Abstract
Purpose
Ocular adverse events have been reported in association with dupilumab, a monoclonal antibody to treat allergic diseases including atopic dermatitis (AD). We describe clinical findings and treatment of dupilumab-related ocular complications.
Patients and Methods
Retrospective study of 19 dupilumab-treated AD patients seen for a new ocular complaint. Primary outcomes were specific ocular exam findings (conjunctival injection, corneal fluorescein staining, blepharitis, meibomian gland dysfunction (MGD)), treatments, and follow-up.
Results
Nineteen dupilumab-treated AD patients were included. Median age was 47 years (range 18–73). Over half were women (11/19) and majority were Caucasian (13/19). Symptom onset occurred at a mean of 99 days (range 23–520 days) from first dupilumab dose. The most common symptoms were redness (63%), tearing (47%), and pruritus (37%). Most common ocular findings were conjunctival injection (75%) and corneal staining (60%). Blepharitis was seen in about a third (30%), and 25% had MGD. Initially, 10% were observed without treatment, while 15% were treated with artificial tears alone. Other treatments included antihistamine drops (20%) and steroid drops alone (15%). In 40% of patients, a combination of steroids and various other topical eye drops were prescribed. Eighty-four percent (16/19) of patients were seen for follow-up. Steroid drops were required at follow-up in 3 out of 4 patients initially treated with antihistamines alone and in two-thirds of patients initially treated with artificial tears only. Mean follow-up period was 88 days (range 5–369). Dupilumab was discontinued in 31.5% (6/19) of patients; of those who discontinued, 3 restarted it later.
Conclusion
Conjunctival injection was the most frequent dupilumab-related ocular symptom and most common exam finding followed by corneal staining. Most patients initially treated with antihistamine drops or artificial tears alone subsequently required steroid drops to control symptoms. Some patients who discontinued dupilumab restarted the medication after achieving adequate control of ocular symptoms.
Introduction
Since 2017, dupilumab has been approved by the Food and Drug Administration (FDA) to treat adults with moderate-to-severe atopic dermatitis (AD) that is refractory to topical therapies. Dupilumab is an IgG-4 human monoclonal antibody that inhibits interleukin-4 and interleukin-13 activity by binding to the interleukin-4 receptor subunit α (IL-4Rα). These cytokines are thought to play a role in the pathophysiology of atopic and allergic diseases. Atopic dermatitis is diagnosed based on physical exam findings of circumscribed eczematous skin lesions that commonly involve the face and extremities and flexural regions. It is a heterogeneous disease that can be persistent or relapsing, with different morphological eruptions and regional manifestations in the body in different demographic groups.Citation1 For our purposes, it is important to note that conjunctivitis and eyelid dermatitis are a common manifestation and part of the 23 clinical signs and symptoms of the disease as defined by Hanifin and Rajka and now used as the diagnostic criteria for AD.Citation2 It can lead to chronic periocular inflammation with resultant damage that can include eyelid malpositioning, keratitis, cicatricial conjunctivitis, as well as cataracts and glaucoma from long-term use of steroids.Citation3
In randomized clinical trials (RCT), dupilumab use was associated with a greater incidence of conjunctivitis compared to placebo in patients with AD.Citation4,Citation5 It has also been found to have higher relative risk of developing conjunctivitis in AD patients compared to other immunomodulatory treatments for AD, such as methotrexate, cyclosporine, and mycophenolate mofetil.Citation6 Other ocular adverse effects reported in association with this medication include blepharitis, herpes simplex keratitis and herpes zoster ophthalmicus,Citation7 and dry eye disease. These clinical trials have provided valuable information about the incidence and risk factors for dupilumab-associated conjunctivitis; however, they were not designed to study the particular presentation and management of this entity.Citation4,Citation8–Citation11 For example, pre-and post-treatment evaluation of ocular symptoms was not done.Citation9,Citation10 In addition, patients were not routinely referred to ophthalmologists as standard of care for evaluation and management in these trials. Moreover, follow-up of dupilumab associated ocular surface pathologies and response to various treatment modalities were not explicitly monitored or reported except where they led to cessation of treatment. Finally, ocular adverse events described were reported by trial investigators who were typically dermatologists or allergists.
