Abstract
Purpose
Peripapillary halos (PPH) are peripapillary changes observed surrounding the optic nerve head in normal eyes and eyes with different disorders. Recognizing the microstructure and mechanism of development of these halos will help clinicians understand the different associated retinal and optic nerve head pathologies. We describe the in vivo histological characteristics of PPH in birdshot chorioretinopathy (BSCR).
Patients and Methods
This was a prospective observational case-series in a single tertiary referral center. Six eyes of three patients with PPH associated with BSCR were determined through clinical examination, fundus photography, and fundus autofluorescence (FAF). Patients underwent swept-source optical coherence tomography (SS-OCT) imaging of the optic nerve head and peripapillary region.
Results
In SS-OCT B-scans across the area of PPH, we observed thinning and interruption of retinal pigment epithelium (RPE)-Bruch’s membrane complex. These halos are a circumferential form of alpha zone RPE-associated crescentic peripapillary atrophy (PPA), unlike the PPH observed with myopia and normal aging.
Conclusion
PPH in BSCR patients may be a sign of prior inflammatory optic neuropathy.
Introduction
Optic nerve head peripapillary halo (PPH) and crescentic peripapillary atrophy (PPA) are common clinical findings in patients with pathologies like glaucoma, high myopia, papillitis, chorioretinitis, and normal aging.Citation1–Citation3
Birdshot chorioretinopathy (BSCR) is an idiopathic bilateral posterior uveitis characterized by multiple yellowish-white choroidal lesions. Ryan and MaumeneeCitation4 introduced the term “birdshot retinochoroidopathy” as the fundus lesions appear like the scatter pattern of birdshot pellets fired from a shotgun. BSCR accounts for 6–8% of posterior uveitis diagnoses. BSCR is most commonly observed in Caucasians in their sixth decade.Citation5 HLA-A29 antigen is strongly associated with BSCR. The prevalence of HLA-A29 antigen in BSCR patients is 80–95%, while in the general population is approximately 7%.Citation6,Citation7 Decreased visual acuity is the most common clinical presentation of BSCR patients, although patients may complain of floaters, nyctalopia, glare, or dyschromatopsia.Citation8 The disease course can be complicated by retinal vasculitis, cystoid macular edema, papillitis, or epiretinal membrane.Citation9
Multimodal imaging techniques are paramount in the assessment and follow-up of BSCR – especially optical coherence tomography (OCT). OCT is a noninvasive imaging technique that can provide high resolution, cross-sectional images of the retina, the retinal nerve fiber layer (RNFL) and the optic nerve head.Citation10 Swept-source optical coherence tomography (SS-OCT) utilizes a tunable laser at speeds of 100,000 to 236,000 A-scans/second, allowing deeper tissue penetration roughly double that of spectral-domain optical coherence tomography (SD-OCT), higher imaging speed, higher detection efficiencies, and better imaging range. This enables an improved assessment of the choroid including the choriocapillaris and the choroidoscleral interface.Citation11,Citation12
Peripapillary atrophy (PPA) is typically a lighter colored, well-demarcated crescent-shaped area on the temporal side of the optic nerve head and can be clinically divided into a central beta zone and peripheral alpha zone.Citation13 These two zones are not always present together. α-zone is characterized by a thinning and interruption of the retinal pigment epithelium (RPE) and thinning of the choroidal tissue. The more important zone suggested to be associated with glaucoma is the β-zone, which is associated with marked atrophy and loss of the RPE with intact Bruch’s membrane and underlying choriocapillaris allowing good visibility of the large choroidal vessels and sclera.Citation14 On angiography using sodium fluorescein or indocyanine green, α-zone shows decreased filling of choroidal vasculature. However, in the β-zone there is a loss of choriocapillaris with no choroidal filling.Citation15 Recently, two more zones related to globe elongation were identified: the gamma zone, which looks histologically similar to the β-zone but with an absent Bruch’s membrane, and the delta zone, which is an area of no blood vessels of at least 50 µm diameter within the gamma zone.Citation16 Focal gamma zone has been reported to be related to glaucoma development, while the conventional gamma zone is not.Citation17
Ophthalmoscopically, peripapillary crescent is peripapillary changes touching a part of the disc circumference, typically observed on the horizontal temporal peripapillary area. On the other hand, when peripapillary retinal changes surround the optic nerve head, we call it a peripapillary halo.Citation18
In this clinical imaging characterization of BSCR optic nerve heads, we use swept-source optical coherence tomography (SS-OCT) to provide in vivo morphological analyses of the peripapillary region following resolution of BSCR lesions. From a pathophysiological aspect, we believe peripapillary haloes in BSCR probably occur from inflammatory optic nerve head edema which stretches and thins the retinal pigmented epithelium and becomes a PPH when the papillitis resolves in the convalescent BSCR state.
