Abstract
Purpose
To create a defocus curve of emerging presbyopic patients of various age groups.
Setting
Single site private practice in Sioux Falls, South Dakota.
Design
This was a non-randomized, prospective study. All subjects were enrolled from healthy volunteers.
Methods
Subjects aged 37–9, 40–42, 43–45 and 46–48 that have 20/20 best-corrected distance visual acuity (BCDVA) were included. Binocular visual acuity at different defocus steps ranging from +0.5 D to −3 D was measured in each age group. Defocus curves were generated from the mean logMAR visual acuities at each defocus step, by age group.
Results
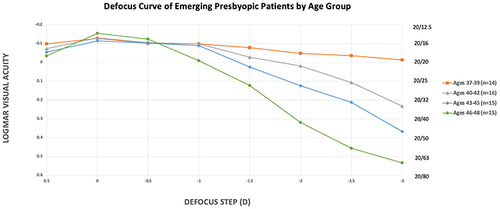
Of the 60 subjects, 23.3% of subjects were between the ages of 37–39, 26.7% were between the ages of 40–42, 25% of subjects were between ages 43–45, and 25% were between the ages of 46–48. Visual acuity significantly decreased from plano to −3 D defocus steps in all groups (p < 0.0002, p = 0, p = 0 and p = 0). The 46–48-year-old group had worse visual acuity compared to the other three groups from the −1.0 to −2.0 D defocus steps (p = 0.037, p = 0.022 and 0.017, respectively). Starting at a near point of 40cm, the 37–39 group had the best logMAR vision and the 46–48 group had the worst vision (p = 0.001).
Conclusion
The defocus curves of emerging presbyopic individuals demonstrate a decreasing visual acuity at near defocus steps that decreases with age. Defocus curves at different age ranges can help doctors explain various presbyopia treatment options in terms of near point capabilities at various ages.
Plain Language Summary
What Was Known:
Defocus curves are used to simulate visual acuity at various distances.
Due to presbyopia, visual acuity at near decreases with age.
What this Paper Adds:
The defocus curve of emerging presbyopic patients demonstrates progressive reduction in near visual acuity with increasing age.
Data sharing statement: No de-identified information regarding the subjects will be shared, nor will further data and study documents be made accessible. If requested, study documents can be obtained from Brian Shafer, MD.
Introduction
Presbyopia is the loss of accommodation of the eye resulting in near-vision impairment.Citation1,Citation2 This is a natural process that occurs due to progressive hardening and loss of viscoelasticity of the lens.Citation3 Patients with presbyopia suffer from blurred vision at near necessitating spectacle correction in most people by the age of 50.Citation1,Citation2
Progressive in nature, presbyopia starts to become clinically apparent around the age of 40.Citation1 With the exponential rise of computer-based occupations, eye strain due to uncorrected or under-corrected presbyopia may lead to potential productivity losses.Citation2 Outside of the workforce, screen-time has dramatically increased for social and recreational purposes. Without near-vision isolation and loss of quality of life can ensue.Citation2
Visually significant presbyopia is nearly ubiquitous in patients with cataracts.Citation2 Additionally, following cataract surgery and implantation of a monofocal intraocular lens (IOL), absolute presbyopia occurs. To combat spectacle-dependence following cataract surgery, IOL technology has evolved to address the complete loss of accommodation.Citation1,Citation4–6 There is a rapidly growing arsenal of multifocal, extended depth of focus (EDOF), extended range of vision, and pseudo-accommodative IOLs, as well as pharmacological presbyopia options.Citation4,Citation5,Citation7,Citation8
Spectacle independence is only possible if visual acuity is functional at distance, intermediate, and near ranges.Citation9–11 Defocus curves are used to demonstrate the expected binocular visual acuity following bilateral implantation of a particular IOL.Citation12,Citation13 To construct a defocus curve, binocular visual acuity is measured at a fixed distance after placing step-wise increases in minus spherical lenses in front of the patient, usually at half or quarter-diopter steps. A defocus step of −1.50 D represents intermediate vision at a distance of 66cm or near point, while −2.50 D represents near vision at a near point of 40cm. Typically, vision is considered functional at logMAR 0.2, or the Snellen equivalent of 20/32.Citation12,Citation13
In preparing a patient for cataract surgery with a presbyopia-correcting IOL, expectations must be properly addressed.Citation1,Citation3,Citation14,Citation15 Though easy to demonstrate a line on a Snellen chart, it is challenging to educate patients on what their vision will be like following surgery or other presbyopia-correcting treatments. While defocus curves are created for all IOLs as part of their approval process, there are no published defocus curves for phakic patients at various stages of presbyopia.
In this study, we address the unmet need of creating defocus curves of emerging presbyopic patients.
Methods
This was a single-site, prospective, non-randomized, non-controlled trial. The protocol was reviewed and approved by an ethics committee (Aspire IRB, Santee, California) on October 8, 2019, with an updated protocol approved by the same committee on July 6, 2020. The protocol was updated to include expanded age groups. The trial was registered to ClinicalTrials.Gov with identifier NCT04474782. All prospective subjects provided written informed consent to participate, and the study was conducted in accordance with the Declaration of Helsinki. No de-identified information regarding the subjects will be shared, nor will further data and study documents be made accessible.
Participants were phakic adults aged 37–39, 40–42, 43–45 and 46–48 years old with best corrected visual acuity (BCVA) of at least 20/20 in each eye. Potential subjects were excluded if they had undergone prior cataract surgery, had a BCVA <20/20 in either eye, or any ocular comorbidity that may reduce the subjects BCVA.
