Abstract
Purpose
This study evaluated real-world treatment of dry eye disease (DED) with lifitegrast.
Patients and Methods
Ophthalmologists and optometrists treating patients with DED were invited to participate through a healthcare provider (HCP)-based panel. HCPs completed a provider survey and contributed data toward a chart review for up to five qualifying patients with DED who initiated lifitegrast ophthalmic solution (index date) between 01/01/2017 (US) or 01/01/2018 (Canada) and 06/30/2019. Patient demographics, treatments, clinical characteristics, and outcomes (ie, severity, signs, symptoms) were collected for the 6-month pre-index period and up to 12-months post-index.
Results
For this study, 517 HCPs contributed 600 patient charts. Among 554 and 281 patients with follow-up at 6 and 12-months post-index, 512 (92.4%) and 238 (84.7%) patients had ongoing lifitegrast treatment, respectively. Other DED-related treatments were less frequently used post-index with lifitegrast vs pre-index: over-the-counter artificial tear use (45.2% vs 75.5%), topical corticosteroids (3.8% vs 18.8%), any cyclosporine (3.0% vs 20.5%). At 3-months (n=571) and 12-months (n=320) post-index vs pre-index, fewer patients had eye dryness (47 [8.2%] and 16 [5.0%] vs 525 [87.5%]), blurred vision (28 [4.9%] and 11 [3.4%] vs 346 [57.7%]), ocular burning/stinging (25 [4.4%] and 8 [2.5%] vs 336 [56.0%]), depression (8 [1.4%] and 9 [2.8%] vs 55 [9.2%]), fatigue (4 [0.7%] and 1 [0.3%] vs 82 [13.7%]), and headache (1 [0.2%] and 0 vs 19 [3.2%]). At 3 and 12-months post-index vs pre-index, average corneal staining score was numerically lower (2.7 and 2.0 vs 6.5), and average Schirmer score (10.6 and 10 vs 6.3) and tear film break-up time (7.3 and 8.0 vs 4.8) higher.
Conclusion
The majority of patients had ongoing lifitegrast treatment 6-months post-index with reduction in overall treatment burden. Improvement in DED signs and symptoms, including QoL impacts, was evident at 3 months and up to 12 months after lifitegrast initiation.
Plain Language Summary
A chart review study was conducted among patients with dry eye disease (DED) in the US and Canada treated with lifitegrast, and a provider survey was administered to the participating ophthalmologists and optometrists who abstracted data from patient medical charts. Five hundred and seventeen ophthalmologists and optometrists contributed a total of 600 patient charts. Patients were very likely to continue treatment with lifitegrast 6 months after initiating treatment, and they experienced a reduction in the use of concomitant DED-related treatment following lifitegrast initiation. Patients had fewer DED symptoms, including quality of life (QoL) impacts, at 3, 6 and 12 months after lifitegrast initiation compared with the period prior to lifitegrast initiation. Corneal staining scores, Schirmer scores, and tear film break-up time were indicative of DED sign improvement starting at 3 months after initiating lifitegrast. Most of the providers who participated reported working in private practice and small practice settings, comprised of two to ten ophthalmologists/optometrists. The majority of providers indicated they typically prescribe lifitegrast within 6 months of DED diagnosis. Observational studies such as the one conducted are especially meaningful to inform the use and impact of lifitegrast in DED patient populations that are more heterogeneous than those included in clinical trials.