This lacuna in our understanding is now beginning to be filled through case reports describing various ocular findings,Citation12–Citation15 as well as case series reporting on dupilumab-associated conjunctivitis.Citation16–Citation20 Typical symptoms are similar to those seen in patients with allergic conjunctivitis, namely ocular surface irritation manifesting as itching, tearing, foreign body sensation, and occasional decrease in vision. The spectrum of findings reported is broad ranging from mild conjunctivitis to severe cicatrizing blepharoconjunctivitis.Citation12 However, given the small numbers of patients in these individual reports, there is still a need to understand the full spectrum of ocular conditions and clinical presentation associated with use of this medication, especially as some case series describe dupilumab-associated conjunctivitis in patients who have pre-existing meibomian gland disease, blepharitis, allergic conjunctivitis, and other syndromes associated with dry eye disease,Citation13,Citation18,Citation19 while many others describe new onset conjunctivitis after initiation on the monoclonal antibody.Citation16 Some publications have described eyelid pathologies,Citation13 while others have focused broadly on ocular surface diseases, or focused specifically on conjunctivitis.Citation5,Citation12,Citation21 All studies have disparate and sometimes conflicting data on incidence of pathologies and response to treatment, though steroids generally have shown a positive therapeutic response. The literature on dupilumab-associated ocular adverse events almost exclusively describes pathologies occurring in patients undergoing treatment with dupilumab for atopic dermatitis, not asthma or nasal polyps which are other clinically approved indications for dupilumab therapy.Citation5,Citation14,Citation18 This may be due to the specific pathophysiologic mechanism underlying AD, wherein structural deficiencies due to downregulation of proteins and biomarkers involved in the integrity of the skin barrier as well as hydration, lipid metabolism, and immune homeostasis.Citation22 Such biological functions are also important in ocular surface homeostasis and maintenance of a healthy tear film, and are disrupted in dry eye disease.Citation23
Notably, none of the case series are patients initially presenting to ophthalmology practices, but are instead patients being followed in dermatology or allergy practices for AD who were then referred for eye examination based on the investigators’ own assessment of the severity of conjunctivitis and need for ophthalmologic evaluation.Citation17,Citation19–Citation21 There is also presently a lack of consensus on the optimal treatment for dupilumab-related ocular complications in the ophthalmology literature given the absence of data on the relative efficacy of various modalities, ie, lubricants, antihistamines, calcineurin inhibitors, topical steroidal and non-steroidal anti-inflammatories.
Here we report the largest case series to date of patients presenting initially to an academic ophthalmology practice with ocular or visual complaints and underlying AD actively being treated with dupilumab. We delineate the ophthalmologic exam findings in these patients, report any underlying history of ophthalmologic conditions, detail the treatment approaches, and describe their follow-up and response to treatment. This will enable ophthalmologists to better understand the presentation of undifferentiated dupilumab-associated ocular disease and guide management.
Methods
Our study was a retrospective case series of 19 dupilumab-treated AD patients seen for a new ocular complaint in an academic ophthalmology practice. Primary outcomes assessed included the presence of specific ocular exam findings, specifically conjunctival injection, eyelid dermatitis, corneal abnormalities blepharitis, and meibomian gland dysfunction (MGD), and treatments prescribed at their initial visit and follow-up visit. The patients were examined by ophthalmologists subspecializing in management of corneal and ocular surface diseases.
Subjects were selected for inclusion within the study through the use of National Drug Code identifiers to identify patients on dupilumab who had been seen at the University of Pennsylvania Dry Eye Center as a part of standard of care during the study period from January 1, 2017 to December 31, 2020. The study was approved by the Institutional Review Board of the University of Pennsylvania.