Methods
The study was approved by the Institutional Review Board of University of Miami Miller School of Medicine and adhered to the tenants of the Declaration of Helsinki. Informed consent was obtained from all subjects. Patients were recruited from the uveitis clinic at the Bascom Palmer Eye Institute at Miami. Inclusion criteria were for patients who were previously diagnosed with birdshot chorioretinopathy according to the recommendations of the international workshop held at the University of California, Los Angeles (UCLA).Citation19
All participants underwent a complete ophthalmological examination including visual acuity assessment, intraocular pressure (IOP) measurement, and slit lamp biomicroscopy. Fundus fluorescein angiography (FFA) and indocyanine green angiography (ICGA) were ordered according to the follow-up plan of each patient.
Six eyes of three patients with peripapillary halos in association with convalescent inactive BSCR were imaged and their retinas and optic nerve heads were characterized. After dilating the pupils with 1% tropicamide and 2.5% phenylephrine, OCT images were obtained using a Plex Elite 9000 SS-OCT (Carl Zeiss Meditec Inc., Dublin, CA). This instrument uses a swept-source tunable laser with a central wavelength of 1,060 nm and has a scanning rate of 100,000 A-scans/second, resulting in an axial resolution of ~5.0 µm in tissue and a lateral resolution of ~20 µm estimated at the retinal surface. A 6×6 mm raster scan centered on the optic nerve head was acquired for each eye in the study. We excluded scans with low signal strength, blink artifacts, poor fixation leading to motion artifacts, media opacity, or segmentation errors.
In addition to SS-OCT imaging, color optic nerve head photos and fundus autofluorescence (FAF) were also performed. Two masked graders (M.K. and R.K.L) analyzed all the SS-OCT scans and optic nerve head imaging.
Results
Six eyes of three patients were included in the study. All participants were white, one female and two males. The mean spherical equivalent was – 0.21. The spherical equivalent was calculated from the phakic eye and for pseudophakic eyes, the pre-operative refraction was used. The patients ranged in age from 48–57 years and the range of disease duration was 3–6 years (). All the patients had normal IOP, ocular motility, and pupillary examinations.
Table 1 Characteristics of the Study Participants. All Spherical Equivalents are in Phakic Eyes
In OCT scans of all eyes, we observed atrophic thinning of the retinal pigment epithelium (RPE) and hyperreflectivity of the underlying choroid in the peripapillary circumferential area. The choroidal tissue was also thinned surrounding the optic nerve head. These findings correspond to the extent of peripapillary halo seen on optic nerve head color photographic images and hypofluorescent areas on FAF ( and ).
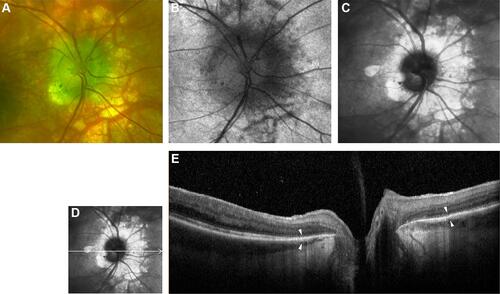
Figure 1 Left optic nerve head of patient 1. (A) Color optic disc photo with a peripapillary halo (PPH). (B) Fundus autofluorescence (FAF) of optic nerve head shows hypo-autofluorescent lesions possibly indicating thinning and interruption of retinal pigment epithelium (RPE). (C) Red free image of the optic nerve head showing the extent of PPH. (D and E) B-scan of swept-source optical coherence tomography (SS-OCT) image showing thinning and focal loss of RPE with hyper-reflectivity of underlying choroid in the peripapillary region with white arrowheads marking the extent of PPH.
