Abstract
Purpose
Currently, varying treatment paradigms and different clinical trial constructs preclude cross-trial comparison between different available vascular endothelial growth factor (VEGF) inhibitors. This study aimed to review the evidence and compare the efficacy of anti-VEGF therapies for neovascular age-related macular degeneration (nAMD), and to develop metrics as a means of facilitating standardized comparison between different anti-VEGF agents within the Advanced VitreoRetinal Analytics (AVRA) model.
Methods
The study analyzed key outcomes in clinical trials of bevacizumab, ranibizumab, aflibercept, and brolucizumab, including best corrected visual acuity (BCVA), number of injections, and duration of follow-up (minimum follow-up of 48 weeks).
Results
The AVRA model includes 1) vision recovery velocity (VRV; letters per unit time), which provides a metric of letters gained or lost over time (or the speed of improvement); 2) injection momentum (InjMom; number of injections multiplied by letters per unit time; units of injections•(letters/time)), which is defined as the number of injections multiplied by VRV and describes the quantity of treatment needed to achieve a vision outcome; and 3) vision recovery acceleration (VRA; letters per unit time squared; units of letters/time2), which denotes final VRV minus initial VRV, per unit time, and describes the rate of change in letters gained or lost over time.
Conclusion
AVRA stipulates that the ideal VEGF inhibitor to treat nAMD would have a higher positive VRV (more letters gained per unit time), low InjMom (lower treatment burden requiring fewer interventions for a given visual acuity outcome), and VRA approximating zero (indicating stable vision over time). AVRA allows comparisons across different trials to determine the optimal anti-VEGF agent for the treatment of nAMD.
Introduction
Anti-vascular endothelial growth factor (VEGF) antagonists have revolutionized the therapeutic approach for neovascular age-related macular degeneration (nAMD) and are the first-line gold standard treatments. The United States Food and Drug Administration (FDA) approved pegaptanib (Macugen®, EyeTech Pharmaceuticals) for the treatment of neovascular age-related macular degeneration (nAMD) in 2004, ranibizumab (Lucentis®, Genentech) in 2006, aflibercept (Eylea®, Regeneron) in 2011, and, most recently, brolucizumab (Beovu®, Novartis) in 2019. Intravitreal bevacizumab (Avastin®, Genentech) has been used as an off-label treatment for nAMD since 2005.
Over the past decade, comparison of different anti-VEGF mainstay agents has continued to be actively debated as new data accumulate. Although clinical registry trials, along with numerous other publications, demonstrate efficacy in the treatment of patients with nAMD, the ability for direct comparison is limited owing to variable treatment intervals and the fact that patients may receive a different number of injections over the course of a study period for different anti-VEGF protocols.
The purpose of this analysis is to review the evidence on the efficacy of anti-VEGF therapies for the treatment of nAMD and use these findings as a basis for the logical theoretical development of new metrics that will facilitate direct comparison between different anti-VEGF agents in a meaningful manner. We believe that, as more agents with varied dosing schedules and mechanisms are approved, this is needed to elucidate the treatment effectiveness of the different agents available to clinicians.
Methods
Ethics Statement
Our study adheres to the Declaration of Helsinki and was exempt from Institutional Review Board approval. Given that our analysis is predicated on existing literature, no informed consent was obtained.
Literature Search
We searched MEDLINE from 2000 to the present and Embase 2000 to the present (on February 1, 2020). The search was restricted to the English language. The search included all of the following study types: clinical trials, comparative studies, controlled clinical trials, meta-analyses, multicenter studies, randomized controlled trials, twin studies, and validation studies. The search excluded case reports, small case series, and non-English language studies.
Study Selection
We included all studies that assessed adult participants with nAMD and a history of anti-VEGF therapy with bevacizumab, ranibizumab, aflibercept, and brolucizumab. All trials must have published the following variables for their participants: number of patients, age, gender, pre- and post-treatment measures of best corrected visual acuity (BCVA), number of injections, and duration of follow-up (with a minimum follow-up of 48 weeks). Two reviewers (DA and JR) independently screened the citations, including titles and abstracts, and reviewed the full text of citations considered relevant. Studies were excluded if patients had alternative causes of macular disease.
Outcome Measures
The primary outcome measure was mean change in BCVA after treatment with the four different VEGF antagonists: bevacizumab, ranibizumab, aflibercept, and brolucizumab. In addition, the number of intravitreal injections for the study period was calculated and verified.