Introduction
Dry eye disease (DED) is a common, multifactorial condition of the ocular surface, which can result in discomfort, visual disturbance, and tear film instability.Citation1–Citation3 Patients with DED often experience symptoms such as foreign body sensation, excessive tearing, pain and redness of the eye, sensitivity to light, discharge from the eye, and blurred vision.Citation3 In the United States (US), approximately 16 million adults have been diagnosed with DED, with an estimated prevalence of 6.8%, while in Canada, approximately 6 million adults are affected by DED, with an estimated prevalence of 21.3%.Citation4,Citation5 These estimates may underrepresent the true burden of DED as diagnosis of DED is challenging due to its multifactorial etiology and given that DED-related signs manifest in only 57% of symptomatic patients.Citation6 DED symptoms can be particularly heterogeneous in patients with immune-mediated diseases, such as inflammatory bowel disease or Sjogren’s syndrome, and the correlation between subjective and objective parameters for DED is not always clear.Citation7,Citation8 The risk of DED increases with age and disproportionately affects women, with some studies finding the prevalence of DED to be two times higher in women compared to men.Citation2,Citation4 Other risk factors associated with DED development include select autoimmune diseases, certain medications (eg, anti-histamines, diuretics, etc), frequent use of electronic devices, and wearing contact lenses.Citation1,Citation2 The COVID-19 pandemic has also exacerbated DED symptoms due to the increase in screen time and face mask use, both of which are implicated in rapid tear evaporation.Citation9
Chronic DED is associated with significant impairments in quality of life (QoL).Citation2 Patients with DED are more likely to have difficulty reading, using a computer, driving, and watching television, which impact their daily and social life as well as professional life.Citation10 DED is associated with a 30% impairment in workplace performance and work productivity.Citation7 Along with DED affecting QoL and work productivity, the average annual direct cost of DED on the US healthcare system is estimated at $3.8 billion.Citation4,Citation10 Therefore, understanding and treating DED properly and timely are of utmost importance to address the needs of all relevant stakeholders, including patients, physicians, the governments, and more broadly the healthcare systems.
Management of DED includes over-the-counter (OTC) artificial tears, lid hygiene, punctal plugs, and short-term prescription treatments such as topical corticosteroid eye drops and topical nonsteroidal anti-inflammatory drug (NSAID) eye drops with antibiotics; however, these short-term prescriptions are not adequate for treating persistent symptoms, and corticosteroids cannot be used long-term due to possible side effects.Citation11 Additional treatments for DED include compounded topical cyclosporine solutions (Klarity-C Drops®, ImprimisRx, San Diego, CA), cyclosporine ophthalmic emulsion (Restasis®, Allergan, Dublin, Ireland), and cyclosporine ophthalmic solution (CEQUA™, Sun Pharmaceuticals Industries Ltd., Goregaon, Mumbai, India), which increase tear production.Citation2,Citation12 Lifitegrast ophthalmic solution (Xiidra®, Novartis AG Pharma, Basel, Switzerland), approved in the US in July 2016 and in Canada in January 2018, is currently the only prescription eye drop indicated for both signs and symptoms of DED.Citation13,Citation14 Lifitegrast is a lymphocyte function-associated antigen-1 agonist that acts by reducing the inflammation associated with DED.Citation2,Citation15
Randomized clinical trials have shown lifitegrast to be a safe and effective therapy in treating DED signs and symptoms.Citation15–Citation17 The current study sought to understand the use and effectiveness of lifitegrast in real-world settings beyond the clinical trial setting. A descriptive healthcare provider (HCP) panel-based chart review study was conducted to assess patient characteristics, treatment patterns, and effectiveness among a large sample of patients with DED treated with lifitegrast in the US and Canada post-market approval.
Methods
Study Design and Study Population
This was a non-interventional, retrospective, longitudinal study among patients with DED treated with lifitegrast in the US and Canada. The study included data from patients’ medical records, which HCPs abstracted into an electronic case record form (eCRF). The eCRF also comprised of a provider survey, which gathered responses relating to HCP and practice-level characteristics. Data were collected via a secure electronic data platform from January – February 2020, where all questions related to study inclusion and exclusion criteria were mandatory. Questions unrelated to study eligibility allowed providers to select the response option “unknown”, if applicable. The target sample size was 600 patient charts. Data were de-identified and complied with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act (HIPAA). All study materials were approved by the New England Independent Review Board (NEIRB) under the exempt category as data were de-identified and patient informed consent was not required. This study was conducted in accordance with the Declaration of Helsinki.
