Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Purpose
The purpose of this study was to evaluate the long-term safety, anatomical, and visual outcomes following intravitreal bevacizumab (Avastin; Genentech) on macular edema (ME) secondary to branch retinal vein occlusion (BRVO).
Methods
A prospective, interventional case series study was conducted among patients with ME due to BRVO, from June 2008 to October 2011. Intravitreal bevacizumab (1.25 mg/0.05 mL) was given at 4–6 weekly intervals until the ME subsided, and cases were followed up for a year. Complete ophthalmic evaluations and measurement of central retinal thickness (CRT) by optical coherence tomography were performed at baseline and follow-up visits.
Results
Sixty-three eyes of 63 patients were included in the study. The mean age was 58.22 years (standard deviation [SD], 12.3). The CRT at baseline was 515.3 ± 189.4 μm, and it significantly improved at each follow-up, with a CRT of 233.6 ± 101.5 μm at 12 months. The best-corrected visual acuity (BCVA) at baseline was 0.82 ± 0.54, and it significantly improved at each follow-up, with a BCVA of 0.40 ± 0.25 at 12 months (P < 0.001). The BCVA was better in 76% of the patients with a more than three-line increase in 55.5% of the eyes. The average number of intravitreal bevacizumab injections was 3.1 (range, 1–6 injections). Recurrent ME occurred in 30.2% of cases. There were no major ocular or systemic adverse events.
Conclusion
Intravitreal bevacizumab appears to be a safe and effective drug for reducing ME and improving visual acuity secondary to BRVO at 12-month follow-up at a tertiary referral eye hospital in Nepal.
Introduction
Retinal vein occlusion (RVO) is a common retinal vascular problem that is second in incidence only to diabetic retinopathy. Many risk factors have been associated with RVO, including age, hypertension, diabetes mellitus, atherosclerotic retinal vascular change, open angle glaucoma, and hypermetropia.Citation1–Citation3 Branch retinal vein occlusion (BRVO) is a type of RVO with potential sight-threatening complications. Macular edema (ME) is often the cause of visual problems in BRVO, and the most common complication of BRVO is the development of cystoid macular edema with a consecutive deterioration in vision. Macular grid laser is a proven treatment modality for reducing ME related to BRVO, but the Branch Vein Occlusion Study has shown a significant visual benefit only in persons with visual acuity of 20/40 or less, compared with the untreated control group.Citation4 Several studies have shown positive results with intravitreal steroids in reducing ME and improving vision in patients with BRVO, but its use has been limited due to side effects, such as cataract formation and increased intraocular pressure.Citation5,Citation6
Vascular endothelial growth factor (VEGF) is a cytokine produced by the hypoxic retina that increases vascular permeability, which leads to ME. VEGF also stimulates endothelial cell hypertrophy, which reduces the capillary lumen and causes more ischemia, thus perpetuating the edema.Citation7 Anti-VEGF treatment could break this cycle and facilitate resolution of ME. Bevacizumab (Avastin; Genentech Inc, San Francisco, CA) is a monoclonal antibody that inhibits all isoforms of VEGF. Its use for RVO was first reported by Rosenfeld in 2005.Citation8 Since then, many case series have been published with better anatomical and visual success in reducing ME secondary to RVO, but they have been limited to developed countries. This prospective clinical study was designed to assess the long-term safety and efficacy of intravitreal bevacizumab and grid laser in selected cases of ME secondary to BRVO at a hospital in Nepal, a developing country with very limited resources.