Advanced VitreoRetinal Analytics (AVRA) Model Construction
We developed three metrics to use as a means of facilitating standardized comparison between different anti-VEGF agents within the novel model, entitled Advanced VitreoRetinal Analytics (AVRA). AVRA is based on a logical mathematical derivation of Newtonian mechanics. The following three variables were constructed.
Vision Recovery Velocity
Vision recovery velocity (VRV; units of letters per unit time) is based on the derivation of the Newtonian mechanics description of velocity, and provides a measure of letters of vision gained (positive value) or lost (negative value) over time (calculated in months herein). This is the simplest AVRA metric to utilize in anti-VEGF agent comparison. VRV can be thought of as the speed at which an agent achieves vision improvement (positive VRV) or vision loss (negative VRV). A greater VRV would translate to a faster speed of vision improvement and thus be favorable to the clinician and patient. Conversely, a lower VRV equates to a relatively slow speed of vision improvement compared to other agents.
Injection Momentum
Injection momentum (InjMom; units of injections•(letters/time)) is derived from physical momentum (mass multiplied by velocity), and denotes the number of injections multiplied by VRV. Conceptually, InjMom is the number of intravitreal injections required to achieve an outcome (ie, improvement in vision). The more injections an anti-VEGF agent requires, the greater the injection momentum needed to achieve an outcome. A favorable VEGF antagonist would therefore have a lower InjMom and consequently require fewer treatments for a given visual acuity outcome.
Vision Recovery Acceleration
Vision recovery acceleration (VRA; units of letters/timeCitation2) is a vector quantity obtained by taking the final VRV and subtracting the initial VRV, per unit time. This calculation compares the final vision recovery velocity to the initial vision recovery velocity over the time course of the study in question; then, the resultant vector is standardized per unit time so as to allow comparison between different anti-VEGF agents and different clinical studies. As VRV described how letters are gained or lost per unit time (month), the value of VRV itself changes by the VRA value per unit of time. Although VRA is the most challenging derivation, what it provides – a measure of the rate of change in vision – is likely the most useful when counseling patients on expected vision changes over a given time period.
Results
We identified 1569 studies from our literature search strategy, from which eight studies were included in our final analysis: comparison of age-related macular degeneration treatments trials (CATT),Citation1 ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration (IVAN),Citation2 inject-and-extend Lucentis compared to Avastin study for exudative age-related macular degeneration (LUCAS),Citation3 minimally classic/occult trial of the anti-VEGF antibody ranibizumab in the treatment of neovascular age-related macular degeneration (MARINA),Citation4 ranibizumab anti-VEGF antibody for the treatment of predominantly classic choroidal neovascularization in age-related macular degeneration (ANCHOR),Citation5 study of 2.0 mg versus 0.5 mg ranibizumab in patients with subfoveal choroidal neovascularization secondary to age-related macular degeneration (HARBOR),Citation6 randomized, double-masked, active-controlled Phase 3 trial of the efficacy and safety of intravitreal VEGF trap-eye in wet age-related macular degeneration (VIEW1, VIEW 2),Citation7 and phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration (HAWK, HARRIER).Citation8 Of note, the open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration (HORIZON),Citation9 prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration (TREX-AMD),Citation10 and seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON cohort study (SEVEN-UP)Citation11 were reviewed but did not meet the inclusion criteria for integrated analysis.
VRV, InjMom, and VRA were calculated for bevacizumab, ranibizumab, aflibercept, and brolucizumab for each of the individual inclusive clinical studies, and are summarized in . A summary across all studies for the different anti-VEGF agents in provided in , with a graphical summary in .
Table 1 Individual Values for Vision Recovery Velocity (VRV), Injection Momentum (InjMom), and Vision Recovery Acceleration (VRA) for Bevacizumab, Ranibizumab, Aflibercept, and Brolucizumab According to Each Clinical Study Included in the Model Analysis
Table 2 Summary Values for Vision Recovery Velocity (VRV), Injection Momentum (InjMom), and Vision Recovery Acceleration (VRA) for Bevacizumab, Ranibizumab, Aflibercept, and Brolucizumab
Figure 1 Graphical summary of vision recovery velocity (VRV), injection momentum (InjMom), and vision recovery acceleration (VRA) for bevacizumab, ranibizumab, aflibercept, and brolucizumab.