Ophthalmologists and optometrists in the US and Canada were recruited to participate in the study through Dynata (Shelton, CT), which maintains panels of HCPs after recruiting and validating HCPs through licensed medical organizations such as the American Medical Association or recruiting HCPs through business-to-business panels and validating their identities through various mechanisms, including government records, which is the industry standard. Ophthalmologists and optometrists were invited to participate if they had at least one qualifying patient who met inclusion criteria. Eligible HCPs completed a provider survey and were permitted to contribute data for up to five patients in this study. Qualifying patients were at least 18 years of age at initiation of lifitegrast and had a diagnosis of DED prior to lifitegrast initiation, had initiated lifitegrast between January 1, 2017 (US)/January 1, 2018 (Canada) and June 30, 2019, had clinical information available both 6-months pre- and post-lifitegrast initiation, and had at least one clinical visit in the 6-month period following lifitegrast initiation; patients were not required to be treated with lifitegrast at the time of their clinical visit in the 6-month period following lifitegrast initiation. The CRF included instructions for the HCP to provide data from a random sample of eligible patients: the HCP was given a series of letters (eg, A to M) and asked to review patient charts with last names starting with those letters, in alphabetical order, and then select the first patient who met all eligibility criteria. The process was repeated up to five times for HCPs with more than one qualifying patient who wished to contribute additional data. The provider survey assessed HCP and practice-level characteristics including clinical practice setting, practice size, number of years in practice, and number of patients with DED treated per year. Providers’ approach to treating DED, including reasons for lifitegrast prescription and discontinuation, and timing of lifitegrast prescription relative to DED diagnosis, were also assessed.
For the chart review part of the study, the index date was defined as the date of lifitegrast initiation. The 6-month period prior to lifitegrast initiation served as the pre-index period. Patient demographic and baseline clinical characteristics (eg, gender, age, health insurance, comorbidities, year of DED diagnosis, cause and type of DED) were assessed in the 6-month pre-index period. The period following lifitegrast initiation was defined as the post-index period, and treatment and DED characteristics until earliest of last known clinic contact or 12-months post-index were identified.
Lifitegrast treatment characteristics, including dose, frequency, and discontinuation, were assessed in the post-index period. Patients were classified as having discontinued lifitegrast if the provider indicated that the patient had ended lifitegrast treatment. Specific reasons for discontinuation were ascertained, including insufficient response, intolerance of treatment, patient preference, and issue with insurance coverage. The proportion of patients continuing lifitegrast treatment at 6 and 12-months post-index time points was calculated among patients who were followed for at least that length of time.
DED-related treatments aside from lifitegrast and clinical outcomes were assessed in both the pre-index and post-index periods. Data on use of DED-related treatments (eg, OTC artificial tears, topical corticosteroids, any cyclosporine) were collected. The scores from DED severity, sign, and symptom assessments within the pre-index period and closest to the index date, and data from these assessments throughout the post-index period were collected. Assessments of interest included DED severity assessments (eg, provider’s severity level measure [mild, moderate, severe], Dry Eye Workshop Scale [DEWS], Canadian Dry Eye Assessment [CDEA], Dry Eye Severity Level [DESL]), assessments for DED signs (eg, corneal staining score, Schirmer score, tear film break-up time [TFBUT]), and DED symptom assessment toolsCitation18 (eg, 5-item Dry Eye Questionnaire [DEQ-5], Dry Eye-related Quality of Life Score [DEQS], Eye Dryness Score [EDS], Impact of Dry Eye on Everyday Live [IDEEL], Ocular Surface Disease Index [OSDI], Standardized Patient Evaluation of Eye Dryness [SPEED] questionnaire). DED symptoms including QoL impacts (eg, eye dryness, blurred vision, ocular burning/stinging, fatigue, depression, headache) as reported in the patient chart and not necessarily part of a questionnaire were also collected. For DED severity, sign, and symptom assessments and DED symptoms, HCPs could report the date of the assessment or symptom report, or indicate if the assessment or report occurred during a given time interval post-index (ie, 3 months [±30 days], 6 months [±30 days], 12 months [±60 days]). DED-related and other ocular surgical procedures (eg, cataract surgery, debridement) recorded in the patient chart for the pre- and post-index periods were also collected.
