Abstract
Wet age-related macular degeneration (AMD) is the most common reason for vision loss in the United States. Many treatments, such as laser therapy and photodynamic therapies, have been used but their efficacy is limited. Emerging anti-vascular endothelial growth factor (VEGF) therapies are now considered the standard of care. Anti-VEGF agents inhibit angiogenesis in the eye by suppressing abnormal blood vessel growth, leading to vision improvement. Ranibizumab and bevacizumab are two examples of anti-VEGF drugs that have been approved; both showed promise based on the visual acuity scale. Aflibercept, another new therapy known to trap VEGF and inhibit multiple growth factors, is promising not only because it can be taken bimonthly based on year 1 of the VIEW trials, but it can also be extended, as demonstrated in year 2 of the VIEW trials. Based on a cost–effect analysis, aflibercept is comparable to other leading therapies. This is a review of relevant clinical trials that have proven the non-inferiority and safety of aflibercept compared to the standard of care and its unique role in the current management of wet AMD.
Introduction
Wet age-related macular degeneration (AMD) is the leading cause of vision loss in adults over the age of 50 years in industrialized nations.Citation1 Wet AMD has an increased incidence in females compared to males and is estimated to affect about 9 million people in the United States alone.Citation1,Citation2 As life expectancy increases and the number of senior citizens escalates, the number of people affected by wet AMD will continue to grow.
Treatment options for macular degeneration have drastically improved over the past 15 years. In the 1980s, conventional heat laser therapy was used for wet AMD, but the repercussions (scarring of the eyes, permanent vision loss) outweighed the benefits in the case of subfoveal choroidal neovascular membrane (CNVM).Citation3 In April 2000, the US Food and Drug Administration (FDA) approved the first treatment for wet AMD: photodynamic therapy using verteporfin (Visudyne®; Novartis International AG, Basel, Switzerland) injections.Citation3 This treatment was groundbreaking at the time because of the substantial decrease of vision loss in patients with subfoveal CNVM where heat laser therapy did poorly. In photodynamic therapy, laser treatment activates verteporfin in the eye and terminates abnormal blood vessels.Citation4 About one in six patients showed improved vision and a slowed progression of disease state with this therapy.Citation5,Citation6
Although photodynamic therapy proved to be effective, its use slowly faded because of more effective anti-vascular endothelial growth factor (VEGF) therapies. In wet AMD, VEGF promotes angiogenesis, the growth of abnormal new blood vessels in the eye. Inhibiting VEGF causes a profound reduction in vision loss.Citation7 The first anti-VEGF breakthrough therapy used for wet AMD was pegatanib (Macugen®; OSI Pharmaceuticals, Farmingdale, NY, USA/Pfizer, New York, NY, USA) followed by ranibizumab (Lucentis®; Genentech Inc, South San Francisco, CA, USA; Novartis International AG), which was approved by the FDA in June 2006.Citation7,Citation8 Ranibizumab is a humanized monoclonal antibody fragment that binds to the VEGF.Citation9 Ranibizumab provided greater visual acuity benefits than photodynamic therapy.
Clinical trials have shown that bevacizumab (Avastin®; Genentech Inc; Roche, Basel, Switzerland), another anti-VEGF agent, is comparable in efficacy to ranibizumab, but it has not been approved for the treatment of wet AMD by the FDA as of yet. Bevacizumab showed similar efficacy compared to ranibizumab in the Comparison of AMD Treatment Trials (CATT), in which 60% of patients in both treatment groups gained visual improvements after 1 year of therapy.Citation10 This was an eye opener for physicians because both treatments are used monthly for the treatment of wet AMD, but bevacizumab is a fraction of the cost of ranibizumab, and therefore bevacizumab is prescribed for off-label use for patients who cannot afford ranibizumab. It is preferred by the majority of ophthalmologists as first-line therapy for AMD because it was first to market, has an overall lower cost, and it has a wider spectrum of reimbursable indications.
