Abstract
Obstructive sleep apnea (OSA) is characterized by frequent episodes of partial or complete obstruction of the airway during sleep causing repeated episodes of apnea. OSA is more prevalent in middle-aged and older adults. OSA is associated with numerous ocular manifestations, including retinal manifestations. Literature highlighted the clear association between OSA and numerous ocular conditions including glaucoma and papilledema. This comprehensive and narrative review aims to summarize up-to-date clinical research concerning the association of OSA and vascular conditions that affect the retina. OSA is associated with the central serous chorioretinopathy (CSC), retinal vein occlusion (RVO), hypertensive retinopathy (HTRP) and development of diabetic retinopathy (DR). Sympathetic activation, hypoxia, and hormonal dysregulation all lead to serious retinal vascular conditions that will worsen OSA patients’ quality of life. It is important to refer patients with newly diagnosed OSA to an ophthalmology clinic for the appropriate test.
Introduction
What’s Obstructive Sleep Apnea?
Obstructive sleep apnea (OSA) is characterized by repeated episodes of partial and complete obstruction of the airway during sleep, resulting in recurrent apnea and hypopnea.Citation1,Citation2 As a result of poor quality of sleeping, OSA associated with excessive daytime sleepiness occurred in 6% of men and 4% of women with OSA.Citation1 According to Franklin KA, Lindberg E in their epidemiological study of OSA, eleven published studies estimated that the prevalence of obstructive sleep apnea to be 22% in men (range, 9–37%) and 17% (range, 4–50%) in women.Citation1
Systemic Response to OSA
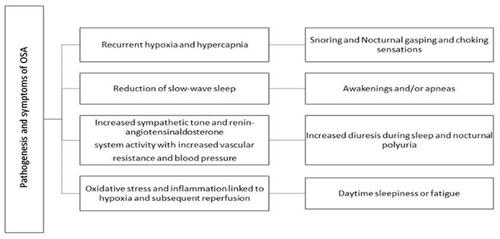
OSA results in hypoxemia and hypercapnia, which in turn lead to enhanced sympathetic vasoconstrictor activity and systemic hypertension. It is associated with a number of cardiovascular complications, such as myocardial ischemia, arrhythmias, and congestive heart failure,Citation3 as well as other life-threatening incidents, such as motor vehicle crashes ().Citation4
Severity of OSA
OSA severity is estimated with apnea–hypopnea index (AHI). AHI which measures the number of apnea or hypopnea per hour during sleep. OSA is defined when the AHI is ≥5. OSA considered to be severe if AHI <30 ().Citation1
Table 1 Severity of OSA According to AHI (Apnea Hypo Apnea Index)
Risk Factors of OSA
The prevalence increases with age, as well as increased body mass index, waist-to-hip ratio, and neck circumference. Moreover, other associations play an important role in the development of sleep apnea, examples of which include abnormalities in mandibular or maxillary size and position, narrow nasal cavities, and enlarged tonsils. Additionally, the increased risk of OSA amongst families with OSA patients suggests a genetic disposition to the disorder.Citation5 Moderate to severe forms of OSA (AHI ≥15) are prevalent amongst men and women aged between thirty- and 70-year old at percentages of 13% and 6%, respectively.Citation6
OSA and Ocular Disorders
OSA is associated with numerous ocular disorders, including floppy eyelid syndrome (FES),Citation7 non-arteritic anterior ischemic optic neuropathy,Citation7 glaucoma,Citation8,Citation9 papilledema,Citation10 central serous chorioretinopathy CSC,Citation11 and central retinal vein occlusions (CRVOs).Citation12 Owing to its high oxygen consumption, the retina is one of the most metabolically active tissues in the human body. Therefore, the retina often manifests changes secondary to hypoxic disorders like OSA earlier than other ocular manifestations in the disease course.Citation13
Retinal vascular disorders represent a substantial economic burden to healthcare systems. In 2019, Moshfeghi expected diabetic retinopathy alone to cost the US healthcare system $4.5 billion in 2020.Citation14 It is of paramount importance to recognize that the association between sight-threatening retinal disorders and OSA is beneficial not only for the decrease of economic burdens but also for the preservation of the patients’ visual function as this would in turn improve OSA patients’ quality of life.
