Abstract
Background:
The purpose of this study was to investigate pathological changes of the corneal cell layer in patients with map-dot-fingerprint (epithelial basement membrane) dystrophy by in vivo laser corneal confocal microscopy.
Methods:
Two patients were evaluated using a cornea-specific in vivo laser scanning confocal microscope (Heidelberg Retina Tomograph 2 Rostock Cornea Module, HRT 2-RCM). The affected corneal areas of both patients were examined. Image analysis was performed to identify corneal epithelial and stromal deposits correlated with this dystrophy.
Results:
Variously shaped (linear, multilaminar, curvilinear, ring-shape, geographic) highly reflective materials were observed in the “map” area, mainly in the basal epithelial cell layer. In “fingerprint” lesions, multiple linear and curvilinear hyporeflective lines were observed. Additionally, in the affected corneas, infiltration of possible Langerhans cells and other inflammatory cells was observed as highly reflective Langerhans cell-like or dot images. Finally, needle-shaped materials were observed in one patient.
Conclusion:
HRT 2-RCM laser confocal microscopy is capable of identifying corneal microstructural changes related to map-dot-fingerprint corneal dystrophy in vivo. The technique may be useful in elucidating the pathogenesis and natural course of map-dot-fingerprint corneal dystrophy and other similar basement membrane abnormalities.
Introduction
Map-dot-fingerprint (epithelial basement membrane) dystrophy (OMIM 121820)Citation1 is a bilateral anterior corneal dystrophy that usually develops between the ages of 20 and 40 years, and is characterized by grayish epithelial fingerprint lines, geographic map-like lines, and dots (or microcysts) on slit-lamp examination.Citation2–Citation4 Findings are variable and can change with time. Although map-dot-fingerprint dystrophy is usually asymptomatic, about 10% of patients develop painful, recurrent epithelial erosions. In some families, this condition appears to segregate as a true autosomal dominant trait.Citation5 However, Werblin et al reported that map-dot-fingerprint dystrophy is present in up to 76% of persons over the age of 50 years, suggesting that most cases of this condition represent age-dependent degeneration of the cornea.Citation6
In vivo white light corneal confocal microscopy allows noninvasive real-time spatial sectioning of living corneal tissues at the cellular level.Citation7,Citation8 The clinical usefulness of this method has been documented in studies of both normal and diseased human corneas.Citation9–Citation11 Recently, cornea-specific in vivo laser confocal microscopy (Heidelberg Retina Tomograph 2 Rostock Cornea Module, HRT 2-RCM, Heidelberg Engineering GmbH, Dossenheim, Germany) has become available and allowed more detailed in vivo observation of corneal microstructure,Citation12,Citation13 including map-dot-fingerprint dystrophy.Citation14,Citation15 In this study, we investigated pathological changes of the corneal cell layer in patients with map-dot-fingerprint dystrophy by in vivo laser corneal confocal microscopy.The possible association of this dystrophy with corneal inflammation is discussed.
Materials and methods
The present study was approved by the ethics committee of Kanazawa University Graduate School of Medical Science and followed the tenets of the Declaration of Helsinki. Two patients (cases 1 and 2) with map-dot-fingerprint dystrophy, neither of whom had a known family history of the disorder, underwent in vivo laser confocal microscopic examination (HRT 2-RCM) at the Department of Ophthalmology, Kanazawa Graduate University of Medical Science.
In vivo laser confocal microscopic observation
Prior to beginning this study, written informed consents were obtained from both subjects after explaining the nature and possible consequences of this study. After applying a large drop of contact gel (Comfort Gel ophthalmic ointment®, Bausch and Lomb, GmbH, Berlin, Germany) on the front surface of the microscope lens and ensuring that no air bubbles had formed, a Tomo-cap® (Heidelberg Engineering GmbH) was mounted on the holder to cover the microscope lens. The center and peripheral cornea were then examined layer by layer using this cornea-specific in vivo laser confocal microscope. HRT 2-RCM uses a 60× water-immersion objective lens (Olympus Europa GmbH, Hamburg, Germany) and a 670 nm diode laser as the light source with an area of observation of 400 μm square section.Citation16
Patients
Case 1
A 60-year-old woman with mild hypertension and background diabetic retinopathy visited our hospital for routine ophthalmic examination. Her corrected visual acuity was 20/40 in both eyes. The intraocular pressure was 11.4 mmHg and 11.8 mmHg in the right and left eye, respectively. By slit-lamp biomicroscopy, geographic-shaped abnormalities were observed in the Bowman’s membrane level in both eyes (). She had a mild cataract in both eyes. Her family history was unremarkable. No other abnormalities were detected by dilated fundus examination. She had no history of recurrent corneal erosions.