The most recently approved treatment for wet AMD is aflibercept (Eylea®),Citation11 co-developed by Sanofi-Aventis (Paris, France) and Regeneron Pharmaceuticals (Tarrytown, NY, USA) and approved by the FDA in November 2011. It works by binding tightly to three isoforms of growth factors (VEGF-A, VEGF-B, and placental growth factor).Citation12 This agent has high binding affinity and a long half-life, which makes afibercept lucrative in terms of the possibility of cost savings and decreased frequency of use. This review will compare the effectiveness of aflibercept versus current therapies based on clinical trials for patients with neovascular (wet) AMD.
Methodology
Using databases such as PubMed, Medline, and ClinicalTrials.gov, data were collected to compare the efficacy of aflibercept versus other therapies in the treatment of wet AMD. Data were also collected from online sources such as the National Eye Institute,Citation13 Macular Degeneration Association,Citation2 other drug-dosing information webpages, and peer-review journals in order to give a fuller description of the pharmaceutical agents and their respective strengths.Citation14,Citation15
Wet AMD pathophysiology
To fully understand macular degeneration the key components that are affected need to be defined. The macula is responsible for fine tuning images within one’s visual field, and impairment of this function can lead to ensuing vision loss.Citation16 Macular degeneration can be broken down into two subsets: dry (nonexudative) AMD and wet (exudative) AMD.Citation17 Dry AMD occurs in about 85% of patients diagnosed with macular degeneration.Citation12 The less serious of the two, dry AMD, is defined by drusen, small yellow deposits located near the retina.Citation18 Dry AMD is categorized into stages based on the size of the drusen, the degree of vision loss, and retinal pigment epithelium hyperpigmentation and atrophy.Citation18
Wet AMD accounts for only 15% of all macular degeneration, but causes up to 90% of blindness in individuals diagnosed with AMD.Citation19 Individuals with wet AMD have weak blood vessels underneath the retina and macula, leading to the leakage of fluids and blood into the eye and consequently causing macular damage.Citation2,Citation20 Cytokines and other inflammatory markers such as VEGF cause ischemia and inflammation, which leads to choroidal neovascularization (CNV).Citation2 In CNV, new blood vessels grow into the subretinal pigment epithelium (type 1) or subretinal space (type 2) by breaking through the Bruch membrane, therefore leading to rapid vision loss of central vision and metamorphopsia on Amsler grid testing.Citation16
VEGF and its role in wet AMD
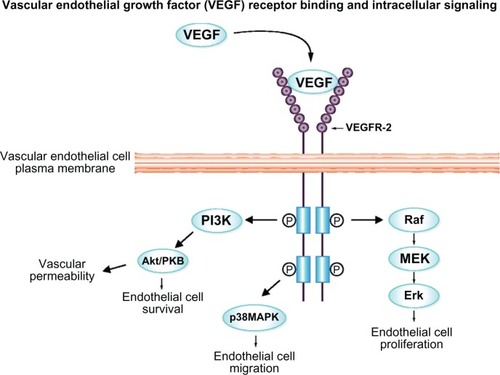
VEGF, which occurs naturally in the body, works by stimulating chemical signals to promote the growth of new blood vessels in areas of the body that are oxygen deficient.Citation21 It does so by binding to a tyrosine kinase receptor, causing activation by phosphorylation (). The VEGF receptor (VEGFR) consists of seven domains, and almost all activation occurs by binding to VEGFR-1 and VEGFR-2 domains.Citation22 Both VEGFR-1 and VEGFR-2 are located in the vascular endothelium, neurosensory retina, and retinal pigment epithelium cells.Citation22 Activation of these receptors is the focal point of angiogenesis and vascular growth where the role of VEGFR-2, but not VEGFR-1, is documented. In other disease states, especially in cancer and tumor growth, overexpression of VEGF can lead to increased blood vessel growth, thus aiding tumor growth by providing an adequate oxygen supply.