Review Purpose
This following review is directed at ophthalmologists, and its purpose is to discuss the proposed mechanism involved in the pathogenesis of retinal vascular manifestation associations and OSA. To find the relevant papers, a search was performed on PubMed and Google Scholar. The keywords were as follows: sleep apnea, obstructive sleep apnea, apnea–hypopnea neuropathy, central retinal vein occlusion, diabetic retinopathy, hypertensive retinopathy and central serous chorioretinopathy.
Diabetic Retinopathy and OSA
Diabetes mellitus is recognized as a serious public health concern with a negative major impact on human life and healthcare systems. Globally, 1 in 11 adults has diabetes mellitus (DM) (90% is having type 2 diabetes mellitus).Citation15 Moreover, around one-third of patients with diabetes mellitus are estimated to have diabetic retinopathy, with one-third of those having severe non-proliferative diabetic retinopathy (NPDR).Citation16 A significant association between diabetic retinopathy and OSA has been noted in the literature.
Two-Way Relationship
Literature describes the relationship between OSA and DM as two-way, as OSA may increase the risk of developing diabetes and diabetes is a risk factor for developing OSA. A study of 360,250 patients has found that the ratio of the incidence of OSA in patients with type 2 DM compared with the incidence of OSA in nondiabetics is 1.48 (P < 0.001).Citation17 At the same time, in their meta-analysis of OSA and DR, Zhu et al find that having OSA is significantly associated with an increased risk of DR.Citation18
Apnea–Hypopnea Index as an Indicator of DR Progression
The severity of OSA is classified according to the Apnea–Hypopnea Index (AHI) value, with an AHI value of 5–15 considered mild, an AHI value of 15–30 considered moderate and an AHI value ≥30 considered severe .Citation19 Studies demonstrate that the increase in the number of apneic and hypoxic events per sleep hour is positively correlated with the progression of non-proliferative DR into proliferative DR.Citation20 However, the AHI is not the only indicator of DR progression in OSA; a drop in the mean SpO2 of below 90% has also been observed to be positively correlated with development of diabetic macular edema.Citation20
Pathogenesis of DR and OSA
It is hypothesized that dysregulation of circadian locomotor output cycles kaput (CLOCK) genes is directly related to the pathogenesis of DR in patients with type 2 DM.Citation21 CLOCK genes play a vital role in the coordination of circadian metabolism and homeostasis. A potential mechanism underlying increased rates of neovascularization in OSA is an increase in the retinal vascular endothelial growth factor expression as a result of hypoxia-induced upregulation of the CLOCK genes.Citation21
Continuous Positive Airway Pressure Stops the Progression DR in OSA
Continuous positive airway pressure (CPAP) is usually indicated in moderate and severe OSA. It has been observed that CPAP improves sleep quality and reduces daytime sleepiness and fatigue along with decreasing mortality and morbidities associated with OSA.Citation19 Studies demonstrate that OSA patients treated with CPAP early have a significantly lower prevalence of retinopathy compared to those who were not treated (OR 0.54, P=0.04).Citation22 Although early treatment with CPAP improves the anatomical outcomes in patients with OSA, retinal nerve fiber layer thickness, and macular thickness, improvement in visual functions of the eye is not observed.Citation23 This indicates that the role of CPAP is to stop the progression of DR in OSA patients, which might help the standard DR treatment achieve the maximum therapeutic goal.Citation23
CRVO and OSA
Literature on CRVO and OSA Association
Epidemiological studies consider CRVO the second most common cause of blindness from vascular disease in the retina, the first most common cause being DR.Citation24 Leroux les Jardins et alCitation25 report three cases of RVO with OSA. Since then, an association between RVO and OSA has been well observed in relevant literature. Notably, by means of screening RVO patients with polysomnography, a prospective and controlled study has concluded that there is a high OSA prevalence among said patients and has sequentially suggested that OSA is a potential risk factor for RVO.Citation26 Wan et al found that OSA incidence was remarkably increased in the RVO patients comparing with the control group.Citation27 Wang et al found the average AHI and was significantly higher in CRVO patients (AHI: 13.86±8.63).Citation28
Mechanisms of CRVO and OSA Association