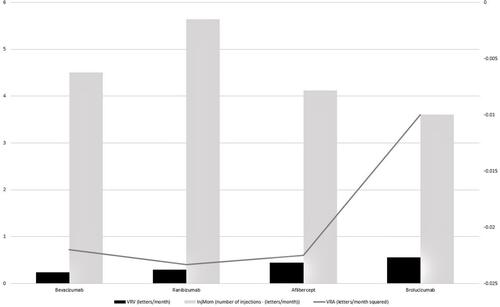
For bevacizumab, ranibizumab, and aflibercept, defined time points and number of injections were clearly present in the aforementioned studies. However, special mention must be made for brolucizumab because both HAWK and HARRIER used a “drop-down” design, making it difficult to exactly calculate the number of injections on q12 week brolucizumab versus q8 week brolucizumab groups. Within the HAWK and HARRIER studies, patients were assessed every 8 weeks to evaluate disease activity; at this time, the investigators would decide whether or not to treat (drop patient into the q8 week interval group) or continue on the original q12 week interval group. This creates a changing number of patients in each group; consequently, the number of injections changes as a function of the time point of the study. Nonetheless, we performed all calculations on the final numbers available at study conclusion.
Discussion
Analysis of the AVRA metrics shows that the agents with the most favorable VRV are aflibercept and brolucizumab, with patients gaining approximately 0.44 and 0.56 letters per month, respectively. This is in contrast to 0.24 and 0.30 for bevacizumab and ranibizumab, respectively. The explanation for using VRV is that, by standardizing time units, one can appreciably prognosticate on what a typical patient can see in terms of letters gained or lost per unit time (months). It must be stressed here that AVRA metrics are not in themselves provided for clinical intrapolation; for example, there is no basis for saying that 0.24 letters gained per month for bevacizumab is inferior to 0.44 letters gained per month for aflibercept, as 0.2 letters in not a decipherable quantity in clinical practice. Rather, the usefulness is by having a means of standardized comparison across agents. In the latter example, a patient can expect a VRV that is 83.3% faster, in letters gained per month, with aflibercept versus bevacizumab for the treatment of nAMD. Prior to AVRA, and to a large part the reason we pursued the development of such an analytical model, one was not able to make such comparisons for the available anti-VEGF agents.
Similarly, both aflibercept and brolucizumab have the most desirable (ie, lowest) InjMom, at 4.13 and 3.61 injections, respectively, owing to the fact that these agents require less frequent dosing. When looking at the bevacizumab and ranibizumab studies with less than monthly dosing (ie, PRN), these had an expected appreciable decrease in InjMom but this came at a cost of VRV. Dramatic examples of this are found for bevacizumab in CATT PRN, with an improved (ie, lower) InjMom of 2.71 injections but a concomitant decrease in VRV to only 0.19 letters gained per month. A similar example can be found with ranibizumab in the IVAN PRN study, with an improved InjMom of 3.25 injections but a significant loss of VRV to only 0.17 letters gained per month. This is an example of the utility of AVRA metrics, in that it facilitates comparison of multiple outcomes – in this case vision and injection burden – with respect to different VEGF antagonists and differing treatment protocols.
VRA, as described above, presents the most challenging conceptual adaption of AVRA but also perhaps is its most useful construct. For all four agents analyzed, VRA was between −0.02 and −0.01 letters lost (negative value) per month squared. This communicates two significant findings regarding the treatment of nAMD with VEGF antagonists. First, for all four agents, over time there is a negative direction to the vector quantity of VRA, indicating the likelihood of patients losing vision over time. Literature over the past decade has shown that patients treated with VEGF antagonists for nAMD tend to lose vision over time, incongruent to registry clinical trial findings. For example, the SEVEN-UP study of ranibizumab in the ANCHOR and MARINA trials showed that one-third of patients had poor outcomes and declined by 15 letters or more. Although undertreatment can be a significant attributable factor in this decline, we can also predict from AVRA that patients are expected to slowly lose vision over time with all four VEGF antagonists available. Progressive geographic atrophy or disciform scarring despite VEGF antagonist therapy could explain deteriorating vision over time.
Second, although all four VEGF antagonists possess negative VRA vectors, they are all very close to zero; in other words, with an acceleration vector close to zero, one would expect overall stability (ie, no rate of change) in maintaining a given VRV. Once again, if we look at the SEVEN-UP study we indeed find this to be true, with almost half of patients remaining stable over the study follow-up period. In fact, because no patient can continue to indefinitely gain vision for a vector approaching infinity, we can decipher that the ideal anti-VEGF agent would have a value of the smallest possible negative value (eg, −0.000001), indicating that, after initial vision gains, there is a maintenance of those vision gains over time, with only minor loss in vision.