Results
A total of 19 dupilumab-treated AD patients were included in our analysis. Demographic characteristics of the cohort are described in . The median age of the cohort was 47 years (range 18–73 years), with the majority being female (58%, 11/19), and Caucasian (68%, 13/19).
Table 1 Demographic Characteristics
Clinical characteristics of patients included in the analysis are outlined in . Most patients (11/19) had no prior history of ocular surface pathology or ocular surgery. However, in those that had pre-existing ocular conditions, 38% (3/8) had a history of MGD. Two other patients had underlying conditions with corneal manifestations, one with history of herpes simplex keratitis, and another with Sjogren’s syndrome. One patient in our case series had a history of bilateral Laser-Assisted In Situ Keratomileusis (LASIK) surgery.
Table 2 Clinical Characteristics, Treatment, and Follow-Up
Mean time to ocular symptom onset was 99 days from first dupilumab dose (range 23 to 520 days). Redness (63%), tearing (47%), and itching (37%) were the most common reported ocular symptoms; other complaints included irritation, blurred vision, photophobia, discharge, foreign body sensation, swelling.
The most common ocular findings were conjunctival injection (75%) and corneal staining (60%). Half of patients had a papillary or follicular reaction of the conjunctiva (). Conjunctival injection was typically diffuse; however, in one case, there was a predilection for the superior conjunctiva, which was initially difficult to distinguish from superior limbic keratoconjunctivitis versus episcleritis with limbal follicles (). Corneal findings were predominantly in the form of punctate epithelial erosions, however, 2 patients presented with marginal stromal infiltrates (). Eyelid pathologies were also frequently present, with blepharitis seen in 30% of patients and MGD in 25% of the total cohort.
Figure 1 (A and B) Slit lamp photography demonstrates thickening of lid margins, follicular reaction, and diffuse conjunctival injection. (C and D) With fluorescein instillation, negative staining reveals bulbar conjunctival follicular elevation.
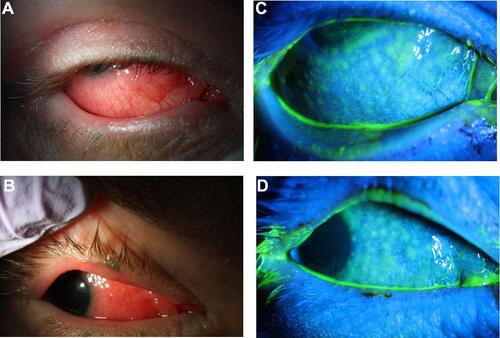
Figure 2 Right eye (left) and left eye (right) slit lamp photographs demonstrating superior conjunctival injection and limbal follicles.
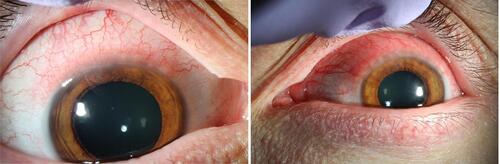
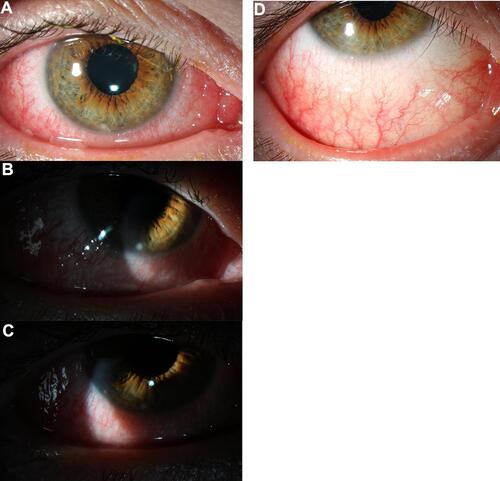
Figure 3 (A–C) Slit lamp photographs demonstrate diffuse conjunctival injection and 2 inferior marginal stromal infiltrates, at 6 o’clock and 8 o’clock. (D) After 1 week of treatment with topical fluorometholone 0.1% there is resolution of marginal stromal infiltrates.