Mechanisms for this correlation derive from the effect of OSA on blood flow autoregulation and microvasculature. Hypertension is associated with OSA and is known as a risk factor for CRVO.Citation4 Additionally, the hypercoagulable state following slow retinal blood flow in OSA secondary to hypoxemia and nocturnal intracranial pressure elevations has been suggested to play a key role in increasing the probabilities of CRVO.Citation29 Last but not least, increased sympathetic activation, peripheral vasoconstriction, oxidative stress, and endothelial damage as a result of hypoxia episodes in patients with OSA have been postulated as crucial in the development of CRVO.Citation27
HTRP and OSA
Hypertension is a well-known major risk factor for cardiovascular disease and all-cause mortality worldwide, with a global burden of 1.38 billion people (31.1% of adult population) in 2010.Citation30 About 83.6% of total hypertensive patients were found to have HTRP, as recorded by Kabedi et al (grade 1: 42.1%; grade 2: 11.3%; grade 3: 23.3%; grade 4: 6.9%).Citation31 A study by Viren et al estimates that 30–40% of hypertensive patients have OSA and 50% of OSA patients are hypertensive.Citation32
AHI as a Predictor of Retinal Arteriolar Changes
In a prospective study of the ocular fundus, patients with severe OSA (as defined by AHI ≥20 and hypoxic burden ≥10%) were three times more likely to have retinal vascular changes in line with mild HTRP, such as arteriolar narrowing, arteriolar sclerosis, and arteriovenous nicking. However, after adjusting for hypertension as a confounder, arteriolar changes remained more common amongst patients with higher AHI (p = 0.04). In conclusion, AHI > 40 doubles the risk of retinal vascular changes independent of blood pressure measurement.Citation33 Furthermore, Jessica Y. et al concluded that there is a significant inverse association between AHI and static markers of retinal arteriolar narrowing, such as arterio-venous ratio (p=0.008) and central retinal arteriolar equivalent (p=0.016), irrespective of mean arterial pressure.Citation34 Such findings may not have been in line with the hypothesis that AHI as a predictor of retinal arteriolar changes. However, despite this limitation, we believe that further studies exploring the association between the two are of paramount importance.
Proposed Mechanism of Hypertensive Retinopathy in OSA
OSA leads to an increase in systemic blood pressure through various mechanisms. A potential but not exhaustive list of examples includes activation of the renin-angiotensin-aldosterone system, decreased stimulation of pulmonary stretch receptors, increased levels of endothelin-1, and bouts of sympathetic activity caused by nocturnal hypoxia and exacerbated by rapid eye movement.Citation35 Various studies of subjects at high altitude have described different ocular changes on fundus photography, such as retinal hemorrhages, cotton wool spots, and optic disc edema. Therefore, it is hypothesized that hypoxia blunts autoregulation and promotes compensatory mechanisms in retinal vasculature.Citation36
CSC and OSA
CSC is an ocular disease characterized by decompensation of the retinal pigment epithelium and hyperpermeability of the choroidal vasculature, resulting in neuroretinal detachment. An incidence of 10 per 100,000 men and 2 per 100,000 women is reported by Xie et al, 2008.Citation36 Patients affected by CSC are typically presented with central scotoma, micropsia, metamorphopsia, or loss of central vision.Citation37
Literature on CSC and OSA Association
A retrospective case-controlled study conducted by Leveque et al concludes that the risk of OSA is significantly greater in CSC patients than in control subjects (odds ratio = 3.67; 95% CI: 1.02, 13.14; P = 0.046). In the study, 17 (58.6%) out of 29 CSC patients had an increased risk for OSA compared to nine (31.0%) of the 29 control subjects. However, potential limitations involve the implementation of the Berlin questionnaire, a self-report tool with a positive predictive value of 89%, a sensitivity of 86 and a specificity of 77%, as opposed to the golden standard of PSG.Citation37
Similarly, Kloos et al screened CSC patients for the risk of OSA with the Epworth Sleep Scale (sensitivity = 66% and specificity = 48% at an Epworth sleep scale score >10), followed by PSG. Kloos et al report that 22% of CSC patients were found to have OSA, which, they cite, is higher than the general population.Citation11 Conversely, a higher prevalence of OSA based on more recent epidemiologic data may not have been taken into account.
