Abstract
Purpose
Intracanalicular dexamethasone insert is a resorbable sustained-release polyethylene glycol-based hydrogel insert delivering a 0.4 mg tapered dose of dexamethasone for up to 30 days to the ocular surface. It is FDA-approved for treating inflammation and pain after ocular surgery. It has also been studied for ocular surface diseases such as allergic conjunctivitis. This study assessed the plasma pharmacokinetic (PK) parameters of dexamethasone following intracanalicular insertion.
Patients and Methods
Study subjects (N=16) were healthy adults. A dexamethasone insert was unilaterally placed into the canaliculus, and blood samples were obtained for analysis 1 hour prior to insertion and 1, 2, 4, 8, 16, 24 hours and 4, 8, 15, 22 and 29 days after insertion. Safety analyses included slit lamp and dilated fundus examinations, best corrected visual acuity, intraocular pressure (IOP) and adverse events (AEs).
Results
Plasma results were below the lower limit of quantitation (LLOQ) at all time points in five subjects (31.3%). Among subjects with quantifiable plasma concentrations, Cmax was <1 ng/mL (range, 0.05 to 0.81 ng/mL), AUC0-last ranged from 0.13 to 7.18 h∙ng/mL, and Tmax ranged from 4.0 to 163.0 hours. Mean (SD) IOP increased from 16.3 (1.4) mmHg at baseline to 19.3 (3.2) at Day 22 but returned to baseline after treatment. No changes occurred in dilated fundus, punctum, or visual acuity examinations.
Conclusion
The dexamethasone 0.4 mg insert results in minimal systemic exposure following intracanalicular administration.
Introduction
Corticosteroid eye drops have long been the standard of care for many ophthalmic conditions including postoperative pain and inflammationCitation1,Citation2 and ocular surface diseases such as dry eyeCitation3 and allergic conjunctivitis.Citation3,Citation4 While therapeutically beneficial, topical corticosteroids may be associated with several ocular complications including glaucoma, posterior subcapsular cataract, corneal thinning,Citation5 infection, and preservative toxicity.Citation6–Citation8 In addition, barriers to effective therapy with ophthalmic drops generally include noncompliance,Citation2,Citation9,Citation10 poor self-administration technique,Citation11,Citation12 and even accidental injury.Citation13
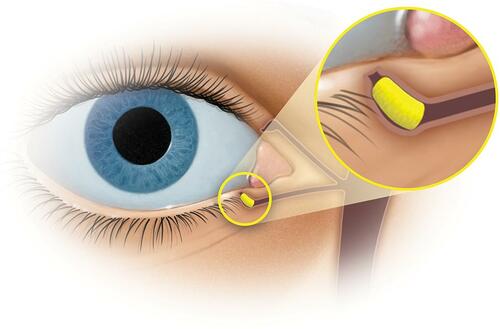
To address these problems, an intracanalicular insert has been developed which delivers dexamethasone to the surface of the eye for up to 30 days (Dextenza® [dexamethasone ophthalmic insert] 0.4 mg, for intracanalicular use; Ocular Therapeutix, Bedford, MA). The insert comprises a biodegradable crosslinked synthetic hydrophilic polyethylene glycol-based hydrogel designed to deliver a tapering dose of preservative-free dexamethasone to the surface of the eye.Citation14 As the insert is administered by the physician, there are no compliance issues and patients do not need to touch their face or eye to receive the medication. Following hydration, the insert consists of mostly water which contributes to its biocompatibility, allowing it to be used in the eye with low potential for inflammation or rejection. As the hydrogel gradually biodegrades, there is no need for removal.Citation15 The dexamethasone hydrogel insert is fluorescein-conjugated to aid with visualization.Citation16
Large placebo-controlled studies have demonstrated the efficacy of the insert for alleviating inflammation and pain following cataract surgeryCitation17,Citation18 leading to FDA approval for the treatment of ocular inflammation and pain following ophthalmic surgery.Citation19 Patients treated with the dexamethasone insert reported favorably on overall satisfaction, convenience and comfort.Citation20 The insert has also been evaluated in Phase 3 studies for treating allergic conjunctivitis.Citation21,Citation22
To address potential safety concerns from systemic absorption of dexamethasone in treated patients, the objective of this study was to evaluate the systemic pharmacokinetic (PK) parameters of dexamethasone following placement of a sustained-release insert in the canaliculus of the eyes of healthy subjects.
