound hyperopic astigmatismJ Cataract Refract Surg200329590090712781273
- Pineda-FernandezARuedaLHuangDNurJJaramilloJLaser in situ keratomileusis for hyperopia and hyperopic astigmatism with the Nidek EC-5000 Excimer laserJ Refract Surg200117667067511758985
- GoldbergDBComparison of myopes and hyperopes after laser in situ keratomileusis monovisionJ Cataract Refract Surg20032991695170114522287
- DitzenKFiedlerJPiegerSLaser in situ keratomileusis for hyperopia and hyperopic astigmatism using the Meditec MEL 70 spot scannerJ Refract Surg200218443043412160151
- Wygledowska-PromienskaDZawojskaIGierek-CiaciuraSNew generation of excimer laser – Asclepion Meditec MEL 70 G ScanKlin Oczna2000102373335 Polish11286116
- DaushDLandeszMThe Aesculap-Meditec laser: results from Germany. Laser correction for hyperopiaSalzJJCorneal Laser SurgerySt Louis, MOMosby1995
- SciscioAHullCCStephensonCGBaldwinHO’BrartDMarshallJFourier analysis of induced irregular astigmatism. Photorefractive keratectomy versus laser in situ keratomileusis in a bilateral cohort of hyperopic patientsJ Cataract Refract Surg20032991709171714522289
- WangLKochDDAnterior corneal optical aberrations induced by laser in situ keratomileusis for hyperopiaJ Cataract Refract Surg20032991702170814522288
- OshikaTKlyceSDApplegateRAHowlandHCEl DanasouryMAComparison of corneal wavefront aberrations after photorefractive keratectomy and laser in situ keratomileusisAm J Ophthalmol19991271179932992
- KanellopoulosAJConwayJPeLHLASIK for hyperopia with the WaveLight excimer laserJ Refract Surg2006221434716447935
- KanellopoulosAJTopography-guided custom retreatments in 27 symptomatic eyesJ Refract Surg2005215513518
- KanellopoulosAJManaging highly distorted corneas with topography-guided treatmentISRS/AAO 2007 Subspecialty Day/Refractive Surgery Syllabus Section II: Ablation StrategiesSan Francisco, CAAmerican Academy of Ophthalmology2007
- BasmakHSahinAYildirimNPapakostasTDKanellopoulosAJMeasurement of angle kappa with synoptophore and Orbscan II in a normal populationJ Refract Surg200723545646017523505
- Gil-CazorlaRTeusMAde Benito-LlopisLMikropoulosDGFemtosecond laser vs mechanical microkeratome for hyperopic laser in situ keratomileusisAm J Ophthalmol20111521162121507378