Although AVRA allows us for the first time to make comparisons across different trials for the available anti-VEGF agents, there are limitations to our analysis. Despite our best efforts and stringent inclusion criteria, there is still heterogeneity within the chosen studies and heterogeneity will continue to be inevitable owing to the varying study designs, treatment paradigms, and follow-up periods of individual studies. Moreover, AVRA is designed to assess treatment efficacy only and it does not provide any insight into safety. Of course, safety is paramount to any clinician and needs to be considered when treating individual patients. However, at this time, there are no studies with the needed large sample sizes to allow for any meaningful analytical model of safety.Citation12 Finally, cost-effectiveness is a real-world consideration when choosing a VEGF antagonist but, given the various publications on economic modeling already available, we have made no attempt to include this in AVRA.
In conclusion, AVRA predicts that the ideal VEGF inhibitor to treat nAMD would have a higher positive VRV, supporting more letters gained per unit time, a lower InjMom, indicating lower treatment burden, and a VRA approximating zero, supporting the stability of vision gains over time. With AVRA, and for the first time, clinicians and researchers alike can make comparisons between the different available anti-VEGF agents with consideration of the speed, momentum, and acceleration of vision improvement (ie, VRV, InjMom, and VRA, respectively). As more VEGF antagonists, likely with a multitude of associated dosing paradigms, become approved, AVRA will be a necessary requisite for meaningful comparison and discussion on the preferred management of nAMD.
Ethics Approval
This study was conducted in accordance with the Declaration of Helsinki. Collection and evaluation of all protected health data were performed in a Health Insurance Portability and Accountability Act (HIPAA)-compliant manner.
Disclosure
DA reports personal fees from Alcon, during the conduct of the study; being an advisory board member, consultant, and speaker for Alcon, Allergan, Bayer, Genentech, Novartis, and Regeneron; and a cofounder and equity holder of Citrus Therapeutics. EC reports being a research subinvestigator for Novartis, Bayer, Allergan, Amgen, Genentech, Chengdu Kanghong Biotechnology, and Ophthotech, outside the submitted work; a consultant for Alcon, Allergan, Chengdu Kanghong Biotechnology, Genentech, Novartis, Ophthotech, and Opthea; and a cofounder and equity holder of Citrus Therapeutics. The authors report no other potential conflicts of interest for this work.
Additional information
Funding
References
- Martin DF, Maguire MG, Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: 2-year results: comparison of age-related macular degeneration Treatments Trials (CATT) research. Ophthalmology. 119;2012:1388–1398. doi:10.1016/j.ophtha.2012.03.053
- Chakravarthy U, Harding SP, Rogers CA, et al.; IVAN Study Investigators. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–1411. doi:10.1016/j.ophtha.2012.04.015
- Berg K, Hadzalic E, Gjertsen I, et al. Ranibizumab or bevacizumab for neovascular age-related macular degeneration according to the lucentis compared to avastin study treat-and-extend protocol two-year results. Ophthalmology. 2016;123:51–59. doi:10.1016/j.ophtha.2015.09.018
- Rosenfeld PJ, Brown DM, Heier JS, et al.; MARINA Study Group. MARINA: ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi:10.1056/NEJMoa054481
- Brown DM, Michels M, Kaiser PKM, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two year results of the ANCHOR study. Ophthalmology. 2009;116:57–65.
- Ho AC, Busbee BG, Regillo CD, et al.; HARBOR Study Group. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular Degeneration. Ophthalmology. 2014;121:2181–2192. doi:10.1016/j.ophtha.2014.05.009
- Heier JS, Brown DM, Chong V, et al., VIEW 1 and VIEW 2 Study Groups. Intravitreal Aflibercept (VEGF Trap-Eye) in Wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548.
- Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER Study Investigators. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127::72–84. doi:10.1016/j.ophtha.2019.04.017
- Singer MA, Awh CC, Sadda S, et al. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119:1175–1183. doi:10.1016/j.ophtha.2011.12.016
- Wykoff CC, Croft DE, Brown DM, et al., TREX-AMD Study Group. Prospective Trial of Treat-and-extend versus monthly dosing for neovascular age-related macular degeneration TREX-AMD 1-Year Results. Ophthalmology. 2015;122(12):2514–2522. doi:10.1016/j.ophtha.2015.08.009
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON a multicenter cohort Study (SEVEN-UP). Ophthalmology. 2013;120:2292–2299. doi:10.1016/j.ophtha.2013.03.046
- Bakri SJ, Thorne JE, Ho AC, et al. Safety and efficacy of anti-vascular endothelial growth factor therapies for neovascular age-related macular degeneration: a report by the american academy of ophthalmology. Ophthalmology. 2019;126:55–63. doi:10.1016/j.ophtha.2018.07.028