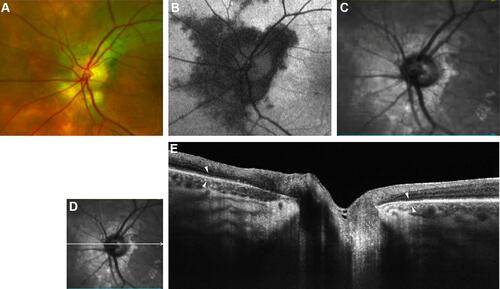
Figure 2 Optic nerve head of right eye of patient 3. (A) Color optic disc photo with peripapillary halo (PPH). (B) Fundus autofluorescence (FAF) of optic nerve head. (C) Red free image of the optic nerve head. (D and E) B-scan of swept-source optical coherence tomography (SS-OCT) image of PPH. White arrowheads point to the margins of RPE-Bruch’s membrane thinning.
Discussion
PPA was first described by ElschnigCitation20 at the beginning of the 20th century. PPA is typically due to choroidal thinning and disruption of RPE in a crescentic area around the optic disc. PPA is observed in several diseases such as glaucoma, high myopia, papillitis, chorioretinitis, as well as being part of the normal aging process. In glaucomatous eyes, the size and area of peripapillary atrophy are believed to closely relate to the pathogenesis of glaucoma with an increasing area of PPA associated with the progression of glaucomatous optic nerve damage and worsening of the visual field.Citation21 The PPA typically associated with glaucoma is a temporal crescent of atrophy. If the PPA is large and encircles the whole optic disc, it is called “Halo glaucomatous” in end-stage glaucomatous eyes.Citation22 In contrast to glaucomatous optic neuropathy, non-glaucomatous optic nerve damage as in non-arteritic anterior ischemic optic neuropathy, does not lead to an enlargement of PPA.Citation23
In highly myopic eyes, PPA is more common than in emmetropic eyes and is estimated to be present in approximately 20% of high myopes. PPA in most of high myopes is in the form of temporal crescentic PPA, but PPH is observed in advanced cases.Citation24,Citation25 The ultrastructure of myopic crescents is similar to the β-zone of PPA, which was confirmed by the SD-OCT findings of these crescents showing complete loss of the RPE layer and a partial loss of the photoreceptor layer. As myopia develops, the axial length increases in size causing retinal stretching, thinning, and pulling of the RPE and choroid away from the optic disc.Citation26 In older patients, PPH is possibly due to age-related degeneration of the RPE–Bruch’s membrane complex with similar changes occurring in the macula and periphery of normal eyes associated with aging. These halos also consist mainly of β-zone component.Citation27
The peripapillary halo is different from the physiological gray crescent of optic nerve which is pigmentation of the neuroretinal rim mostly temporal and within the disc border, unlike the pigmentary changes outside the disc borders in PPH. The clinical significance of the gray crescent of ONH is the appearance of pseudocupping as a physiological variant in order to avoid falsely diagnosing normal eyes as having glaucomatous damage.Citation28–Citation30
SS-OCT plays a role in the diagnosis and monitoring of the course of BSCR. In SS-OCT B-scans of BSCR patients, thinner choroids and retinas compared to normal subjects were observed.Citation31 B-scans also showed loss of retinal architecture, outer retinal hyperreflective foci, choroidal focal depigmentation, generalized thinning, and choroidal hyperreflective foci have been observed.Citation31 In a retrospective study of 42 eyes of 21 BSCR patients, peripapillary atrophy was the most common lesion (90% of patients) observed in OCT scans of these patients.Citation32 OCT is crucial in the qualitative and quantitative assessment of cystoid macular edema, which is the most common cause of vision loss in BSCR patients.Citation33
To the best of our knowledge, this is the first study to characterize the microstructure of peripapillary halos in inflammatory uveitis using SS-OCT. We observed in our study that the PPH observed in BSCR patients is a result of the thinning and interruption of the RPE and Bruch’s membrane, but not a complete loss of these layers. The BSCR PPH is mainly of an α-zone-like component, unlike myopic and age-related halos which are mainly associated with β-zone type atrophy.
BSCR patients are believed to be at higher risk for glaucomatous optic neuropathy as steroids are commonly used in the management of noninfectious uveitis leading to elevation of IOP.Citation34 In addition, hypoperfusion of the optic nerve head also occurs as a result of decreased choroidal blood flow in BSCR patients.Citation35 Foci of inflammatory cells observed in prelaminar optic nerve may also contribute to the optic nerve head damage.Citation36 Clinicians should be aware of these peripapillary halos in BSCR patients with glaucoma as it is harder to properly identify the optic disc margins in such patients and retinal nerve fiber layer (RNFL) thickness analysis can be misleading is patients with prominent PPA and PPH, thereby leading to overestimation of glaucomatous damage.Citation37
We postulate that the observed RPE-Bruch’s complex changes are related to mechanical factors during inflammation of the optic nerve head. During the stage of active inflammation, all retinal layers including RPE-Bruch’s complex are elevated and swollen. RPE, Bruch’s membrane, and the optic nerve sheath are thought to be the load-bearing structures of the optic nerve head.Citation38
After edema is resorbed and inflammation subsides, the mechanical stretch that was exerted on RPE-Bruch’s membrane causes atrophy, thinning, and breaks in the peripapillary area while the remainder of retinal layers return to their original position. These changes may be explained by the embryological origins of retinal pigment epithelium and the neurosensory retina as RPE develops from the outer layer of the optic cup, whereas the neural retina originates from the inner layer of the optic cup.