Materials and methods
This prospective, interventional, nonrandomized, case series study was conducted at Tilganga Institute of Ophthalmology, a tertiary eye care center in Nepal. Consecutive cases of BRVO with ME, central retinal thickness (CRT) > 249 μm, and visual acuity worse than 6/12, who could come for regular follow-up visits were invited to participate in the study. The study was conducted from July 2008 until September 2011. Patients excluded from the study were those with a history of treatment with laser therapy or intravitreal injections, age-related macular degeneration, diabetic retinopathy, macular scar, pre-existing glaucoma, or neovascular glaucoma secondary to RVO. Patients with uncontrolled hypertension, diabetes mellitus, myocardial infarction, or cerebrovascular accident within three months of presentation also were excluded. Informed consent was obtained from the patients before enrollment in the study, after fully explaining the possible risks and benefits of intravitreal bevacizumab. Ethical approval was obtained from the Institutional Review Board of the Institute, and the study was conducted according to the tenets of the Declaration of Helsinki.
A detailed history was taken to ascertain each patient’s demographics and chief complaints, including duration of the problem and presence of systemic diseases, such as hypertension, diabetes mellitus, cardiac diseases, and hyperlipidemia.
Ocular evaluation included presenting and best-corrected visual acuity (BCVA) on a Snellen chart and anterior and posterior segment examinations using a Haag–Streit slit-lamp and 90 D lens, as well as an indirect ophthalmoscope and a 20 D lens. Color fundus photography documented the affected regions of the retina. Fundus fluorescein angiography was not performed routinely in our series.
Central retinal thickness was assessed objectively with optical coherence tomography (Stratus OCT; Carl Ziess Meditec, Dublin, CA) at baseline and at every follow-up visit, at 4–6 weeks intervals, until the ME subsided, and then every 2–3 months until 12 months of follow-up were completed. Intraocular pressure was taken by Goldman applanation tonometry. Systemic blood pressure was measured at baseline and at each follow-up visit. Fasting and postprandial blood sugar and lipid panels were recommended in all cases to search for underlying systemic risk factors and to assess the level of metabolic control before intravitreal injection. Likewise, patients were advised to consult their physician and/or cardiologist for evaluation and control of systemic diseases.
The intravitreal bevacizumab was injected in a dose of 1.25 mg/0.05 mL through the pars plana with a 27 G or 30 G needle, at baseline and repeated at 4–6-week intervals, until the ME subsided. The intravitreal injections were administered aseptically in the operating theater. Afterwards, patients used a topical antibiotic (ciprofloxacin) four times a day and ciprofloxacin ointment at bedtime for a week. Detailed ophthalmic evaluation was performed at each follow-up including visual acuity, anterior and posterior segment evaluation, and assessment of ME. Recurrent ME was defined as any increase in central macular thickness (CMT) relative to the previous follow-up values. Retreatment with intravitreal bevacizumab was indicated in recurrent cases whenever the CMT increased 100 μm or more, with or without vision deterioration of at least one line (five ETDRS letters). In such cases, if there was a presence of exudates, grid laser was also used along with the intravitreal bevacizumab. Recurrent ME cases with CMT less than 100 μm from the previous follow-up value were treated with grid laser only, but only observed such cases if there were no exudates. Visual acuity was converted to LogMAR for visual outcome analysis. The data was analyzed in SPSS software (version 11.5; SPSS Inc, Chicago, IL). Paired t-tests were used for statistical analysis. P values less than 0.05 were considered statistically significant in this study.
Results
A total of 63 eyes of 63 patients were included in the study. The mean age was 58.22 years (12.39 SD), with a range of 28–79 years. Males outnumbered females, comprising 57% and 43% of the population, respectively. The mean duration of symptoms prior to presentation was 2.8 months (1.8 SD), with a range of two weeks to six months. The average number of intravitreal bevacizumab injections was 3.1 (1 SD), with a range of 1–6 (). Forty-one eyes had superotemporal BRVO, 19 eyes had inferotemporal BRVO, and three eyes had macular BRVO. Concurrent systemic hypertension was found in 31 cases (48%), and nine patients (14.2%) had diabetes mellitus.