Figure 1 VEGF binds to the vEGFR-2 receptor, activating angiogenic response by phosphorylating domains within the receptor and below the endothelial membrane.
Abbreviations: VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Wet AMD is illustrated by the development of CNV. In CNV the innermost layer, the Bruch’s membrane, becomes inflamed and causes thickening of the vascular endothelium.Citation23,Citation24 Irregular growth of blood vessels under the macula supports this thickening and causes rapid central vision loss. Even in advanced stages of wet AMD partial vision is still retained, and severe loss of peripheral vision is uncommon.Citation25 The thickening and addition of new blood vessels leads to inflammation of the macula and eventual scarring of the retina and macula.Citation26 The vessels are undeveloped and cause a leakage of fluids into the eye, leading to inflammation.
CNV has a strong link to the increased expression of the VEGF gene.Citation27 VEGF is often considered the main growth factor leading to the increased angiogenesis within the eye. VEGF maintains this vessel growth, along with other cytokines such as tumor necrosis factor-alpha and a host of interleukins.Citation8 In wet AMD, inflammatory events that cause damage to the eye happen at a much faster rate than in dry AMD. Additionally, oxidative stress, complement factor H gene, and lifestyle factors such as diet and smoking might play a role in the etiology of AMD.Citation28,Citation29
Current management of wet AMD
Wet AMD treatment options continue to expand, with new and more effective agents. Photodynamic therapy is no longer recommended as a monotherapy regimen, but success has been shown in combination with pharmacological treatment.Citation30 Currently, the standard of care for wet AMD is either a monthly dose of bevacizumab or ranibizumab.Citation31 Ranibizumab shows promising results in patients with wet AMD, improving vision in over one-third of the patients tested.Citation32 It has relatively few side effects, such as eye irritation and redness, most of which are due to the injection itself.Citation33 Bevacizumab has shown non-inferior results when compared to ranibizumab in the CATTs.Citation10 Many physicians tend to use this medication off-label due to its cost effectiveness versus ranibizumab.
Aflibercept
Aflibercept, unlike other VEGF inhibitors, is a recombinant protein in which VEGFR-1 (second binding domain) and VEGFR-2 (third binding domain) are attached to the Fc portion of human immunoglobulin G.Citation34 Aflibercept differs from ranibizumab and bevacizumab in the mode of action by targeting VEGF via acting as a dummy receptor for VEGF, thus effectively inhibiting the angiogenic response.Citation35 This targeted response traps VEGF by binding to it even more tightly than its native receptor, triggering no angiogenic action from the VEGF and leaving it inactive.Citation12 Without the cascade of angiogenic responses, fewer leaky vessels grow into the macula. Aflibercept slows the progression of wet AMD, allowing patients to retain central vision for much longer.
VEGF is categorized into VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placenta growth factor (PIGF).Citation14,Citation35 Aflibercept, a fusion protein with binding domains from native VEGF receptors, binds with high affinity to VEGF-A, VEGF-B, PIGF1, and PIGF2.Citation14 Preclinical studies demonstrated that aflibercept suppressed CNV in several animal models.Citation34 The result of Phase III trials in humans showed effective short-term suppression of CNV in patients with exudative AMD and suggested longer durability of aflibercept compared with ranibizumab and bevacizumab.Citation36,Citation37 A pivotal Phase III VEGF trial in patients with wet AMD showed that aflibercept was noninferior to ranibizumab in preventing vision loss with comparable vision gains, safety, and perhaps at lower cost than ranibizumab.Citation35
Pharmacokinetics
Aflibercept has the highest affinity for VEGF165 (Kd 0.49 Pm) compared to ranibizumab (Kd 46 Pm) and bevacizumab (Kd 58 Pm).Citation19,Citation37 Moreover, aflibercept is the only approved therapy for exudative AMD that inhibits VEGF-B and PIGF, giving it further claim to be a promising solution for wet AMD. Using aflibercept can potentially inhibit more angiogenic factors, possibly treating wet AMD more effectively than current therapies.