In contrast, Frank et al did not detect a statistically significant association between CSC and OSA in their study, which controlled for BMI and used a larger cohort of patients. This indicates that previous results reported by Brodie et al may have been attributed to the confounder of BMI.Citation38 However, an association between CSC and OSA has been established in more recent studies. For example, a nationwide population-based study with the Taiwan National Health Insurance Database has identified 10,753 OSA patients and 322,590 control subjects and confirmed that, between the two, the incidence of CSC was significantly higher in OSA (adjusted incident rate ratio for probable SA: 1.2 [95% CI: 1.1–1.4], P < 0.0001).Citation39 Similarly, Pan et al queried over 59 million OSA patients and identified those with a more restrictive definition of OSA (patients who had undergone a sleep study and had a record of receiving a CPAP device) and concluded that the risk of CSC is significantly increased with OSA diagnosis (HR = 1.081, P < 0.033).Citation40
Proposed Mechanism of CSC in OSA
Increased levels of epinephrine, norepinephrine and corticosteroids are hypothesized to be the main pathophysiology underlying the association between CSC and OSA. Increased catecholamines are thought to be due to intermittent asphyxia and sudden arousal from sleep. Similarly, pulsatile corticosteroids release occurs during nocturnal arousals, and corticosteroid levels are believed to be increased in sleep-deprived patients. Furthermore, norepinephrine stimulates corticotrophin and leads to the additive effect of further cortisol release.Citation37
CPAP and CSC
A case report on bilateral CSC that resolved rapidly after starting CPAP machine therapy further supports the proposed association between CSC and OSA.Citation41 Furthermore, Liu et al have concluded that suspected OSA patients who received CPAP had a significantly decreased CSC incidence rate, as opposed to the non-CPAP group.Citation39
Conclusion
OSA is common, and its prevalence is climbing with the increased prevalence of obesity, alongside other risk factors. OSA is still underdiagnosed and only 15% of those who are symptomatic receive OSA treatment. There is a growing body of literature showing a relationship between OSA and various sight-threatening ocular conditions, including retinal vascular manifestation. Sympathetic activation, hypoxia, and hormonal dysregulation all lead to a number of retinal vascular conditions that will worsen OSA patients’ quality of life. Early detection with appropriate intervention of any retinal vascular condition can decrease the burden of OSA. This necessitates an early referral to undergo sleep studies for those with poorly controlled hypertensive retinopathy and non-arteritic anterior ischemic optic neuropathy.
Abbreviation
OSA, obstructive sleep apnea; CRVO, central retinal vein occlusion; HTR, hypertensive retinopathy; DR, deiabetic retinopathy; CSC, central serous chorioretinopathy; AHI, apnea hypo apnea index; DM, diabetes mellitus; CPAP, continuous positive airway pressure.
Disclosure
The authors report no conflicts of interest for this work.
Additional information
Funding
References
- Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311–1322. doi:10.3978/j.issn.2072-1439.2015.06.11
- Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea [published correction appears in Physiol Rev.2010 Apr; 90(2):797–8]. Physiol Rev. 2010;90(1):47–112. doi:10.1152/physrev.00043.2008
- Dorasamy P. Obstructive sleep apnea and cardiovascular risk. Ther Clin Risk Manag. 2007;3(6):1105–1111.