Alternatively, these RPE changes may represent a direct consequence of the birdshot inflammatory process as they predominantly affect outer retinal layers. Once the peripapillary inflammation resolves, a peripapillary halo develops, affecting mostly the RPE-Bruch’s layer, as shown on the B-scans of the SS-OCT. The peripapillary halo could be a result of previous episodes of papillitis in asymptomatic patients and serve as a clinical indicator of previous attacks.
This study has limitations, mainly because of the small number of patients with chronic inflammatory disease at a single tertiary center in addition to the cross-sectional design of the study. But, as the aim of this study was to describe the microstructure of a common clinical observation, a small number of cases is only needed to be imaged to describe the same anatomical findings.
Prospective studies including a larger number of BSCR patients with imaging studies at baseline, active, and convalescent phases will be needed to confirm and clarify the significance of our findings, but this would be difficult because of the low incidence of BSCR and most patients with baseline imaging may never develop PPH.
Additionally, it is worth mentioning that the peripapillary halos are not only described in patients with a history of BSCR but also in other inflammatory conditions involving the optic nerve head as in serpiginous choroiditis and Vogt-Koyanagi-Harada (VKH) disease.Citation39,Citation40
Conclusion
Peripapillary halo in BSCR is a frequent clinical finding of the optic nerve head and the in-vivo anatomy and possible clinical significance of this halo are not fully understood. In this study we observed that peripapillary halo is formed mainly by a circumferential alpha zone of peripapillary atrophy. We demonstrate with in vivo SS-OCT histological analysis that the histological change of RPE-Bruch’s membrane is secondary to thinning and interruption of peripapillary RPE and Bruch’s membrane with window defects associated with hyperreflectivity to the underlying choroid.
We hypothesize circumferential PPH (versus crescentic PPA) is the result of swelling of the optic nerve head secondary to BSCR papillitis secondary to stretching, thinning, and tearing of the retinal layers attached to and surrounding the border tissue of Elschnig which is believed to be the optic disc border. After resolution of optic nerve head edema, the retinal layers no longer are fully attached to the disc border and have an atrophic circumferential ring around the optic nerve head (a halo) that is PPH.
Through examination and imaging of the optic nerve head when retinal inflammatory disease is present, the presence of PPH may offer insight and possibly serve as a clinical indicator of previous episodes of papillitis in BSCR patients.
Abbreviations
PPH, peripapillary halo; BSCR, birdshot chorioretinopathy; SS-OCT, swept-source optical coherence tomography; PPA, peripapillary atrophy; RPE, retinal pigment epithelium; SD-OCT, spectral-domain optical coherence tomography; IOP, intraocular pressure; FFA, fundus fluorescein angiography; ICGA, indocyanine green angiography; FAF, fundus autofluorescence; RNFL, retinal nerve fiber layer.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Jonas JB, Fernández MC, Naumann GO. Glaucomatous parapapillary atrophy: occurrence and correlations. Arch Ophthalmol. 1992;110(2):214–222.