The most captivating aspect of aflibercept is its extended half-life. According to reviews of aflibercept by StewartCitation8 and Stewart et al,Citation35 the estimated serum half-life of intravitreal aflibercept is approximately 18 days versus ranibizumab and bevacizumab, which have half-lives of approximately 4.75 days and 8.25 days, respectively. Stewart et al estimated intravitreal half-life for aflibercept to be 7.1 days.Citation35 The reason for aflibercept’s unusually long half-life is due to its relatively large molecular weight of 115 Kd, which allows it to circulate within the eye for a longer time.Citation22 Owing to its prolonged half-life, bimonthly dosing is possible and has altered current regimens of monthly dosing for exudative AMD. Dosing in such a way can provide immediate benefits to patients who have a difficult time with intravitreal dosage administration. Also with the extended dosing intervals, patients are less likely to face side effects from the dosage regimen and are less likely to have medication administration errors.
Clinical evidence
Aflibercept gained FDA approval after two randomized, double-blind Phase III trials were conducted: VIEW 1 and VIEW 2 (VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet Age-Related Macular Degeneration).Citation36,Citation37 These studies were done to measure the safety and efficacy of aflibercept compared to the current standard of care for wet AMD, ranibizumab. It is important to note that the VIEW studies were run concurrently in different parts of the world and both studies had the same endpoints, treatment group population, and primary outcome measures. The only difference was that VIEW 1 was conducted in North America, whereas VIEW 2 was conducted internationally. Both trials had a set outcome goal at 52 weeks of treatment and the primary outcome was identified as the percentage of patients who maintained vision at week 52. Maintaining vision was defined as losing less than 15 letters based on the best-corrected visual acuity scale compared to baseline measurements.Citation36 There were four treatment groups into which patients were randomly placed ().Citation36,Citation37
Table 1 Treatment groups in the VIEW 1 and VIEW 2 clinical trials
The results in all four treatment groups yielded similar conclusions. The primary endpoint showed noninferiority in all four treatment groups where noninferiority was defined in comparison to the standard of care ranibizumab and concluding that the three aflibercept groups were noninferior.Citation36,Citation37 The most pertinent data for the use of aflibercept show the success of aflibercept dosed every 8 weeks. In year 2 of the VIEW trials, when dosing was switched to as-needed, aflibercept again proved noninferior to ranibizumab.Citation37 The safety analysis in both VIEW trials found aflibercept to be a well-tolerated drug.Citation38 When compared to ranibizumab, the safety profile was of approximately equivalent measures.Citation36,Citation39 In conclusion, aflibercept dosed intravitreally each month or every 2 months after three initial monthly doses resulted in comparable efficacy and safety to monthly ranibizumab. Side effects, either ocular or systemic, were similar across treatment groups, with no differences between aflibercept administered every 2 months and monthly ranibizumab.Citation38
These studies provided a foundation for using aflibercept therapy in wet AMD. Potential advantages of aflibercept might include extended dosing periods that might lead to fewer hospital and physician visits and less financial cost to the patient due to less frequent dosing.
Cost–effect analysis
The recommended dose for aflibercept is 2 mg every 4 weeks for 3 months, followed by 2 mg every 8 weeks.Citation40 More frequent dosing was not found to be advantageous to patients.Citation41 Ranibizumab is currently dosed at 0.5 mg monthly as per guidelines.Citation9 Although there are only three FDA-approved therapies for wet AMD, it should also be noted that bevacizumab is the most-used therapy for wet AMD because of its relatively low cost and comparable efficacy to ranibizumab. Bevacizumab is most often dosed at 1.25–2.5 mg per monthly treatment for macular degeneration.Citation42 All three therapies are considered to have equal efficacy across the board based on studies and clinical data that have been evaluated.Citation31
For one treatment of ranibizumab, the cost of medication totals USD1950 per vial/dose.Citation43 If a patient is on ranibizumab for 1 year, the cost of therapy is USD23,400 (12 doses).Citation44 For aflibercept, due to the less intensive dosing regimen, clear savings can be seen. A single dose/treatment of aflibercept costs approximately USD1850 and the yearly cost averages USD14,800 (eight doses). Bevacizumab is the cheapest, costing roughly USD50 per dose/treatment. A yearly treatment of one bevacizumab dose per month would cost about USD600.Citation45,Citation46 Not included in these cost analyses is the cost of treatment (physician visits, optical coherence tomography costs, and injection costs), which varies from center to center and is not readily available.