Statistical Analysis
A total of 600 patient charts met pre-specified quality control measures and were included in the analysis. Descriptive statistics were calculated using frequencies and proportions (including two-sided 95% confidence intervals [CIs]) for categorical variables and means, standard deviations (SDs), and medians for continuous variables. Ninety five percent CIs for categorical variables were calculated using the Clopper–Pearson method. For categorical variables, missing data were considered as a separate category. Summary statistics for severity, signs, and symptoms were calculated for the 6-months pre-index period and at 3-months (±30 days), 6-months (±30 days) and 12-months (±60 days) post-index among patients with ongoing lifitegrast treatment and with follow-up until at least the specified time points. The frequency and proportion of patients with reported symptoms at any time up to 6 months post-index period were also described. Analyses were conducted overall (pooled for US and Canada). All analyses were performed using SAS Enterprise Guide software Version 7.1 (SAS Institute, Cary, NC).
Results
Healthcare Provider (HCP) Survey
A total of 517 HCPs contributed 600 patient charts, with an average of 1.2 patient charts per HCP. Among the 517 providers who participated in this study, 477 were from the US (including 287 ophthalmologists and 190 optometrists), and 40 were from Canada (including 13 ophthalmologists and 27 optometrists). Of the 517 HCPs who contributed patient chart data, 501 (96.9%) completed the HCP survey (). Most HCPs reported working in private practice (416 [83.0%]) and small practice settings of 2 to 10 ophthalmologists/optometrists (312 [62.3%]). The majority of HCPs indicated prescribing lifitegrast within 6 months of DED diagnosis. The most common reason for lifitegrast prescription was lifitegrast’s efficacy, while the most common reason for lifitegrast discontinuation was issues related to cost and insurance coverage.
Table 1 Healthcare Provider (HCP) Survey (n=501)
Patient Chart Review
Baseline Demographic and Clinical Characteristics
Characteristics of the 600 patients treated with lifitegrast who met the eligibility criteria are described in . The majority of patients were female (455 [75.8%]) with a mean age of 57.1 years at the time of lifitegrast initiation. Two hundred and sixty three patients (263, 43.8%) were diagnosed with DED after 2017. For cause or type of DED, 376 patients (62.7%) had evaporative deficiency only, and 32 patients (5.3%) had aqueous deficiency only, while 160 patients (26.7%) had both aqueous and evaporative deficiencies. The most common ocular diseases reported pre-index were cataract (203 [33.8%]), glaucoma (33 [5.5%]), and macular degeneration (24 [4.0%]). The most prevalent comorbidities reported pre-index were hypertension (163 [27.2%]), allergies (114 [19.0%]), diabetes (any type) (82 [13.7%]) and depression (63 [10.5%]).
Table 2 Demographic and Clinical Characteristics in the Pre-Index Period Among Patients with DED Treated with Lifitegrast (n=600)
Lifitegrast Utilization
Lifitegrast utilization is described in . Average time (± SD [median]) from DED diagnosis to lifitegrast initiation was 23.3 ± 42.7 [11.6] months. Among 554 patients with at least 6 months of observation, 512 patients (92.4%) were still on lifitegrast treatment at 6 months post-index. Among 281 patients with 12 months of observation, 238 patients (84.7%) were still on lifitegrast treatment at 12 months post-index. As patients were required to have one clinical visit in the 6-month period following lifitegrast initiation and half of patients initiated lifitegrast in the year prior to data collection, most patients did not have 12 months of observation. Seventy six patients (76, 12.7%) who discontinued lifitegrast during the post-index period had a lifitegrast treatment duration (mean ± SD [median]) of 5.5 ± 3.8 [5.0] months. The most common reasons for lifitegrast treatment discontinuation included insufficient response (34 [44.7%]), intolerance to treatment (25 [32.9%]), patient preference (22 [28.9%]), and issues with insurance coverage (18 [23.7%]).