Table 1 General characteristics of patients
Follow-up outcomes
Visual acuity
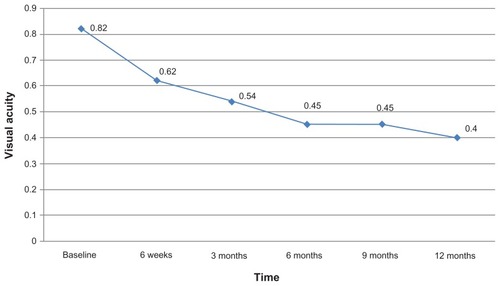
The mean BCVA at baseline was 0.82 ± 0.54. After intravitreal bevacizumab, the mean BCVA was 0.62 ± 0.37 at 6 weeks, 0.54 ± 0.33 at 3 months, 0.45 ± 0.27 at 6 months, 0.45 ± 0.25 at 9 months, and 0.40 ± 0.25 at 12 months. The improvement in BCVA was statistically significant at each follow-up (P < 0.001) compared to baseline values ().
BCVA improved in 76% of the eyes, and more than three lines of improvement were seen in 55.5%. BCVA was the same in 21% of the eyes, and it deteriorated in 3% at the 12-month follow-up.
Subgroup analysis on visual outcome
Further analysis was conducted to assess visual outcome at 12 months compared to the baseline values. The improvement in visual acuity was statistically significant in all cases, regardless of age at presentation being 59 years or less (P < 0.001) or more than 59 years (P < 0.001), whether there was concurrent systemic hypertension (P < 0.001), absence of hypertension (P = 0.01), duration of chief complain of less than 3 months (P < 0.001) or equal to or greater than 3 months (P < 0.001), grid laser (P < 0.004) or without grid laser (P < 0.001), and BCVA less than 1 (P < 0.001) or greater or equal to 1 (P < 0.001) ().
Table 2 Visual outcome analysis at 12 months
Central retinal thickness
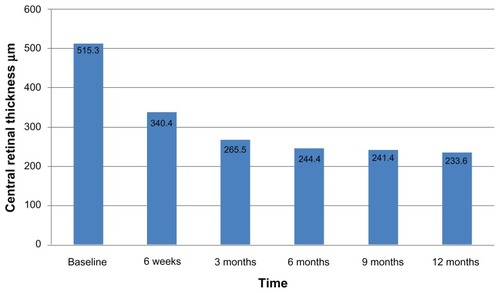
The mean CRT at baseline was 515.3 ± 189.4 μm. The mean CRT was 340.4 ± 135.6 μm at 6 weeks, 265.8 ± 123.9 μm at 3 months, 244.4 ± 126.7 μm at 6 months, 241.2 ± 109.3 μm at 9 months, and 233.6 ± 101.5 at 12 months follow-up. A statistically significant reduction in CRT was observed at each follow-up visit (P < 0.001) relative to baseline values ().
Recurrent macular edema
Recurrent ME was found in 19 cases (30.2%) within the 12-month period. The rate of recurrent edema was not statistically different among the patients with presenting duration of greater than or less than 3 months (P = 0.979), presence or absence of concurrent systemic hypertension (P = 0.893), age less than or more than 59 years (P = 0.601), or BCVA at presentation >1 or less than or equal to 1 LogMAR (P = 0.44).
Including the 12-month follow-up, a total of 19 cases were treated with grid laser. In two cases, grid laser was administered twice. The grid laser was administered in one case at 2 months, in three cases at 3 months, in five cases at 4 months, in two cases at 5 months, in two cases at 6 months, in one case each at 7, 8, 9, and 10 months, and in two cases at 12 months.
Recurrent ME was found in 10.52% of cases at 3 months follow-up, 42.10% at 6 months follow-up, 26.3% at 9 months follow-up and 21.05% at 12 months follow-up. In one case, there was recurrent ME at 6 and 9 months follow-up, and in one other case there was recurrent edema at 4, 9, and 12 months follow-up. Persistent ME was present in two cases. Among the cases with recurrent ME, the repeat intravitreal bevacizumab was given in 6.4%, combined intravitreal bevacizumab and grid laser in 4.9%, grid laser only in 11%, and was observed without intervention in 7.9% of cases.