Other indications for aflibercept
Experiments to use intravitreal aflibercept for the treatment of diabetic macular edema have shown promising results in clinical trials.Citation47–Citation49 The results of a Phase II clinical trial showed that anti-VEGF therapies improved vision compared to the standard of care for diabetic macular edema, which is photocoagulation therapy.
Conclusion
It is obvious that aflibercept has a role in macular degeneration therapy, but its use is currently limited. The major limiting factor is the cost of the therapy and administration. With bevacizumab already being the most-used anti-VEGF drug for macular degeneration, even though it is being used off-label, its use is not anticipated to subside. The CATT research group found the monthly use of either bevacizumab or ranibizumab results in the same visual acuity outcome.Citation10 Such comparisons have raised questions about which drug to choose. Physicians tend to prescribe bevacizumab due to its extremely low cost compared to aflibercept and ranibizumab.
Aflibercept is an intriguing choice for patients who have a difficult time with the intravitreal injections. After the initial three loading doses, bimonthly dosing can be beneficial for noncompliant patients. Compared to current treatments, aflibercept has shown equal efficacy and safety. Its unique pharmacokinetics and binding to multiple receptors may allow it to expand its role in other disease states as well.
Disclosure
The authors report no conflicts of interest in this work.
References
- HaddrillMMacular degeneration treatment [webpage on the Internet]San Diego, CAAccess Media Group LLC2011 [updated Nov 2011; cited Oct 2012]. Available from: http://www.allaboutvision.com/conditions/amd-treatments.htmAccessed October 23, 2012
- About macular degeneration [webpage on the Internet]Sarasota, FLMacular Degeneration Association2011 [cited October 26, 2012]. Available from: http://www.maculardegenerationassociation.org/about-md/Accessed February 25, 2013
- MedlinePlus: Verteporfin injection [webpage on the Internet]Bethesda, MDUS National Library of Medicine [updated September 1, 2010]. Available from: http://www.nlm.nih.gov/medlineplus/druginfo/meds/a607060.htmlAccessed February 25, 2013
- AhnJCChungPSThe activity of G-ROS and the predominant role of Type II reaction in the photodynamic therapy using 9-hydroxypheophorbide-alpha for HeLa cell linesGen Physiol Bio-phys2012313343350
- YooJJKimCChungCWJeongYIKangDH5-aminolevulinic acid-incorporated poly(vinyl alcohol) nanofiber-coated metal stent for application in photodynamic therapyInt J Nanomedicine201271997200522619537
- Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 2. Early Treatment Diabetic Retinopathy Study Research GroupOphthalmology19879477617743658348
- ChenYHanFProfile of ranibizumab: efficacy and safety for the treatment of wet age-related macular degenerationTher Clin Risk Manag2012834335122911433
- StewartMWClinical and differential utility of VEGF inhibitors in wet age-related macular degeneration: focus on afliberceptClin Ophthalmol201261175118622973088
- ChenEBrownDMWongTPLucentis using Visudyne study: determining the threshold-dose fluence of verteporfin photodynamic therapy combined with intravitreal ranibizumab for exudative macular degenerationClin Ophthalmol201041073107920957143
- CATT Research GroupMartinDFMaguireMGRanibizumab and bevacizumab for neovascular age-related macular degenerationN Engl J Med2011364201897190821526923