Table 3 Lifitegrast Utilization Among Patients with DED
DED-Related Treatments and Ocular Surgeries in the Pre- and Post-Index Periods
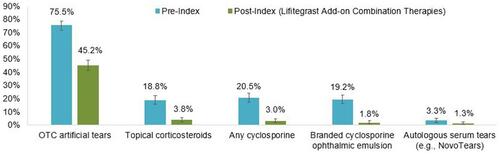
Two hundred and twenty six patients (226, 37.7%) were treated with lifitegrast alone post-index. Overall, the use of non-lifitegrast DED-related treatments was lower following lifitegrast initiation. displays other DED-related treatment used pre-index and concomitantly with lifitegrast in the post-index period. OTC artificial tear use was reported for 453 patients (75.5%) pre-index and for 271 patients (45.2%) at some point in the post-index period. One hundred and thirteen patients (113, 18.8%) were documented as treated with topical corticosteroids pre-index, whereas 23 patients (3.8%) were documented as treated with topical corticosteroids post-index. One hundred and twenty three patients (123, 20.5%) were treated with any cyclosporine pre-index vs 18 patients (3.0%) post-index. One hundred and fifteen patients (115, 19.2%) and 11 patients (1.8%) were treated with branded cyclosporine ophthalmic emulsion pre-index and post-index with lifitegrast, respectively. The frequency and proportion of patients who had ocular and DED-related surgical procedures during the pre- and post-index periods are summarized in Table S1. Cataract surgery was the most common surgery in the pre-index period.
Figure 1 Proportion of patients with DED-related treatments in the 6-month pre-index period and added to lifitegrast in the post-index period (n=600)*. Vertical bars indicate 95% confidence intervals. Patients may have multiple DED-related treatments; therefore, the sum of percentages may exceed 100%. *11 patients (1.8%) switched from lifitegrast to other therapies during the post-index period. These patients are not included in .
DED Severity in the Pre- and Post-Index Periods
Information on DED severity was scarce. Four hundred and forty four patients (444, 74.0%) had no severity assessments pre-index. Data were less widely available post-index (results not shown), with no severity information reported for 546 (95.6%), 509 (95.3%), and 266 (83.1%) patients at 3, 6, and 12-months post-index, respectively.
DED Symptoms in the Pre-Index and Post-Index Periods
Compared with patients in the 6-month pre-index period, patients with ongoing lifitegrast treatment had fewer DED symptoms reported at 3, 6, and 12-months post-index (). Likewise, a lower proportion of patients had DED symptoms reported at any time up to 6 months post-index compared to the 6-month pre-index period (). Symptom questionnaires were more common in the 6-month pre-index period vs the 3, 6, and 12-month time points in the post-index period with no particular symptom questionnaire used predominantly (). The average scores for DEQ-5, OSDI, and SPEED questionnaires were numerically lower at 3, 6, and 12-months post-index vs pre-index ().
Table 4 Patients with DED Symptoms Reported in the Pre- and Post-Index Periods
Table 5 DED Questionnaires in the Pre- and Post-Index Periods
DED Signs in the Pre- and Post-Index Periods
Results from the most common DED clinical sign assessments (ie, corneal staining score, Schirmer score, and TFBUT) are displayed in . Among patients with corneal staining scores, the averages were numerically lower at 3, 6, and 12-months post-index than in the pre-index period. Among patients with Schirmer scores and TFBUT, patients had higher average scores at these post-index time points than in the pre-index period.
Table 6 DED Sign Assessments in the Pre- and Post-Index Periods
Discussion
This retrospective analysis of DED patients treated with lifitegrast in real-world settings in the US and Canada suggests that DED symptoms, including QoL impacts, and signs improved following lifitegrast initiation. In addition, the use of other DED-related treatments (particularly, OTC artificial tears, branded cyclosporine ophthalmic emulsion, any cyclosporine, and topical corticosteroids) decreased following lifitegrast initiation. Other DED-related treatment use was lower following lifitegrast initiation, and over one-third of patients were reported to be treated with lifitegrast alone; this was observed despite the expansion of treatment options for DED in recent years. These findings highlight the potential effectiveness of lifitegrast on symptoms and signs both as a standalone and concurrent treatment.