Safety
In one case, the patient developed severe intraocular inflammation (uveitis) that was resolved with a topical steroid and cycloplegics. There were no other major ocular or systemic problems, such as increased intraocular pressure, endophthalmitis, retinal detachment, or thromboembolic events during the 12 months of follow-up.
Discussion
In our series of 63 BRVO eyes treated with intravitreal bevacizumab, ME and visual acuity significantly improved at the 12-month follow-up without any major ocular or systemic adverse effects. To our knowledge, this is one of the largest case series of BRVO patients that assesses the safety and effectiveness of intravitreal bevacizumab.
The mean age of patients (58.22 years) and male predominance were consistent with the previous study on the demographic profile of RVO.Citation1 The mean age was slightly higher than the series by Demir et alCitation11 but was lower than the other published series.Citation9–Citation12 The male predominance in our case series could be due to the privilege of males in Nepalese society, which ensures better access to health facilities.
The average number of intravitreal bevacizumab in our series was 3.1, ranging from one to six injections. The average number needed was less in our series than in the other reported series at 12 months,Citation10,Citation11 but it was similar to the series by Ahmadi et al.Citation12 ME, as assessed by CRT measurements, was significantly improved at each follow-up visit compared to baseline values. Our findings were consistent with other reported short- and long-term series.Citation9–Citation16 Nineteen cases (30.2%) developed recurrent ME during a 1-year period, with slightly higher recurrence before 6 months. The addition of grid laser to recurrent cases with plenty of exudates may decrease recurrence after 6 months, but it needs further comparative study. Unlike other studies, we were not able to follow the patients at monthly intervals after the resolution of ME, which means we could have missed transient ME that may have subsided by the time of the next follow-up. In our series, there were no significant differences in the rate of recurrent ME among the cases with or without hypertension, duration of complaints longer or shorter than 3 months, age of the patient above or below 59 years, or BCVA less than 1 or greater than or equal to 1 LogMAR. Like the reduction in central macular thickness, BCVA also improved significantly in all follow-up visits, including the 12-month follow-up (P < 0.005). This finding in our series was consistent with other reported series.Citation9–Citation16
BCVA improved in more than four-fifths of the cases, with more than three lines of improvement in 55% of patients at the 12-month follow-up. In our series, BCVA improved significantly in all cases, without any differences among the groups regarding concurrent systemic hypertension, duration of the presentation (greater than or less than three months), grid laser or without grid laser, age group more than or less than 59 years, or presenting BCVA less than <1 or ≥1 LogMAR. Our observation contradicts the findings of Ahmadi et al,Citation12 who found that visual outcome was better among those without concurrent hypertension, in younger age groups, and with better presenting visual acuity.
As reported in other series,Citation9–Citation16 we did not observe any major ocular or systemic problems after intravitreal bevacizumab, such as endophthalmitis, cataract, glaucoma, retinal detachment, or thromboembolic events, except for one case of uveitis that was completely resolved with topical steroids and cycloplegics, with good visual recovery. Although intravitreal injections are routinely administered on an outpatient basis in developed countries, all of the intravitreal injections in our series were administered in the operating theater, using all appropriate aseptic precautions. Our safety results were consistent with other series from developed countries, so we cannot comment on intraocular infections as an outpatient procedure risk in developing countries such as Nepal.Citation9–Citation16
The other limitation was that, except for a few cases, fundus fluorescein angiography was not performed in our series at baseline or at follow-up visits to assess the change in capillary nonperfusion areas. Although this study confirms the effectiveness and safety of intravitreal bevacizumab as reported in other countries, it also provides guidelines for treating ME secondary to BRVO in countries like Nepal, which have limited resources.