- Aflibercept (Eylea) for age-related macular degeneration [webpage on the Internet]New Rochelle, NYThe Medical Letter, Inc2012 [updated February 6, 2012]. Available from: http://secure.medicalletter.org/TML-article-1383aAccessed November 13, 2012
- BrowningDJKaiserPKRosenfeldPJStewartMWAflibercept for age-related macular degeneration: a game-changer or quiet addition?Am J Ophthalmol2012154222222622813448
- NEI: National Eye Institute [homepage on the Internet]Bethesda, MDNational Institutes of Health [updated Sep 2009]. Available from: http://www.nei.nih.gov/Accessed November 13, 2012
- StewartMWThe expanding role of vascular endothelial growth factor inhibitors in ophthalmologyMayo Clin Proc2012871778822212972
- ChongVBiological, preclinical and clinical characteristics of inhibitors of vascular endothelial growth factorsOphthalmologica2012227Suppl 121022517120
- Facts about age-related macular degeneration [webpage on the Internet]Bethesda, MDNational Institutes of Health2009 [updated Sep 2009]. Available from: http://www.nei.nih.gov/health/maculardegen/armd_facts.aspAccessed November 13, 2012
- CampochiaroPAAnti-vascular endothelial growth factor treatment for retinal vein occlusionsOphthalmologica2012227Suppl 1303522517123
- CostagliolaCAgnifiliLArcidiaconoBDuseSFasanellaVMastropasquaRSystemic thromboembolic adverse events in patients treated with intravitreal anti-VEGF drugs for neovascular age-related macular degenerationExpert Opin Biol Ther201212101299131322866908
- ChristoforidisJBWilliamsMMKothandaramanSKumarKEpitropoulosFJKnoppMVPharmacokinetic properties of intravitreal I-124-aflibercept in a rabbit model using PET/CTCurr Eye Res201237121171117422991959
- GolikPTońskaKComparison of the biological principles underlying the action of monoclonal antibody (mAb) and decoy receptor anti-VEGF agents – on the example of ranibizumab (anti-VEGF-A mAb) and aflibercept (decoy VEGFR1-2 receptor)Klin Oczna201211417983 Polish22783753
- PapadopoulosNMartinJRuanQBinding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumabAngiogenesis201215217118522302382
- Verner-ColeEADavisSJLauerAKAflibercept for the treatment of neovascular age-related macular degenerationDrugs Today (Barc)201248531732922645720
- VerittiDSaraoVLanzettaPNeovascular age-related macular degenerationOphthalmologica2012227Suppl 1112022517121
- OhrMKaiserPKIntravitreal aflibercept injection for neovascular (wet) age-related macular degenerationExpert Opin Pharmacother201213458559122300011
- NorkTMDubielzigRRChristianBJPrevention of experimental choroidal neovascularization and resolution of active lesions by VEGF trap in nonhuman primatesArch Ophthalmol201112981042105221825187
- IdaHTobeTNambuHMatsumuraMUyamaMCampochiaroPARPE cells modulate subretinal neovascularization, but do not cause regression in mice with sustained expression of VEGFInvest Ophthalmol Vis Sci200344125430543714638748
- NagineniCNKommineniVKWilliamADetrickBHooksJJRegulation of VEGF expression in human retinal cells by cytokines: implications for the role of inflammation in age-related macular degenerationJ Cell Physiol2012227111612621374591
- JarrettSGBoultonMEConsequences of oxidative stress in age-related macular degenerationMol Aspects Med201233439941722510306
- SparrowJRUedaKZhouJComplement dysregulation in AMD: RPE-Bruch’s membrane-choroidMol Aspects Med201233443644522504022
- CouchSMBakriSJReview of combination therapies for neovascular age-related macular degenerationSemin Ophthalmol201126311412021609223