This is the first real-world study to assess the long-term (ie, up to 12 months) effectiveness of lifitegrast among a large patient population treated for DED in clinical practice. Observational research such as this is critical in describing the treatment experience among patients in real-world settings, which include a broader population than those eligible for trials.Citation15–Citation17 The current study reported a quarter of patients with contact lenses usage, and nearly half with OTC artificial tears use in combination with lifitegrast; patients with these characteristics were excluded from controlled trials to control for confounding. Studies such as this are important to inform HCPs about real-world experiences and enable them to improve the patient journey for the population of patients they treat.
This study shows that lower proportions of patients reported DED symptoms including QoL impacts at 3, 6, and 12-months after initiating lifitegrast treatment relative to prior to lifitegrast use. Furthermore, the summary statistics show that compared with the pre-index period, the average corneal staining score was numerically lower as early as 3 months after initiating lifitegrast, which is indicative of an improvement in DED signs.Citation19 The average TFBUT was less than 5 seconds during the pre-index period and was above 7 at 3, 6, and 12-months after initiating lifitegrast treatment. These findings are consistent with prior studies that report an association between lifitegrast treatment and DED symptom and sign improvement.Citation16,Citation17,Citation20 The OPUS-1 Phase III study demonstrated DED sign improvement with lifitegrast by reduced corneal fluorescein and conjunctival lissamine staining, and the chart review study by Tong et al that described the characteristics and outcomes of 121 patients with DED treated with lifitegrast also showed lifitegrast was associated with an improvement in corneal staining and TFBUT.Citation16,Citation20
A high proportion of patients had ongoing lifitegrast treatment at the end of follow-up. The observational study by Tong et al that included 121 patients reported 80.2% of patients were still on treatment at the end of follow-up, where average follow-up was 88.1 days.Citation20 An observational study by White et alCitation21 using claims data reported that among 3235 patients, 2082 (64.4%) discontinued lifitegrast within 12 months of initiation and had a median of 29 days from lifitegrast initiation to discontinuation. That study was unable to elaborate on the reasons why patients discontinued treatment as claims data do not have this information. Notably, nearly three quarters of the patients in that study initiated lifitegrast in the year it was approved, and insurance coverage issues may have impacted discontinuation among patients who initiated lifitegrast soon after its approval. When lifitegrast was first marketed, coupons were initially offered that would cover the first 6 weeks of treatment, and patients frequently discontinued treatment following the free supply due to lack of insurance coverage for lifitegrast at that time. This was a universal issue with patients covered by Medicare but has since improved for patients with commercial insurance (D White, Department of Ophthalmology, SkyVision Centers, personal communication, December 2020).
There were some differences in findings obtained from the chart review and provider survey. This could be due to differences in experiences among patients included in the chart review who initiated lifitegrast as far back as 2017 vs current practice patterns that providers described in the provider survey. There may have been differences in reasons for treatment discontinuation captured in the provider survey vs what was reported in the patient chart if the information in the chart reflected what the patient reported, which could differ from HCPs’ perceptions about why patients discontinue.
Observational studies are non-interventional and therefore limited by heterogeneity in patient follow-up and variations in treatment regimens. There were lower proportions of patients with symptom score and sign assessments available at the pre-specified post-index time points in this study as compared to prospective studies. However, the data collection tool for this study was designed to capture a wide array of symptoms, including those not necessarily ascertained through a questionnaire, which the patient may have reported to the HCP while being treated in a clinical practice setting. The HCPs who participated in this study provided real-world retrospective data for patients who were not enrolled in clinical trials, demonstrating the diversity of diagnostic tests as well as DED-related symptom questionnaires that are currently being used in clinical practices across the US and Canada. Further research could focus on reaching a consensus on best practices for diagnosing and monitoring progress while on treatment.