Intravitreal bevacizumab is an effective and safe drug for reducing ME and improving visual acuityCitation9–Citation16 compared to intravitreal triamcinolone, which is more likely to raise intraocular pressure and enhance cataract formation,Citation5,Citation6 and relative to grid laser alone, which has limited potential for visual recovery.Citation4 However, the main drawback of intravitreal bevacizumab is its short duration of action and recurrent ME.Citation9–Citation16
We recommend further long-term, randomized, case control studies of intravitreal bevacizumab, with or without addition of grid laser, for the role of grid laser in reducing recurrent ME and for long-term safety and effectiveness of intravitreal bevacizumab in the future.
Conclusion
Intravitreal bevacizumab appears to be an effective and safe drug for reducing ME and improving visual acuity secondary to BRVO during 12 months of follow-up at a hospital setting in a developing country, such as Nepal.
Acknowledgement
The authors are grateful to Professor Dr Paul S Bernstein from the University of Utah, for final editing of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- ThapaRPaudyalGBernsteinPSDemographic characteristics, patterns, and risk factors for retinal vein occlusion in Nepal: a hospital-based case-control studyClinic Experiment Ophthalmol2010386583590
- LimLLCheungNWangJJPrevalence and risk factors of retinal vein occlusion in an Asian populationBr J Ophthalmol200892101316131918684751
- KleinRKleinBEMossSEMeuerSMThe epidemiology of retinal vein occlusion: The Beaver Dam Eye StudyTrans Am Ophthalmol Soc20099813314111190017
- The Branch Vein Occlusion Study GroupArgon laser photocoagulation for macular edema in branch vein occlusionAm J Ophthalmol19849832712826383055
- HouJTaoYJiangYRLiXXGaoLIntravitreal bevacizumab versus triamcinolone acetonide for macular edema due to branch retinal vein occlusion: a matched studyChin Med J (Engl)2009122222695269919951598
- IpMSGottliebJLKahanaAIntravitreal triamcinolone for the treatment of macular edema associated with central retinal vein occlusionArch Ophthalmol200412281131113615302652
- NomaHMinamotoAFunatsuHIntravitreal levels of vascular endothelial growth factor and interleukin-6 are correlated with macular edema in branch retinal vein occlusionGraefes Arch Clin Exp Ophthalmol2006244330931516133018
- RosenfeldPHFungAEPuliafitoCAOptical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for macular edema from central vein occlusionOphthalmic Surg Lasers Imaging200536433633916156153
- RabenaMDPieramiciDJCastellarinAANasirMAAveryRLIntravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to branch retinal vein occlusionRetina200727441942517420692
- PragerFMichelsSKriechbaumKIntravitreal bevacizumab (Avastin) for macular oedema secondary to retinal vein occlusion: 12-month results of a prospective clinical trialBr J Ophthalmol200993445245619074916
- DemirMObaEGulkilikGOdabasiMOzdalEIntravitreal bevacizumab for macular edema due to branch retinal vein occlusion: 12-month resultsClin Ophthalmol2011574574921750607
- AhmadiAAChuoJYBanashkevichAMaPEMaberleyDAThe effects of intravitreal bevacizumab on patients with macular edema secondary to branch retinal vein occlusionCan J Ophthalmol200944215415919491948
- GutièrrezJCBarquetLACaminalJMIntravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to retinal vein occlusionClin Ophthalmol20082478779119668432
- AbeggMTappeinerCWolf-SchnurrbuschUBarthelmesDWolfSFleischhauerJTreatment of branch retinal vein occlusion induced macular edema with bevacizumabBMC Ophthalmol200881818823536
- FigueroaMSContrerasINovalCSArruabarrenaCResults of bevacizumab as the primary treatment for retinal vein occlusionsBr J Ophthalmol20109481052105620679089
- KriechbaumKMichelsSPragerFIntravitreal Avastin for macular oedema secondary to retinal vein occlusion: a prospective studyBr J Ophthalmol200892451852218211942