- Xu L, Li Y, Wang S, Wang Y, Wang Y, Jonas JB. Characteristics of highly myopic eyes: the Beijing Eye Study. Ophthalmology. 2007;114(1):121–126. doi:10.1016/j.ophtha.2006.05.071
- See JL, Nicolela MT, Chauhan BC. Rates of neuroretinal rim and peripapillary atrophy area change: a comparative study of glaucoma patients and normal controls. Ophthalmology. 2009;116(5):840–847. doi:10.1016/j.ophtha.2008.12.005
- Ryan SJ, Maumenee AE. Birdshot retinochoroidopathy. Am J Ophthalmol. 1980;89(1):31–45. doi:10.1016/0002-9394(80)90226-3
- Silpa-Archa S, Cao JH, Boonsopon S, Lee J, Preble JM, Foster CS. Birdshot retinochoroidopathy: differences in clinical characteristics between patients with early and late age of onset. Ocul Immunol Inflamm. 2017;25(5):594–600. doi:10.3109/09273948.2016.1158278
- Nussenblatt RB, Mittal KK, Ryan S, Green WR, Maumenee AE. Birdshot retinochoroidopathy associated with HLA-A29 antigen and immune responsiveness to retinal S-antigen. Am J Ophthalmol. 1982;94(2):147–158. doi:10.1016/0002-9394(82)90069-1
- Brézin AP, Monnet D, Cohen JH, Levinson RD. HLA-A29 and birdshot chorioretinopathy. Ocul Immunol Inflamm. 2011;19(6):397–400. doi:10.3109/09273948.2011.619295
- Rothova A, Berendschot TT, Probst K, van Kooij B, Baarsma GS. Birdshot chorioretinopathy: long-term manifestations and visual prognosis. Ophthalmology. 2004;111(5):954–959. doi:10.1016/j.ophtha.2003.09.031
- Shah KH, Levinson RD, Yu F, et al. Birdshot chorioretinopathy. Surv Ophthalmol. 2005;50(6):519–541. doi:10.1016/j.survophthal.2005.08.004
- Adhi M, Duker JS. Optical coherence tomography–current and future applications. Curr Opin Ophthalmol. 2013;24(3):213. doi:10.1097/ICU.0b013e32835f8bf8
- Copete S, Flores-Moreno I, Montero JA, Duker JS, Ruiz-Moreno JM. Direct comparison of spectral-domain and swept-source OCT in the measurement of choroidal thickness in normal eyes. Br J Ophthalmol. 2014;98(3):334–338. doi:10.1136/bjophthalmol-2013-303904
- Liu X, Khodeiry MM, Lin D, et al. The association of acute cerebrospinal fluid pressure reduction with choroidal thickness. Curr Eye Res;2021. 1–8. doi:10.1080/02713683.2021.1874024
- Jonas JB. Clinical implications of peripapillary atrophy in glaucoma. Curr Opin Ophthalmol. 2005;16(2):84–88. doi:10.1097/01.icu.0000156135.20570.30
- Jonas JB, Martus P, Horn FK, Jünemann A, Korth M, Budde WM. Predictive factors of the optic nerve head for development or progression of glaucomatous visual field loss. Invest Ophthalmol Vis Sci. 2004;45(8):2613–2618. doi:10.1167/iovs.03-1274
- Spaide RF. Age-related choroidal atrophy. Am J Ophthalmol. 2009;147(5):801–810. doi:10.1016/j.ajo.2008.12.010
- Jonas JB, Jonas SB, Jonas RA, et al. Parapapillary atrophy: histological gamma zone and delta zone. PLoS One. 2012;7(10):e47237. doi:10.1371/journal.pone.0047237
- Kim HR, Weinreb RN, Zangwill LM, Suh MH. Characteristics of focal gamma zone parapapillary atrophy. Invest Ophthalmol Vis Sci. 2020;61(3):17. doi:10.1167/iovs.61.3.17
- Rockwood EJ, Anderson DR. Acquired peripapillary changes and progression in glaucoma. Graefes Arch Clin Exp Ophthalmol. 1988;226(6):510–515. doi:10.1007/BF02169197
- Levinson RD, Brezin A, Rothova A, Accorinti M, Holland GN. Research criteria for the diagnosis of birdshot chorioretinopathy: results of an international consensus conference. Am J Ophthalmol. 2006;141(1):185–187. doi:10.1016/j.ajo.2005.08.025
- Elschnig A. Das Colobom am Sehnerveneintritte und der Conus nach unten. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1900;51(3):391–430. doi:10.1007/BF01938806
- Uchida H, Ugurlu S, Caprioli J. Increasing peripapillary atrophy is associated with progressive glaucoma. Ophthalmology. 1998;105(8):1541–1545. doi:10.1016/S0161-6420(98)98044-7