There are limitations to the current study. Data were collected retrospectively and are limited to the information available in the patients’ medical charts. OTC treatments were those reported by the patient to the provider, and medications were those prescribed or given by the provider, but adherence was not verified. The presence of symptoms reported here was based upon information that was reported by the patient to the provider. As with all analyses of retrospective non-randomized studies, this study is subject to potential biases including selection bias, measurement error, and non-random missing data. To minimize the chance of selection bias, a randomization scheme was implemented for HCPs to include data from patients who initiated lifitegrast treatment regardless of how long they continued their treatment or their experience with their treatment. Across the different ophthalmologist and optometrist practices, clinical assessments of DED in real-world settings are based on heterogeneous criteria and assessment schedules. Findings from this study may not be generalizable to countries outside of the US and Canada. The self-selection of ophthalmologists and optometrists who joined the panel and who responded to the online chart review invitation (providing data on an average of one patient per HCP) may also limit generalizability.
Conclusions
Findings from this study suggest that patients are likely to continue treatment with lifitegrast for at least 6 months. Treatment with lifitegrast was indicative of improvements in both signs and symptoms, including QoL impacts, in real-world settings as early as 3 months and lasting as long as 12 months, and with a reduction in the use of concomitant DED-related treatments. Observational research like this study is helpful to inform the effectiveness of lifitegrast in a heterogeneous real-world patient population.
Abbreviations
CI, confidence interval; CDEA, Canadian Dry Eye Assessment; DED, dry eye disease; DEQ-5, 5-Item Dry Eye Questionnaire; DEQS, Dry Eye-related Quality of Life Score; DESL, Dry Eye Severity Level; DEWS, Dry Eye Workshop Scale; eCRF, electronic case report form; EDS, Eye Dryness Score; HCP, healthcare provider; HIPAA, Health Insurance Portability and Accountability Act; IDEEL, Impact of Dry Eye on Everyday Life; NEIRB, New England Independent Review Board; NSAID, nonsteroidal anti-inflammatory drug; OSDI, Ocular Surface Disease Index; OTC, over-the-counter; QoL, quality of life; SD, standard deviation; SPEED, Standardized Patient Evaluation of Eye Dryness; TFBUT, tear film break-up time; US, United States.
Acknowledgments
This research was funded by Novartis Pharma AG, Basel, Switzerland. The authors would like to thank Dr. Mei Sheng Duh and Dr. Felicia Castriota from Analysis Group, Inc. for their contributions to this study.
Disclosure
Ms. Catherine Nguyen and Dr. Caroline Korves are employees of Analysis Group, Inc., a consulting company that has received funding from Novartis Pharma AG for this and other research. Ms. Annie Syntosi and Ms. Brigitte Sloesen are employees of Novartis Pharma AG. Dr. Mrudula B Glassberg and Dr. Arthur Chan are employees of Novartis Pharmaceuticals Corporation. Dr. John A Hovanesian, Dr. Kelly K Nichols, Dr. Mitchell Jackson and Dr. James Katz have been consultants to Novartis for this and other work. Dr. Kelly K Nichols reports personal fees from Bruder, Dompe, HanAll, Osmotica, Oyster Point, SightSciences, Alcon/Acquiom, Thea, Tarsus, and Topivert; personal fees, non-financial support from Kala; grants from Allergan, Tear Science, and NIH NEI, outside the submitted work. Dr. John A Hovanesian has received research grant support from Novartis. The authors report no other conflicts of interest in this work.