After the initial visit, 10% were observed without treatment, whereas 15% patients were treated with artificial tears (AT) alone. Other treatments initiated included antihistamine drops (20%), or steroid drops alone (15%). In the remaining 40% of patients, a combination of topical steroids and other topical treatments were initiated, which included mast cell stabilizers, calcineurin inhibitors and anti-inflammatories (lifitegrast or cyclosporine). Combination steroid-antibiotic drops or ointments were used primarily in cases of MGD or blepharitis, or where there was severe conjunctival injection with a follicular or papillary reaction.
Out of the 19 patients, 16 were seen for follow-up. The mean follow-up period was 89 days (range 5–369). Among patients seen for follow-up who were treated with conservative measures initially (ATs and antihistamines), the majority required topical steroids in some form. These were often supplemented with topical calcineurin inhibitors and anti-inflammatory drops such as cyclosporine. Dupilumab treatment was empirically discontinued in 6 out of 19 patients; of the 6 who discontinued, 3 were able to restart treatment without recurrence of symptoms during the study period.
Discussion
In our study of AD patients on dupilumab presenting with visual changes or ocular discomfort to an ophthalmology practice, there were high incidences of conjunctival injection with a papillary or follicular reaction, corneal punctate epithelial erosions, and MGD/blepharitis. Patients typically presented with symptoms of redness, pruritus, tearing; severe cases also had blurred vision and light sensitivity. Our findings confirmed data from the limited literature noting the presence of pre-existing meibomian gland disease and ocular surface disease in some but not all patients. Interestingly, two cases presented with marginal stromal infiltrates in one eye—not hitherto described as a finding in dupilumab-associated ocular surface disease—though symptoms of conjunctivitis were present in both eyes.
As compared to most other similar case series,Citation14,Citation17 initial treatment in our study was typically conservative with observation, artificial tears, and antihistamine drops. This enables us to show that in the majority of cases, conservative treatment failed to control symptoms, and topical steroid with or without anti-inflammatory treatments was invariably needed to control the ocular side effects. This stands in contrast to the finding by Rial et al, however in their case series, artificial tears were started in AD patients who were asymptomatic, and they started using them prior to the initiation of dupilumab.Citation18 In our case series, we found that a higher number of patients failed over-the-counter treatments and required subsequent topical prescription, highlighting the need for closer ophthalmologic evaluation of AD patients on dupilumab. This confirms the findings of Achten et al who found that many patients remained symptomatic after treatment even with the use of anti-inflammatory eye drops, though only a minority worsened.Citation19
Relative to other case series,Citation7,Citation16–Citation19 ours is unique in that these patients presented initially to an ophthalmology practice with undifferentiated visual changes or ocular surface discomfort and were not necessarily referred for what may have likely presumed to be a dupilumab-associated adverse effect. Thus, a temporal relationship with dupilumab use was not immediately evident or expected. This may be a better reflection of how patients may present to ophthalmology clinics. This is notable, as study subjects in prior similar-sized case series were initially seen in dermatology or allergy practices, with a subset referred for ophthalmologic evaluation, and criteria for referral and for initiation of various therapies was ambiguous.Citation16,Citation17,Citation19,Citation20
In our experience, patients with dupilumab-associated ocular issues may have underlying MGD, which could potentially worsen. This suggests that patients with severe atopic dermatitis or a history of conjunctivitis should be evaluated by an ophthalmologist before dupilumab initiation to treat underlying predisposing conditions for ocular surface inflammation. Interestingly, the severity of conjunctivitis in patients with atopic dermatitis has been found to be correlated with the severity of their dermatitis, suggesting that these patients may be at highest risk.Citation24 The continued use of dupilumab must be evaluated jointly by the ophthalmologist and dermatologist or allergist based on the ocular risk versus systemic benefit. Our finding that often topical anti-inflammatory therapies are required would demand closer monitoring by an ophthalmologist.