- KovachJLSchwartzSGFlynnHWJrScottIUAnti-VEGF treatment strategies for wet AMDJ Ophthalmol2012201278687022523653
- MeyerCHHolzFGPreclinical aspects of anti-VEGF agents for the treatment of wet AMD: ranibizumab and bevacizumabEye (Lond)201125666167221455242
- FigurskaMRobaszkiewiczJWierzbowskaJSafety of ranibizumab therapy in wet AMD and the role of vascular endothelial growth factors in physiological angiogenesisKlin Oczna20101124–614715020825071
- TraynorKAflibercept approved for macular degenerationAm J Health Syst Pharm20126916
- StewartMWGripponSKirkpatrickPAfliberceptNat Rev Drug Discov201211426927022460118
- Regeneron Pharmaceuticals Vascular Endothelial Growth Factor (VEGF) Trap-Eye: Investigation of Efficacy and Safety in Wet Age-Related Macular Degeneration (AMD) (VIEW 1)ClinicalTrials gov [website on the Internet]Bethesda, MDUS National Library of Medicine2007 [updated December 20, 2012]. Available from: http://clinicaltrials.gov/ct2/show/NCT00509795NLM identifer: NCT00509795. Accessed November 13, 2012
- Bayer Vascular Endothelial Growth Factor (VEGF) Trap-Eye: Investigation of Efficacy and Safety in Wet Age-Related Macular Degeneration (AMD) (VIEW 2)ClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2008 [updated February 27, 2012]. Available from: http://clinicaltrials.gov/ct2/show/NCT00637377NLM identifer: NCT00637377. Accessed November 13, 2012
- HeierJSBrownDMChongVVIEW 1 and VIEW 2 Study GroupsIntravitreal aflibercept (VEGF Trap-Eye) in wet age-related macular degenerationOphthalmology2012119122537254823084240
- FramptonJEAflibercept for intravitreal injection: in neovascular age-related macular degenerationDrugs Aging2012291083984623038609
- NguyenQDCampochiaroPAShahSMClear-It 1 InvestigatorsEvaluation of very high- and very low-dose intravitreal aflibercept in patients with neovascular age-related macular degenerationJ Ocul Pharmacol Ther201228658158822775078
- StewartMWRosenfeldPJPenhaFMPharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor Trap-eye)Retina201232343445722374154
- CostaRAJorgeRCalucciDCardilloJAMeloLAJrScottIUIntravitreal bevacizumab for choroidal neovascularization caused by AMD (IbeNA Study): results of a phase 1 dose-escalation studyInvest Ophthalmol Vis Sci200647104569457817003454
- JacksonTLKirkpatrickLCost comparison of ranibizumab and bevacizumabBMJ2011343d505821862536
- HurleySFMatthewsJPGuymerRHCost-effectiveness of ranibizumab for neovascular age-related macular degenerationCost Eff Resour Alloc200861218573218
- MitchellPAnnemansLGallagherMCost-effectiveness of ranibizumab in treatment of diabetic macular oedema (DME) causing visual impairment: evidence from the RESTORE trialBr J Ophthalmol201296568869322399690
- RafteryJCleggAJonesJTanSCLoteryARanibizumab (Lucentis) versus bevacizumab (Avastin): modelling cost effectivenessBr J Ophthalmol20079191244124617431015
- LangGEDiabetic macular edemaOphthalmologica2012227Suppl 1212922517122
- BandelloFBerchicciLLa SpinaCBattaglia ParodiMIaconoPEvidence for anti-VEGF treatment of diabetic macular edemaOphthalmic Res201248Suppl 1162022907145
- Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Study of Intravitreal Aflibercept, Bevacizumab, and Ranibizumab for DME (Protocol T)ClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2012 [updated August 21, 2012]. Available from: http://clinicaltrials.gov/ct2/show/NCT01627249NLM identifer: NCT01627249. Accessed November 14, 2012
- RiniBISmallEJBiology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinomaJ Clin Oncol20052351028104315534359