- Primrose J. Early signs of the glaucomatous disc. Br J Ophthalmol. 1971;55(12):820. doi:10.1136/bjo.55.12.820
- Rath E, Rehany U, Linn S, Rumelt S. Correlation between optic disc atrophy and aetiology: anterior ischaemic optic neuropathy vs optic neuritis. Eye. 2003;17(9):1019–1024. doi:10.1038/sj.eye.6700691
- Brasil OFM, Brasil MVOM, Japiassú RM, et al. Fundus changes evaluation in degenerative myopia. Arq Bras Oftalmol. 2006;69(2):203–206. doi:10.1590/S0004-27492006000200013
- Nonaka A, Hangai M, Akagi T, et al. Biometric features of peripapillary atrophy beta in eyes with high myopia. Invest Ophthalmol Vis Sci. 2011;52(9):6706–6713. doi:10.1167/iovs.11-7580
- Chui TY, Zhong Z, Burns SA. The relationship between peripapillary crescent and axial length: implications for differential eye growth. Vision Res. 2011;51(19):2132–2138. doi:10.1016/j.visres.2011.08.008
- Curcio CA, Saunders PL, Younger PW, Malek G. Peripapillary chorioretinal atrophy: bruch’s membrane changes and photoreceptor loss. Ophthalmology. 2000;107(2):334–343. doi:10.1016/S0161-6420(99)00037-8
- Sayed MS, Margolis M, Chen JL, Gregori G, Lee RK. Shields gray crescents masquerading as glaucomatous cupping of the optic nerve head. Ophthalmol Glaucoma. 2018;1(2):99–107. doi:10.1016/j.ogla.2018.08.001
- Torres LA, Sharpe GP, Vianna JR, Nicolela MT, Chauhan BC. Anatomical features of gray crescent. JAMA Ophthalmol. 2018;136(12):1419–1420. doi:10.1001/jamaophthalmol.2018.3403
- Davies IJ, Muir KW, Halabis JA, Stinnett SS, Allingham RR, Shields MB. Gray optic disc crescent: evaluation of anatomic correlate by spectral-domain OCT. Ophthalmol Glaucoma. 2019;2(2):120–125. doi:10.1016/j.ogla.2018.11.005
- Garcia-Garcia O, Jordan-Cumplido S, Subira-Gonzalez O, Garcia-Bru P, Arias L, Caminal-Mitjana JM. Feasibility of swept-source OCT for active birdshot chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255(8):1493–1502. doi:10.1007/s00417-017-3655-4
- Teussink MM, Huis In Het Veld PI, de Vries LA, Hoyng CB, Klevering BJ, Theelen T. Multimodal imaging of the disease progression of birdshot chorioretinopathy. Acta Ophthalmol (Copenh). 2016;94(8):815–823. doi:10.1111/aos.13114
- Thorne JE, Jabs DA, Peters GB, Hair D, Dunn JP, Kempen JH. Birdshot retinochoroidopathy: ocular complications and visual impairment. Am J Ophthalmol. 2005;140(1):45. e41–45. e48. doi:10.1016/j.ajo.2005.01.035
- Rush RB, Goldstein DA, Callanan DG, Meghpara B, Feuer WJ, Davis JL. Outcomes of birdshot chorioretinopathy treated with an intravitreal sustained-release fluocinolone acetonide–containing device. Am J Ophthalmol. 2011;151(4):630–636. doi:10.1016/j.ajo.2010.10.005
- Talisa E, Bonini Filho MA, Adhi M, Duker JS. Retinal and choroidal vasculature in birdshot chorioretinopathy analyzed using spectral domain optical coherence tomography angiography. Retina. 2015;35(11):2392–2399. doi:10.1097/IAE.0000000000000744
- Gaudio P, Kaye D, Crawford JB. Histopathology of birdshot retinochoroidopathy. Br J Ophthalmol. 2002;86(12):1439–1441. doi:10.1136/bjo.86.12.1439
- Chong GT, Lee RK. Glaucoma versus red disease: imaging and glaucoma diagnosis. Curr Opin Ophthalmol. 2012;23(2):79–88. doi:10.1097/ICU.0b013e32834ff431
- Burgoyne CF, Downs JC, Bellezza AJ, Suh J-KF, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24(1):39–73. doi:10.1016/j.preteyeres.2004.06.001
- Khanamiri HN, Rao NA. Serpiginous choroiditis and infectious multifocal serpiginoid choroiditis. Surv Ophthalmol. 2013;58(3):203–232. doi:10.1016/j.survophthal.2012.08.008
- Lavezzo MM, Sakata VM, Morita C, et al. Vogt-Koyanagi-Harada disease: review of a rare autoimmune disease targeting antigens of melanocytes. Orphanet J Rare Dis. 2016;11(1):1–21. doi:10.1186/s13023-016-0412-4