The various modalities of treatment for dupilumab-associated ocular surface disease that have been reported in the literature include topical steroids, cyclosporine, lifitegrast, tacrolimus, and oral prednisone.Citation15,Citation25,Citation26 We found success with topical therapies alone, and our patients did not require oral steroids. Once inflammation is controlled by topical steroids, changing to a less potent steroid, such as loteprednol, or topical steroid-sparing agent, such as cyclosporine, is reasonable.
All physicians should be aware of the possibility of significant ocular surface adverse effects associated with dupilumab treatment, which can become debilitating. Given the findings seen in our case series, we hypothesize that dupilumab may initiate or exacerbate ocular surface inflammation, due to underlying predisposition to ocular surface inflammation or due to atopy exacerbated by a drug-induced reaction. Supporting this hypothesis are findings from clinical trials of patients with underlying AD, where dupilumab use was significantly associated with higher incidence of conjunctivitis compared to placebo.Citation4,Citation5 However, studies using dupilumab for the treatment of conditions other than atopic dermatitis, namely asthma, chronic rhinosinusitis with nasal polyps, and eosinophilic esophagitis, showed no difference in the rate of conjunctivitis between treatment and placebo.Citation27–Citation30 Thus, atopic dermatitis may itself be a risk factor associated with the development of conjunctivitis in patients treated with dupilumab, in comparison to patients treated with dupilumab for other conditions. Several hypotheses have been proposed for mechanisms driving conjunctivitis in dupilumab-treated patients with AD, including alterations in cytokine activity leading to increased presence and proliferation of Demodex mites increased OX40 ligand activity, eosinophilia, disruption of immune responses from conjunctival‐associated lymphoid tissue, decreased IL-13 related mucus production, and IL-13 related reduction of conjunctival goblet cells.Citation24,Citation31–Citation33
In the LIBERTY AD CAFE study of dupilumab for the treatment of atopic dermatitis, the duration-adjusted incidence rates for the development of conjunctivitis with dupilumab compared with placebo were 81.13 per 100 patient years versus 38.94 patients per 100 patient years, respectively.Citation10 As reported in a meta-analysis of conjunctivitis in clinical trials of dupilumab, the incidence and hazard ratio of conjunctivitis for dupilumab monotherapy versus placebo was found to be 8.6% versus 2.1% (hazard ratio 4.13, 95% CI 2.21–7.72).Citation5 Risk factors that have been identified for developing conjunctivitis are severe atopic dermatitis at baseline, a history of conjunctivitis, and certain biomarkers (thymus and activation-regulated chemokine, IgE, and eosinophils).Citation5
Conclusions
In our study, we attempted to better characterize the specific changes of the ocular surface that can be observed with slit lamp examination in AD patients. We also characterize pre-existing ocular pathologies and find a sizeable number of patients with underlying dry eye disease, whether evaporative or aqueous-deficient, or eyelid pathologies that put them at risk of developing ocular surface issues. Our case series had 1 patient with herpes keratitis, which is notable as rare cases of ocular herpes simplex were reported in dupilumab clinical trials, albeit with both placebo and dupilumab use. We show empiric treatment response from more than 1 follow-up visit.
In our practice, we treated patients initially with conservative therapies, typically over-the-counter artificial tears and antihistamines, prior to initiating prescription-strength topical anti-inflammatories. Thus, we are able provide data demonstrating that patients presenting to eye clinic tend to fail conservative treatment with non-prescription lubricants and over-the-counter antihistamines, in contrast to prior reports.Citation18 Moreover, we report findings not previously described in the literature, including marginal keratitis and possible episcleritis.
In summary, the ocular manifestations of dupilumab-associated conjunctivitis can be severe and will often require immunosuppressive topical therapies. Identification of patients at higher risk is crucial and ophthalmologists should be aware of this important medication-induced condition and how to manage ocular complications in these patients.