- Tregear S, Reston J, Schoelles K, Phillips B, Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5(6):573–581. doi:10.5664/jcsm.27662
- Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291(16):2013–2016. doi:10.1001/jama.291.16
- Rana D, Torrilus C, Ahmad W, et al. Obstructive sleep apnea and cardiovascular morbidities: a review article. Cureus. 2020;12(9):e10424. doi:10.7759/cureus.10424
- Palombi K, Renard E, Levy P, et al., Non-arteritic anterior ischaemic optic neuropathy is nearly systematically associated with obstructive sleep apnoea. Br J Ophthalmol. 2006;90(7):879–882. doi:10.1136/bjo.2005.087452
- Mojon DS, Hess CW, Goldblum D, et al. High prevalence of glaucoma in patients with sleep apnea syndrome. Ophthalmology. 1999;106(5):1009–1012. doi:10.1016/S0161-6420(99)00525-4
- Sergi M, Salerno DE, Rizzi M, et al. Prevalence of normal tension glaucoma in obstructive sleep apnea syndrome patients. J Glaucoma. 2007;16(1):42–46. doi:10.1097/01.ijg.0000243472.51461.24
- Purvin VA, Kawasaki A, Yee RD. Papilledema and obstructive sleep apnea syndrome. Arch Ophthalmol. 2000;118(12):1626–1630. doi:10.1001/archopht.118.12.1626
- Kloos P, Laube I, Thoelen A. Obstructive sleep apnea in patients with central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2008;246(9):1225–1228. doi:10.1007/s00417-008-0837-0
- Shah SM, Bakri SJ. Obstructive sleep apnea evaluation in retinal vein occlusion patients: an opportunity for multidisciplinary care? Can J Ophthalmol. 2020;55(4):284–285. doi:10.1016/j.jcjo.2020.03.004
- Wang XY, Wang S, Liu X, et al. Retinal vascular morphological changes in patients with extremely severe obstructive sleep apnea syndrome. Chin Med J (Engl). 2017;130(7):805–810. doi:10.4103/0366-6999.202728
- Moshfeghi A. Evaluating the social costs of blindness from AMD. Presentation at: Retina World Congress; March 2019; Fort Lauderdale, FL. Available from: https://www.healio.com/news/ophthalmology/20190323/social-cost-of-retinal-blindness-in-us-will-be-billions-of-dollars-by-2020. Accessed July 29, 2021.
- Sapra A, Bhandari P. Diabetes mellitus. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; Jan, 2020 [updated November 19, 2020]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551501/. Accessed July 29, 2021.
- Lee R, Wong TY, Sabanayagam C, Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vision. 2015;2(1):17. doi:10.1186/s40662-015-0026-2
- Subramanian A, Adderley NJ, Tracy A, et al. Risk of incident obstructive sleep apnea among patients with type 2 diabetes. Diabetes Care. 2019;42(5):954–963.
- Zhu Z, Zhang F, Liu Y, et al. Relationship of obstructive sleep apnoea with diabetic retinopathy: a meta-analysis. Biomed Res Int. 2017;2017:4737064. doi:10.1155/2017/4737064
- Epstein LJ, Kristo D, Strollo PJ Jr, et al. Adult obstructive sleep apnea task force of the American academy of sleep medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276.
- Shiba T, Sato Y, Takahashi M. Relationship between diabetic retinopathy and sleep-disordered breathing. Am J Ophthalmol. 2009;147(6):1017–1021. doi:10.1016/j.ajo.2008.12.027
- Kusunose N, Akamine T, Kobayashi Y, et al. Contribution of the clock gene DEC2 to VEGF mRNA upregulation by modulation of HIF1a protein levels in hypoxic MIO-M1 cells, a human cell line of retinal glial (Müller) cells. Jpn J Ophthalmol. 2018;62(6):677–685.
- Nishimura A, Kasai T, Kikuno S, et al. Apnea hypopnea index during rapid eye movement sleep with diabetic retinopathy in patients with type 2 diabetes. J Clin Endocrinol Metab. 2019;104(6):2075–2082.