Patients and Methods
Study Participants
Eligible subjects were healthy adults with a best-corrected visual acuity (BCVA) of 0.6 logMAR (20/80 Snellen) or better in each eye. Reasons for study exclusion included hypersensitivity to any component of the study product; recent ocular trauma, infection or inflammation; glaucoma or intraocular pressure <5 mmHg or >22 mmHg; women of childbearing potential who were currently pregnant, nursing or planning a pregnancy during the study.
Ethics
The study protocol and related materials were approved by an Institutional Review Board (Salus IRB, Austin, TX). The study was conducted in accordance with the United States Title 21 Code of Federal Regulations, Part 56 and in accordance with the Declaration of Helsinki.Citation23 Enrolled subjects provided written informed consent prior to participating in any study-related activities.
Dexamethasone Intracanalicular Insert
Each polyethylene glycol-based hydrogel ocular insert contains 0.4 mg of dexamethasone (Dextenza® [dexamethasone ophthalmic insert] 0.4 mg, for intracanalicular use; Ocular Therapeutix, Inc., Bedford, MA).Citation24 A single insert releases a 0.4 mg dose of dexamethasone for up to 30 days. The resorbable insert does not require removal; however, saline irrigation or manual expression can be performed to remove the insert if necessary. The dexamethasone inserts are conjugated with fluorescein to permit visualization through the tissue when illuminated with a blue light source.
Procedures
Within 14 days of an initial screening visit, a dexamethasone insert was unilaterally placed into the inferior vertical canaliculus on study Day 1 (). Subjects with punctum size <0.4 mm or ≥1.0 mm, unsuccessful transient dilation to 0.7 mm prior to insertion or unsuccessful insertion were excluded from the study.
Blood samples were obtained for plasma PK analysis at 1 hour (±30 min) prior to insertion and following insertion after 1 hour (±10 min), 2 hours (±10 min), 4 hours (±20 min), 8 hours (±20 min), 16 hours (±60 min), 24 hours (±60 min) and on Days 4, 8, 15, 22 and 29. If the insert was still present at the Day 29 Visit, it was removed via saline irrigation or by applying manual pressure.
Study Endpoint
The primary PK parameters assessed were peak plasma concentration (Cmax), area under the plasma concentration time curve (AUC) and time to reach peak drug plasma concentration (Tmax). Human plasma samples and quality control standards containing dipotassium EDTA were stored at −20°C until analysis. Quantitation of dexamethasone was performed using validated high-pressure liquid chromatography with tandem mass spectrometry (LC/MS/MS) detection.
Safety Assessments
Best-corrected visual acuity (BCVA) testing, slit lamp biomicroscopy including IOP measurement and dilated fundus examination were performed. The presence of the test article was confirmed with a blue light and yellow filter. Subjects were queried about potential adverse events during each evaluation.
Statistical Analyses
Descriptive statistics were calculated for each PK parameter at each time point. The AUC was calculated using the log-linear trapezoidal rule: linear trapezoidal rule to Tmax and log trapezoidal rule thereafter. Subject demographics are presented using continuous summary statistics. The results from safety evaluations were tabulated using descriptive statistics. IOP from each visit was summarized using continuous summary statistics for actual values and change from baseline. Discrete summary statistics were determined for any subjects with baseline IOP increases ≥10 mmHg.
Results
Among the 16 enrolled subjects, 14 completed the study through Day 29. The dexamethasone insert was removed from one subject due to an AE (intraocular pressure increased) following the Day 15 visit and the insert was not noted to be present on Day 15 in another subject. Enrolled subjects had a mean age of 31.7 years (range, 19 to 55 years) and most (68.7%) were female. The demographics and clinical characteristics of enrolled subjects are summarized in .
Table 1 Demographics and Clinical Characteristics
Pharmacokinetic Analysis
Dexamethasone plasma assay results were below the lower limit of quantitation (LLOQ; 0.05 ng/mL) at all time points in five (31.3%) of the 16 subjects, 100% (16/16) of the subjects at 1 and 2 hours, 87.5% (14/16) of the subjects at 4 hours, 75% (12/16) of the subjects at 8, 16 hours and on Day 4, and 66.67% (10/15) of the subjects on Day 8, were below LLOQ. Individual dexamethasone plasma concentrations are shown in .