References
- Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. 2009;3:405–412. doi:10.2147/OPTH.S5555
- Semba CP, Gadek TR. Development of lifitegrast: a novel T-cell inhibitor for the treatment of dry eye disease. Clin Ophthalmol. 2016;10:1083–1094. doi:10.2147/OPTH.S110557
- Phadatare SP, Momin M, Nighojkar P, Askarkar S, Singh KK. A comprehensive review on dry eye disease: diagnosis, medical management, recent developments, and future challenges. Adv Pharm. 2015;2015:1–12. doi:10.1155/2015/704946
- Farrand KF, Fridman M, Stillman IO, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90–98. doi:10.1016/j.ajo.2017.06.033
- Caffery B, Srinivasan S, Reaume CJ, et al. Prevalence of dry eye disease in Ontario, Canada: a population-based survey. Ocul Surf. 2019;17(3):526–531. doi:10.1016/j.jtos.2019.02.011
- Elhusseiny AM, Khalil AA, El Sheikh RH, Bakr MA, Eissa MG, El Sayed YM. New approaches for diagnosis of dry eye disease. Int J Ophthalmol. 2019;12(10):1618–1628. doi:10.18240/ijo.2019.10.15
- Barta Z, Czompa L, Rentka A, et al. Evaluation of objective signs and subjective symptoms of dry eye disease in patients with inflammatory bowel disease. Biomed Res Int. 2019;2019:8310583. doi:10.1155/2019/8310583
- Alunno A, Bartoloni E, Valentini V, et al. Discrepancy between subjective symptoms, objective measures and disease activity indexes: the lesson of primary sjogren’s syndrome. Clin Exp Rheumatol. 2018;36 Suppl 112(3):210–214.
- Giannaccare G, Vaccaro S, Mancini A, Scorcia V. Dry eye in the COVID-19 era: how the measures for controlling pandemic might harm ocular surface. Graefes Arch Clin Exp Ophthalmol. 2020;258(11):2567–2568. doi:10.1007/s00417-020-04808-3
- Uchino M, Schaumberg DA. Dry eye disease: impact on quality of life and vision. Curr Ophthalmol Rep. 2013;1(2):51–57. doi:10.1007/s40135-013-0009-1
- Dry eyes. Mayo clinic. September 24, 2020. Available from: https://www.mayoclinic.org/diseases-conditions/dry-eyes/diagnosis-treatment/drc-20371869. Accessed November 18, 2020.
- FDA. Drug approval package: CEQUA. Drug Approvals and Databases. 2019. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210913Orig1s000TOC.cfm. Accessed November 15, 2020.
- SHIRE. Optometrists vs. ophthalmologists. Dry Eye Diagnosis. 2019. Available from: https://www.xiidra.com/dry-eye-diagnosis/optometrist-vs-ophthalmologist. Accessed November 15, 2020.
- FDA. FDA approves new medication for dry eye disease. Press Announcements. 2016. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-medication-dry-eye-disease. Accessed November 15, 2020.
- Holland EJ, Luchs J, Karpecki PM, et al. Lifitegrast for the treatment of dry eye disease: results of a Phase III, randomized, double-masked, placebo-controlled trial (OPUS-3). Ophthalmology. 2017;124(1):53–60. doi:10.1016/j.ophtha.2016.09.025
- Sheppard JD, Torkildsen GL, Lonsdale JD, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 Phase 3 study. Ophthalmology. 2014;121(2):475–483. doi:10.1016/j.ophtha.2013.09.015
- Tauber J, Karpecki P, Latkany R, et al. Lifitegrast ophthalmic solution 5.0% versus placebo for treatment of dry eye disease: results of the randomized phase III OPUS-2 study. Ophthalmology. 2015;122(12):2423–2431. doi:10.1016/j.ophtha.2015.08.001
- Okumura Y, Inomata T, Iwata N, et al. A review of dry eye questionnaires: measuring patient-reported outcomes and health-related quality of life. Diagnostics. 2020;10(8):559.
- Bron AJEV, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;7:640–650. doi:10.1097/00003226-200310000-00008
- Tong AY, Passi SF, Gupta PK. Clinical outcomes of lifitegrast 5% ophthalmic solution in the treatment of dry eye disease. Eye Contact Lens. 2020;46(Suppl 1):S20–S24. doi:10.1097/ICL.0000000000000601
- White DE, Zhao Y, Ogundele A, et al. Real-world treatment patterns of cyclosporine ophthalmic emulsion and lifitegrast ophthalmic solution among patients with dry eye. Clin Ophthalmol. 2019;13:2285–2292. doi:10.2147/OPTH.S226168