- Lin PW, Lin HC, Friedman M, et al. Effects of CPAP for patients with OSA on visual sensitivity and retinal thickness. Sleep Med. 2020;67:156–163. doi:10.1016/j.sleep.2019.10.019
- Huon LK, Liu SY, Camacho M, Guilleminault C, The association between ophthalmologic diseases and obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath. 2016;20(4):1145–1154. doi:10.1007/s11325-016-1358-4
- Leroux Les Jardins G, Glacet-Bernard A, Lasry S, Housset B, Coscas G, Soubrane G, Retinal vein occlusion and obstructive sleep apnea syndrome. J Fr Ophtalmol. 2009;32(6):420–424. doi:10.1016/j.jfo.2009.04.012
- Felfeli T, Alon R, Adel FA. Screening for obstructive sleep apnea amongst patients with retinal vein occlusion. Can J Ophthalmol. 2020;10:1016.
- Wan W, Wu Z, Lu J, et al. Obstructive sleep apnea is related with the risk of retinal vein occlusion. Nat Sci Sleep. 2021;13:273–281. doi:10.2147/NSS.S290583
- Wang YH, Zhang P, Chen L, et al. Correlation between obstructive sleep apnea and central retinal vein occlusion. Int J Ophthalmol. 2019;12(10):1634–1636. doi:10.18240/ijo.2019.10.17
- Grover DP, Obstructive sleep apnea and ocular disorders. Curr Opin Ophthalmol. 2010;21(6):454–458. doi:10.1097/ICU.0b013e32833f00dc
- mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223–237. doi:10.1038/s41581-019-0244-2
- Kabedi NN, Mwanza JC, Lepira FB, Kayembe TK, Kayembe DL. Hypertensive retinopathy and its association with cardiovascular, renal and cerebrovascular morbidity in Congolese patients. Cardiovasc J Afr. 2014;25(5):228–232. doi:10.5830/CVJA-2014-045
- Viren SK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology foundation scientific statement from the American heart association council for high blood pressure research professional education committee, council on clinical cardiology, stroke council, and council on cardiovascular nursing. J Am Coll Cardiol. 2008;52(8):686–717. doi:10.1016/j.jacc.2008.05.002
- Fraser CL, Bliwise DL, Newman NJ, et al. A prospective photographic study of the ocular fundus in obstructive sleep apnea. J Neuroophthalmol. 2013;33(3):241–246. doi:10.1097/WNO.0b013e318290194f
- Tong JY, Golzan M, Georgevsky D, et al. Quantitative retinal vascular changes in obstructive sleep apnea. Am J Ophthalmol. 2017;182:72–80. ISSN 0002-9394. doi:10.1016/j.ajo.2017.07.012
- Kario K. Obstructive sleep apnea syndrome and hypertension: mechanism of the linkage and 24-h blood pressure control. Hypertens Res. 2009;32(7):537–541. doi:10.1038/hr.2009.73
- Xie Y, Wang N. The eye and high altitude. In: Wang N, editor. Integrative Ophthalmology. Advances in Visual Science and Eye Diseases. Vol. 3. Singapore: Springer; 2020. doi:10.1007/978-981-13-7896-6_15
- Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980–2002. Ophthalmology. 2008;115(1):169–173. doi:10.1016/j.ophtha.2007.02.032
- Brodie FL, Charlson ES, Aleman TS, et al. Obstructive sleep apnea and central serous chorioretinopathy. Retina. 2015;35(2):238–243. doi:10.1097/IAE.0000000000000326
- Liu PK, Chang YC, Tai MH, et al. The association between central serous chorioretinopathy and sleep apnea: a Nationwide Population-Based Study. Retina. 2020;40(10):2034–2044. doi:10.1097/iae.0000000000002702
- Pan CK, Vail D, Bhattacharya J, Cao M, Mruthyunjaya P. The effect of obstructive sleep apnea on absolute risk of central serous chorioretinopathy. Am J Ophthalmol. 2020;218:148–155. doi:10.1016/j.ajo.2020.05.040
- Jain AK, Kaines A, Schwartz S. Bilateral central serous chorioretinopathy resolving rapidly with treatment for obstructive sleep apnea. Graefes Arch Clin Exp Ophthalmol. 2010;248(7):1037–1039. doi:10.1007/s00417-009-1257-5