Table 2 Individual Dexamethasone Plasma Concentrations
Plasma concentrations of dexamethasone were detectable in 11% of samples (21 of 189). For plasma samples with quantifiable drug concentrations, mean Cmax was <1 ng/mL (range, 0.05 to 0.81 ng/mL), AUC0-last ranged from 0.13 to 7.18 h∙ng/mL, and Tmax ranged from 4.0 to 163.0 hours. The PK results from each subject are summarized in .
Table 3 Pharmacokinetic Parameter Results by Subject
Ease of Insertion, Visualization and Removal
Ease of inserting the dexamethasone insert was judged by the investigator to be easy in 15 eyes (93.7%) and moderate in one eye (6.3%).
Safety
There were no serious adverse events (AEs). There were three ocular and one non-ocular AEs. In three subjects (18.7%), there was an increase in baseline IOP that were considered mild in severity and were deemed related to study treatment but resolved without treatment following insert removal at Day 15 (1/3) and at the end of the study (2/3). There was no change in mean IOP in the fellow eyes (). Nasopharyngitis (n=1, 6.3%) was also a reported AE (non-ocular) but was unrelated to study treatment. No changes were observed in visual acuity measures (), punctum, slip lamp or dilated fundus examinations at any time point during the study.
Table 4 Changes in Intraocular Pressurea
Table 5 Changes in Best-Corrected Visual Acuitya
Discussion
The present study was performed to assess any potential systemic absorption of dexamethasone in patients receiving intracanalicular dexamethasone. The results indicate very little systemic absorption of dexamethasone as most plasma samples did not contain measurable drug. Among the 16 treated subjects, plasma drug concentrations were <0.05 ng/mL at all time-points in five subjects (31.3%). Among the remaining 11 subjects, plasma drug concentrations were <0.05 ng/mL at 1 and 2 hours post-insertion and at Days 15, 22 and 29. In the 21 plasma samples above the LLOQ between 4 hours and Day 8, maximum plasma dexamethasone concentrations ranged from 0.05 to 0.81 ng/mL. Overall, the safety profile of the dexamethasone insert is consistent with the ocular administration of dexamethasone using other dosage forms, such as drops and implants.
When instilling typical eye drops, the lacrimal fluid drains through the nasolacrimal duct from the conjunctival sac to the nasal cavity. There, absorption can also occur through the highly vascularized nasopharyngeal mucosa. It has been estimated that up to 80% of instilled drug may reach the systemic circulation.Citation25 The insert is designed to deliver a single 0.4 mg tapered dose of dexamethasone to the ocular surface for a period of up to 30 days. In contrast, dexamethasone phosphate 0.1% eye drops contain 1 mg/mL of medication. A standard eyedropper dispenses 0.05 mL per drop delivering 0.05 mg of drug/drop, although the volume of commercial ophthalmic products will vary.Citation26 Initial therapy with dexamethasone ophthalmic drops may deliver 1.0 to 2.0 mg of drug to the surface of the eye during the first day of therapy alone.
In contrast, the insert delivers a daily dexamethasone dose of approximately 0.02 mg per day directly to the ocular surface. This daily rate is regulated by the solubility of the hydrogel matrix and drug transfer at the interface proximal to the ocular surface in contact with the tear fluid lavage. The drug release from the dexamethasone insert will remain constant until sufficiently depleted at the interface between the hydrogel and the tear fluid leading to a gradual tapering effect observed in the tear fluid pharmacokinetic profiles. The daily systemic exposure from eye drops exceeds the insert. Eye drops require more frequent administrations and a greater total daily dose to achieve similar therapeutic levels. The lower systemic absorption of dexamethasone from intracanalicular insert (0.4 mg) supports these observations.
Our results are similar to other ocular dexamethasone PK studies. While we understand that the intravitreal space has a different blood supply, administration of a 0.7 mg dexamthasone implant in patients with macular edema produced plasma drug concentrations ranging from 0.052 to 0.102 ng/mL during 90-day post-treatment measurements.Citation27 Mean (SD) steady-state drug serum concentrations of 0.7 (0.4) ng/mL (range, 0.0 to 1.2 ng/mL) were reported following topical administration of dexamethasone sodium phosphate 0.1% eye drops.Citation28 Following bilateral administration of a tobramycin 0.3%/dexamethasone 0.1% ophthalmic suspension four times daily for 2 days, the peak plasma dexamethasone concentrations was <1 ng/mL.Citation29
There was a higher incidence of subjects who reported increased IOP in the treated eye (18.7%) than observed in previous studies for treating patients with postoperative pain and inflammation (6%).Citation24 No changes in the conjunctiva, cornea, anterior chamber, iris, eyelid or lens were observed. Similar to the clinical trial experience,Citation30 investigators rated the dexamethasone insert as easy to place, visualize and remove when necessary.