Patient Consent
Written consent to publish this case has not been obtained. This report does not contain any personal identifying information. An IRB exemption was granted for this study by the University of Pennsylvania Institutional Review Board, IRB Protocol # 827060, on the grounds of involving no more than minimal risk to patients. All research maintained patient data confidentiality and was performed in accordance with the principles stated in the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Acknowledgment
The abstract of this paper was presented at the Association for Research in Vision and Ophthalmology 2021 Annual Meeting as a poster presentation with interim findings. The poster’s abstract was published in the June 2021 Abstract Issue of IOVS: https://iovs.arvojournals.org/article.aspx?articleid=2774801.
Disclosure
Mina Massaro-Giordano reports being on advisory boards for Dompe and Lynthera; non-financial support from Lynthera, being an investor in PRN neutraceuticals (owns minor stock) and being a consultant for Lynthera and Claris, outside the submitted work. The authors report no other potential conflicts of interest for this work.
Additional information
Funding
References
- Nettis E, Ortoncelli M, Pellacani G, et al. A multicenter study on the prevalence of clinical patterns and clinical phenotypes in adult atopic dermatitis. J Investig Allergol Clin Immunol. 2020;30:448–450. doi:10.18176/jiaci.0519
- Ständer S, Ropper AH. Atopic dermatitis. N Engl J Med.2021;384:1136–1143. doi:10.1056/NEJMra2023911
- Girolomoni G, de Bruin-weller M, Aoki V, et al. Nomenclature and clinical phenotypes of atopic dermatitis. Ther Adv Chronic Dis. 2021;12:204062232110029. doi:10.1177/20406223211002979
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two Phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–2348. doi:10.1056/NEJMoa1610020
- Akinlade B, Guttman-Yassky E, de Bruin-weller M, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019;181:459–473. doi:10.1111/bjd.17869
- Schneeweiss MC, Kim SC, Wyss R, et al. Dupilumab and the risk of conjunctivitis and serious infection in patients with atopic dermatitis: a propensity score-matched cohort study. J Am Acad Dermatol. 2021;84:300–311. doi:10.1016/j.jaad.2020.09.084
- Ivert LU, Wahlgren C-F, Ivert L, et al. Eye complications during dupilumab treatment for severe atopic dermatitis. Acta Derm Venereol. 2019;99:375–378. doi:10.2340/00015555-3121
- Thaçi D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging Phase 2b trial. Lancet Lond Engl. 2016;387:40–52. doi:10.1016/S0140-6736(15)00388-8
- Blauvelt A, de Bruin-weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet Lond Engl. 2017;389:2287–2303. doi:10.1016/S0140-6736(17)31191-1
- de Bruin-weller M, Thaçi D, Smith CH, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized Phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol. 2018;178:1083–1101. doi:10.1111/bjd.16156
- Worm M, Simpson EL, Thaçi D, et al. Efficacy and safety of multiple dupilumab dose regimens after initial successful treatment in patients with atopic dermatitis: a Randomized Clinical Trial. JAMA Dermatol. 2020;156:131–143. doi:10.1001/jamadermatol.2019.3617
- Liberman P, Shifera AS, Berkenstock M. Dupilumab-associated conjunctivitis in patients with atopic dermatitis. Cornea. 2020;39:784–786. doi:10.1097/ICO.0000000000002262
- Paulose SA, Sherman SW, Dagi Glass LR, Suh LH. Dupilumab-associated blepharoconjunctivitis. Am J Ophthalmol Case Rep. 2019;16:100550. doi:10.1016/j.ajoc.2019.100550