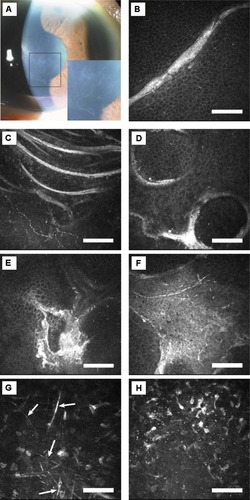
Figure 1 Case 1. (A) Slit-lamp photograph of right cornea. Geographic-shaped abnormalities in the central cornea are magnified in the lower right box. (B) In vivo laser confocal microscopic images of corneal lesions. Highly reflective linear images in the basal epithelium (depth 28 μm). (C) Highly reflective curvilinear, multilaminar lines in the basal epithelium (depth 20 μm). (D) Ring-shaped highly reflective extracellular deposits in the basal epithelium (depth 25 μm). (E) Highly reflective irregular deposit in the basal epithelium (depth 29 μm). (F) Highly reflective geographic opacities in the basal epithelial layer (depth 22 μm). (G) Highly reflective needle-shaped structures were also observed in the stroma (arrows, depth 445 μm). (H) Highly reflective microdots were observed in the stroma (depth 74 μm). Note: Bar 100 μm.
Case 2
A 44-year-old man who was otherwise healthy visited our hospital for further evaluation of recurrent corneal erosions. His corrected visual acuity was 20/20 in both eyes. The intraocular pressure was 14.8 mmHg and 16.0 mmHg in the right and left eye, respectively. By slit-lamp biomicroscopy, fingerprint-shaped abnormalities were observed in the Bowman’s membrane level in both eyes (). He had had a painful corneal erosion in his right eye two years prior to our appointment, and he had a similar event in his left eye one month prior to his appointment. His family history was also unremarkable. No other abnormalities were detected by dilated fundus examination.
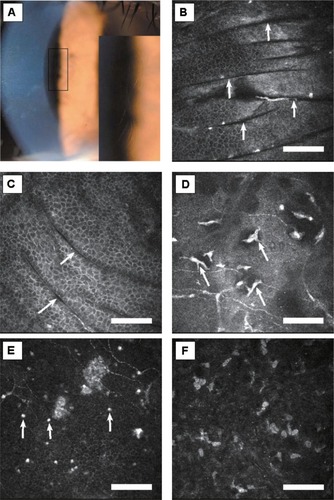
Figure 2 Case 2. (A) Slit-lamp photograph of right cornea. Fingerprint-shaped abnormalities in the central cornea are magnified in the lower right box. (B and C) In vivo laser confocal microscopic images of corneal lesions. Multiple dark parallel striae in the superficial/basal epithelium and Bowman’s membrane layer (arrows, depth 22 μm and 25 μm, respectively). (D) Infiltration of possible Langerhans cells in Bowman’s membrane cell layer (depth 51 μm, arrows). (E) Highly reflective dots were observed in the basal epithelial layer (depth 60 μm, arrows). (F) Highly reflective microdots were observed in the stroma (depth 80 μm).
Results
In vivo laser confocal microscopy
In case 1, microscopy of the affected lesions (“map”) of the cornea revealed various shapes of highly reflective extracellular deposits in the superficial/basal epithelium and Bowman’s membrane layer (). In the stroma, highly reflective needle-shaped structures ( together with microdots in ) were observed. Descemet’s membrane and the endothelial cell layer appeared normal.
In case 2, microscopy of the affected lesions (“fingerprints”) of the cornea revealed multiple dark striae in the superficial/basal epithelium and Bowman’s membrane layer (). Infiltration of possible Langerhans cells and highly reflective dots were also observed in Bowman’s membrane layer (). In the stroma, highly reflective microdots were observed (). Descemet’s membrane and the endothelial cell layer appeared normal.
Discussion
We have now reported in vivo laser confocal microscopic images in patients with map-dot-fingerprint (epithelial basement membrane) dystrophy. In the epithelial/Bowman’s membrane layer, variously shaped (linear, multilaminar, curvilinear, ring-shape, geographic) highly reflective materials were observed in the “map” area. In the same layer as the “fingerprint” lesions, multiple linear and curvilinear hyporeflective lines were observed. The novel aspect of this study is that we reported stromal changes of this dystrophy, such as highly reflective microdots and needle-shaped materials; these changes have not been reported previously.Citation14,Citation15
The histopathologic finding of “map” and “fingerprint” lesions represents a thickened epithelial basement membrane that has invaginated into the epithelium in the form of multilaminar sheets of fibrogranular material.Citation3,Citation17–Citation19 We surmise that the various shapes of highly reflective images in the epithelial/Bowman’s membrane layer identified on HRT 2-RCM might correspond with fibrogranular materials at the scalloped borders or inside the lesions of map-shaped subepithelial opacities. Also, the multiple linear and curvilinear hyporeflective lines in the fingerprint lesions might correspond with invagination of the abnormal redundant basement membrane. These lesions could be due to the artifacts during operation; however, we did not observe these artifacts among hundreds of patients studied previously. Taken altogether, in vivo laser confocal microscopic images obtained by HRT 2-RCM seem to correlate very well with previously reported histopathological changes in map-dot-fingerprint dystrophy.
Classically, two clinically and histopathologically distinct types of “dot” lesions have been reported, ie, Cogan cysts and the cysts reported by Bron and Brown.Citation18,Citation20,Citation21 Cogan cysts are characterized by intraepithelial cystic aggregations of degenerating cells underneath an intraepithelial sheet.