Conclusion
The 0.4 mg dexamethasone intracanalicular insert results in minimal systemic exposure following intracanalicular administration.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgment
The authors acknowledge the editorial assistance of Dr. Carl S. Hornfeldt, Apothekon, Inc., during the preparation of this manuscript and David Evans, OD, Total Eye Care, Memphis, TN, Deepa Mulani, MS, and Jonathan H Talamo, MD, Ocular Therapeutix, Inc., and Robert Noecker, MD, Ophthalmic Consultants of Connecticut, Fairfield, CT for their early contributions to this study. This study was sponsored by Ocular Therapeutix, Inc., Bedford, MA. The abstract of this paper was presented at the 2017 ARVO Annual Meeting, Baltimore, MD; May 7–11, 2017 which appeared in Invest Ophthalmol Vis Sci. 2017;58. Available from: https://iovs.arvojournals.org/article.aspx?articleid=2638122.
Disclosure
Michael H Goldstein, Srilatha Vantipalli, Jamie Lynne Hart, Fabiana Q Silva, Charles Blizzard, and Arthur Driscoll are employees of and owned stocks for Ocular Therapeutix who was the sponsor for the study and who commercializes DEXTENZA. Mr Charles Blizzard has a patent issued: US20160331738A1 - Drug Delivery Through Hydrogels. Eugene B McLaurin is affiliated with Total Eye Care and is an investigator in a clinical trial sponsored by Ocular Therapeutix. The authors report no other conflicts of interest in this work.
References
- Zhang G, Liu S, Yang L, Li Y. The role of dexamethasone in clinical pharmaceutical treatment for patients with cataract surgery. Exp Ther Med. 2018;15(1):2177–2181. doi:10.3892/etm.2017.5639
- Brooks CC, Jabbehdari S, Gupta PK. Dexamethasone 0.4mg sustained-release intracanalicular insert in the management of ocular inflammation and pain following ophthalmic surgery: design, development and place in therapy. Clin Ophthalmol. 2020;14:89–94. doi:10.2147/OPTH.S238756
- Yang CQ, Sun W, Gu YS. A clinical study of the efficacy of topical corticosteroids on dry eye. J Zhejiang Univ Sci B. 2006;7(8):675–678. doi:10.1631/jzus.2006.B0675
- Sacchetti M, Abicca I, Bruscolini A, Cavaliere C, Nebbioso M, Lambiase A. Allergic conjunctivitis: current concepts on pathogenesis and management. J Biol Regul Homeost Agents. 2018;32:49–60.
- McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Safety. 2002;25(1):33–55. doi:10.2165/00002018-200225010-00004
- Rosin LM, Bell NP. Preservative toxicity in glaucoma medication: clinical evaluation of benzalkonium chloride-free 0.5% timolol eye drops. Clin Ophthalmol. 2013;7:2131–2135.
- Zhang R, Park M, Richardson A, et al. Dose-dependent benzalkonium chloride toxicity imparts ocular surface epithelial changes with features of dry eye disease. Ocul Surf. 2020;18(1):158–169. doi:10.1016/j.jtos.2019.11.006
- Singh G, Kaur J. Iatrogenic dry eye: late effect of topical steroid formulations. J Indian Med Assoc. 1992;90:235–237.
- Robin A, Grover DS. Compliance and adherence in glaucoma management. Indian J Ophthalmol. 2011;59(7):S93–96. doi:10.4103/0301-4738.73693
- Olthoff CM, Schouten JS, van de Borne BW, Webers CA. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology. 2005;112:953–961. doi:10.1016/j.ophtha.2004.12.035
- Gomes BF, Paredes AF, Madeira N, Moraes HV, Santhiago MR. Assessment of eye drop instillation technique in glaucoma patients. Arq Bras Oftalmol. 2017;80(4):238–241. doi:10.5935/0004-2749.20170058
- Gupta R, Patil B, Shah BM, Bali SJ, Mishra SK, Dada T. Evaluating eye drop instillation technique in glaucoma patients. J Glaucoma. 2012;21(3):189–192. doi:10.1097/IJG.0b013e31820bd2e1
- Solomon A, Chowers I, Raiskup F, Siganos CS, Frucht-Pery J. Inadvertent conjunctival trauma related to contact with drug container tips: a masquerade syndrome. Ophthalmology. 2003;110(4):796–800. doi:10.1016/S0161-6420(02)01967-X
- Sawhney AS, Bassett M, Blizzard C. Drug delivery through hydrogel plugs. US Patent 8409606B2; 2013. Available from: https://patentimages.storage.googleapis.com/8e/df/8d/27cebf6bb8bbee/US8409606.pdf. Accessed April 14, 2021.