- Shen E, Xie K, Jwo K, et al. Dupilumab-induced follicular conjunctivitis. Ocul Immunol Inflamm. 2019;27:1339–1341. doi:10.1080/09273948.2018.1533567
- Roca-Ginés J, Rahhal-Ortuño M, Torres-Navarro I, et al. Cyclosporine 0.1% (Ikervis®) treatment in steroid-dependent dupilumab-associated conjunctivitis. Arch Soc Espanola Oftalmol. 2019;94:396–399. doi:10.1016/j.oftal.2019.04.013
- Wollenberg A, Ariens L, Thurau S, et al. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J Allergy Clin Immunol Pract. 2018;6:1778–1780.e1. doi:10.1016/j.jaip.2018.01.034
- Bohner A, Topham C, Strunck J, et al. Dupilumab-associated ocular surface disease: clinical characteristics, treatment, and follow-up. Cornea. 2021;40:584–589. doi:10.1097/ICO.0000000000002461
- Rial MJ, Barroso B, Rodríguez-Bermejo C, Sastre J. Letter regarding “Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J Allergy Clin Immunol Pract. 2019;7:753. doi:10.1016/j.jaip.2018.10.055
- Achten R, Bakker D, Ariens L, et al. Long-term follow-up and treatment outcomes of conjunctivitis during dupilumab treatment in patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol Pract. 2021;9:1389–1392.e2. doi:10.1016/j.jaip.2020.09.042
- Popiela MZ, Barbara R, Turnbull AMJ, et al. Dupilumab-associated ocular surface disease: presentation, management and long-term sequelae. Eye. 2021;35:3277–3284. doi:10.1038/s41433-020-01379-9
- Nettis E, Bonzano L, Patella V, et al. Dupilumab-associated conjunctivitis in patients with atopic dermatitis: a multicenter real-life experience. J Investig Allergol Clin Immunol. 2020;30:201–204. doi:10.18176/jiaci.0481
- Morelli P, Gaspari M, Gabriele C, et al. Proteomic analysis from skin swabs reveals a new set of proteins identifying skin impairment in atopic dermatitis. Exp Dermatol. 2021;30:811–819. doi:10.1111/exd.14276
- Pflugfelder SC. Tear dysfunction and the Cornea: LXVIII Edward Jackson memorial lecture. Am J Ophthalmol. 2011;152:900–909.e1. doi:10.1016/j.ajo.2011.08.023
- Treister AD, Kraff-Cooper C, Lio PA. Risk factors for dupilumab-associated conjunctivitis in patients with atopic dermatitis. JAMA Dermatol. 2018;154:1208–1211. doi:10.1001/jamadermatol.2018.2690
- Zirwas MJ, Wulff K, Beckman K. Lifitegrast add-on treatment for dupilumab-induced ocular surface disease (DIOSD): a novel case report. JAAD Case Rep. 2019;5:34–36. doi:10.1016/j.jdcr.2018.10.016
- Aszodi N, Thurau S, Seegräber M, et al. Management of dupilumab-associated conjunctivitis in atopic dermatitis. JDDG. 2019;17:488–491.
- Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet Lond Engl. 2016;388:31–44. doi:10.1016/S0140-6736(16)30307-5
- Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378:2475–2485. doi:10.1056/NEJMoa1804093
- Bachert C, Mannent L, Naclerio RM, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a Randomized Clinical Trial. JAMA. 2016;315:469–479. doi:10.1001/jama.2015.19330
- Hirano I, Dellon ES, Hamilton JD, et al. Efficacy of dupilumab in a Phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterology. 2020;158:111–122.e10. doi:10.1053/j.gastro.2019.09.042
- Thyssen JP. Could conjunctivitis in patients with atopic dermatitis treated with dupilumab be caused by colonization with Demodex and increased interleukin-17 levels? Br J Dermatol. 2018;178:1220. doi:10.1111/bjd.16330
- Thyssen JP, de Bruin-weller MS, Paller AS, et al. Conjunctivitis in atopic dermatitis patients with and without dupilumab therapy - international eczema council survey and opinion. JEADV. 2019;33:1224–1231. doi:10.1111/jdv.15608
- Bakker DS, Ariens LFM, van Luijk C, et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. 2019;180:1248–1249. doi:10.1111/bjd.17538