The histopathology of the second cyst type is thought to be a continuous layer of fibrillogranular material located between the epithelial basement membrane and Bowman’s layer, not a product of cellular degeneration. Neither patient we examined for this study had any microcysts on slit-lamp examination. Therefore, in vivo confocal images of microcysts could not be obtained in this study.
The precise origin of the needle-shaped material has not been identified; however, it can be seen in the early and late phase of corneal inflammation.Citation11 We also observed possible Langerhans cells and other inflammatory cells in the superficial corneas of both patients. Taken together, these potential inflammatory components observed in corneas affected by map-dot-fingerprint dystrophy lead us to suspect an association of this dystrophy with inflammation. A possible explanation of the inflammatory changes is that they might be due to a secondary response caused by previous corneal erosions and/or deposition of fibrogranular materials.
In conclusion, HRT 2-RCM is capable of identifying in vivo corneal microstructural changes related to map-dot-fingerprint corneal dystrophy with higher resolution than is possible with slit-lamp biomicroscopy. We could identify unique and characteristic images of corneal deposits not only in the epithelial cell/Bowman’s layer level, but also in the stroma. Thus, HRT 2-RCM may be useful in elucidating the pathogenesis and natural course of map-dot-fingerprint corneal dystrophy and other similar basement membrane abnormalities.
Disclosure
The authors report no conflicts of interest in this work.
References
- Online Mendelian Inheritance in ManBaltimore, MDJohns Hopkins University2004 Available from: http://www.ncbi.nlm.nih.gov/omim/. Accessed July 4, 2012.
- CoganDGDonaldsonDDKuwabaraTMarshallDMicrocystic dystrophy of the corneal epitheliumTrans Am Ophthalmol Soc19646221322514269893
- CoganDGKuwabaraTDonaldsonDDMicrocystic dystrophy of the cornea: a partial explanation for its pathogenesisArch Ophthalmol1974924704744547958
- IrvineADWangMXMcLeanWHEpithelial, basement membrane, and Bowman’s membrane layer dystrophiesWangMXCorneal Dystrophies and Degenerations: A Molecular Genetic ApproachNew York, NYOxford University Press2003
- LaibsonPRKrachmerJHFamilial occurrence of dot (microcystic), map, fingerprint dystrophy of the corneaInvest Ophthalmol1975143973991079207
- WerblinTPHirstLWStarkWJMaumeneeIHPrevalence of map-dot-fingerprint changes in the corneaBr J Ophthalmol1981654014097260010
- CavanaghHDPetrollWMAlizadehHClinical and diagnostic use of in vivo confocal microscopy in patients with corneal diseaseOphthalmology1993100144414548414403
- KaufmanSCMuschDCBelinMWConfocal microscopy: a report by the American Academy of OphthalmologyOphthalmology200411139640615019397
- KobayashiASugiyamaKIn vivo corneal confocal microscopic findings of palisades of Vogt and its underlying limbal stromaCornea20052443543715829801
- KobayashiASugiyamaKHuangAJIn vivo confocal microscopy in patients with central cloudy dystrophy of FrancoisArch Ophthalmol20041221676167915534129
- KobayashiAMaedaASugiyamaKIn vivo confocal microscopy in the acute phase of corneal inflammationOphthalmic Surg Lasers Imaging20033443343614509473
- StaveJZinserGGrummerGGuthoffRModified Heidelberg Retinal Tomograph HRT. Initial results of in vivo presentation of corneal structuresOphthalmologe200299276280 German.12058503
- KobayashiAYoshitaTSugiyamaKIn vivo findings of bulbar/palpebral conjunctiva and presumed meibomian gland by laser scanning confocal microscopyCornea20052498598816227847
- LabbéANicolaRDDupasBAuclinFBaudouinCEpithelial basement membrane dystrophy: evaluation with the HRT II Rostock Cornea ModuleOphthalmology20061131301130816877069
- RosenbergMETervoTMPetrollWMVesaluomaMHIn vivo confocal microscopy of patients with corneal recurrent erosion syndrome or epithelial basement membrane dystrophyOphthalmology200010756557310711897
- Heidelberg Retina Tomograph II (Rostock Cornea Module) Operating Instructions of Software Version 1.1Dossenheim, GermanyHeidelberg Engineering GmbH2004
- LaibsonPRMicrocystic corneal dystrophyTrans Am Ophthalmol Soc197674488531301322
- BronAJBrownNASome superficial corneal disordersTrans Ophthalmol Soc U K197191XII+5291575
- RodriguesMMFineBSLaibsonPRDisorders of the corneal epithelium: a clinicopathologic study of dot, geographic, and fingerprint patternsArch Ophthalmol1974924754824139941
- BronAJTripathiRCCystic disorders of the corneal epithelium I: clinical aspectsBr J Ophthalmol1973573613754541667
- TripathiRCBronAJCystic disorders of the corneal epithelium II: pathogenesisBr J Ophthalmol1973573763904541668