- Kołodyńska D, Skiba A, Górecka B, Hubicki Z. Hydrogels from fundaments to application. In: Majee SB, editor. Emerging Concepts in Analysis and Applications of Hydrogels. London: IntechOpen Limited; 2016.
- Tyson SL, Campbell P, Biggins J, et al. Punctum and canalicular anatomy for hydrogel-based intracanalicular insert technology. Ther Deliv. 2020;11(3):173–182. doi:10.4155/tde-2020-0010
- Tyson SL, Bafna S, Gira JP, et al. Multicenter randomized phase 3 study of a sustained-release intracanalicular dexamethasone insert for treatment of ocular inflammation and pain after cataract surgery. J Cataract Refract Surg. 2019;45(2):204–212. doi:10.1016/j.jcrs.2018.09.023
- Walters T, Bafna S, Vold S, et al. Efficacy and safety of sustained release dexamethasone for the treatment of ocular pain and inflammation after cataract surgery: results from two phase 3 studies. J Clin Exp Ophthalmol. 2016;7(4):572.
- Ocular Therapeutix, Inc. Dextenza® (Dexamethasone Insert) for Intracanalicular Use. Prescribing Information. Bedford, MA: Ocular Therapeutix, Inc.; 2019.
- Gira JP, Walters TR. Evaluating the patient experience after implantation of a 0.4mg sustained release dexamethasone intracanalicular insert (Dextenza): results of a qualitative survey. Patient Prefer Adherence. 2017;11:487–494. doi:10.2147/PPA.S126283
- Torkildsen G, Abelson MB, Gomes PJ, McLaurin E, Potts SL, Mah FS. Vehicle-controlled, Phase 2 clinical trial of a sustained-release dexamethasone intracanalicular insert in a chronic allergen challenge model. J Ocul Pharmacol Ther. 2017;33(2):79–90. doi:10.1089/jop.2016.0154
- ClinicalTrials.gov. OTX-15-002: a Phase 3 study evaluating the safety and efficacy of OTX-DP for the treatment of chronic allergic conjunctivitis. Identifier: NCT02988882. Available from: https://clinicaltrials.gov/ct2/show/NCT02988882?term=ocular+therapeutix&draw=3&rank=17. Accessed April 14, 2021.
- World Medical Association. WMA declaration of Helsinki – ethical principles for medical research involving human subjects; 2018. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed 16 March 2021.
- Ocular Therapeutix, Inc. Dextenza® (Dexamethasone Ophthalmic Insert) 0.4 Mg for Intracanalicular Use [Prescribing Information 2019]. Bedford, MA: Ocular Therapeutix, Inc.; 2019.
- Farkouh A, Frigo P, Czejka M. Systemic side effects of eye drops: a pharmacokinetic perspective. Clin Ophthalmol. 2016;10:2433–2441. doi:10.2147/OPTH.S118409
- Moore DB, Beck J, Kryscio RJ. An objective assessment of the variability in number of drops per bottle of glaucoma medication. BMC Ophthalmol. 2017;17:78. doi:10.1186/s12886-017-0473-8
- Allergan USA, Inc. Ozurdex® (Dexamethasone Intravitreal Implant) for Intravitreal Injection [Prescribing Information 2018]. Madison, NJ: Allergan USA, Inc.; 2018.
- Weijtens O, Schoemaker RC, Romijn FP, Cohen AF, Lentjes EG, van Meurs JC. Intraocular penetration and systemic absorption after topical application of dexamethasone disodium phosphate. Ophthalmol. 2002;109:1887–1891. doi:10.1016/S0161-6420(02)01176-4
- Alcon Laboratories, Inc. Tobradex® ST (Tobramycin/Dexamethasone Ophthalmic Suspension) 0.3%/0.05% [Prescribing Information 2011]. Fort Worth, TX: Alcon Laboratories, Inc.; 2011.
- Lee A, Blair HA. Dexamethasone intracanalicular insert: a review in treating post-surgical ocular pain and inflammation. Drugs. 2020;80(11):1101–1108. doi:10.1007/s40265-020-01344-6