Potential subjects had their uncorrected distance visual acuity (UCVA) measured using the Early Treatment of Diabetic Retinopathy (ETDRS) chart at a distance of 20 feet. A manifest refraction was performed using a phoropter to determine the subject’s BCVA on the ETDRS chart at 20 feet and this refraction was placed in a trial frame. Loose lenses were placed in front of both eyes in the trial frame ranging from +0.50 D to −3.00 D and the visual acuity was measured using the ETDRS chart at 20 feet. All visual acuities were converted to logMAR for ease of reporting.
For each of the age groups of 37–39, 40–42, 43–45 and 46–48 years old, a defocus curve was created using mean logMAR visual acuities on the y-axis of a graph and the defocus step ranging from +0.50 D to −0.50 D. Differences in visual acuity (measured in logMAR units) by age group at each defocus step was compared using a One-Way ANOVA test and, if a difference was detected, significance was confirmed using a Pairwise Tukey-HSD post-hoc test. The level of significance was chosen as 0.05. No formal power calculations were conducted.
Results
A total of 60 subjects (120 eyes) participated in this trial. Of these, 58 (96.7%) were Caucasian, 1 (1.6%) was Asian, and 1 (1.6%) was Native American. There were 30 (50%) females and 30 (50%) males. 23.3% of subjects were between the ages of 37–39, 26.7% were between the ages of 40–42, 25% of subjects were between ages 43–45, and 25% were between the ages of 46–48.
shows the mean logMAR visual acuity at each defocus step from +0.5 to −3.0D for each group. demonstrates the defocus curve of each age group. There was no statistically significant difference in logMAR vision at the +0.5, 0, or −0.5 D defocus step. Visual acuity significantly decreased from −0.5 to −3 D defocus steps in all groups (p < 0.0002, p = 0, p = 0 and p = 0). The 46–48-year-old group had worse visual acuity compared to the other three groups from the −1.0 to −2.0 D defocus steps (p = 0.037, p = 0.022 and 0.017, respectively). Starting at a near point of 40cm, the 37–39 group had the best logMAR vision and the 46–48 group had the worst vision (p = 0.001).
Table 1 Mean LogMAR Visual Acuity by Age and Defocus Step with Standard Deviation
Discussion
To our knowledge, this is the first series of binocular defocus curves for emerging presbyopic individuals of different ages. Evident from the curves in , intermediate and near vision rapidly worsen between the ages of 46 and 48. Additionally, very-near vision begins to significantly decline at the age of 40.
With an ever-growing armamentarium of presbyopia-correcting intraocular lenses, managing patient expectations is of paramount importance. By comparing the defocus curves of IOLs or other presbyopia-correcting therapies to that of emerging presbyopic individuals of different age ranges, patients can be more effectively counseled on visual outcomes. Eye care providers will now be able to directly inform patients that following cataract surgery, vision at different ranges will be most similar to when they were a certain age.
A limitation of our study is that we have included only 4 distinct age groups. A second limitation is that there was minimal ethnic diversity among the subjects. In future studies, more ages will be included to determine the defocus curves for a wider set of individuals. Future studies with larger sample sizes may help prove reproducibility. Additionally, future studies will evaluate the impact of refractive error on vision at different defocus steps.
In the aging population, presbyopia is inevitable. By comparing defocus curves of individuals at different stages of life, patient expectations following premium cataract surgery with implantation of a presbyopia-correcting IOL and other therapies will be more accurate. With proper expectations met, patients will be better informed for potentially life-long decisions regarding presbyopia.
Disclosures
Drs. Shafer, Thompson, and Berdahl are all consultants for Alcon®. Dr Berdahl reports personal fees for consulting and/or ownership from Alcon, Bausch and Lomb, Expert Opinion, Johnson and Johnson Surgical Vision, Ocular Surgical Data, RxSight, Zeiss, during the conduct of the study; personal fees for consulting and/or ownership from AbbVie, Aerie, Aerpio, Aldeyra, Aurea Medical, Aurion Biotech/CorneaGen, Dakota Lions Eye Bank, Elios Vision INC, Equinox, Expert Opinion, Glaukos, Gore, Imprimis/Harrow Health, iRenix, Iacta Pharmaceuticals, Kala, Kedalion, MELT Pharmaceuticals, MicroOptx, New World Medical, Ocular Therapeutix, Omega Ophthalmic, Orasis, Oyster Point, Santen, Sight Sciences, Surface INC, Tarsus, Tear Clear, Vertex Ventures, ViaLase, Vittamed, Vance Thompson Viison, Verana Health, Versa Biologics, Visionary Ventures, Visus, and Zeiss, outside the submitted work. Dr Vance Thompson reports personal fees for research from Alcon, Acufocus, Bausch & Lomb, Johnson & Johnson, RxSight, Zeiss, and Allergan, during the conduct of the study; personal fees for consulting and/or research from AdOM, Allotex, Avellino, Avisi Technologies, Inc, BRIM Biotechnology, Inc, BVI, Centricity, Conjtac, Crystilex, CSO, Equinox, Euclid Systems, Expert Opinion, eyeBrain Medical Inc, Eyedetec, Eyesafe, Forsight Robotics, Glaukos, Imprimis, iVeena, LayerBio, Medevise, Melt Pharmaceuticals, NanoDrops, Ocular Innovations, Ocular Therapeutix, ORA, Oyster Point Pharmaceuticals, Rayner, Reopia, SightSciences, Stepwise Medical, Stuart Therapeutics, Tarsus Rx, TearClear, TearOptix, Thea, TherOptix, Treehouse Health, Vance Thompson Vision, Visant, Visionary Ventures, Visus, Vivior AG, and 2EyesVision, outside the submitted work. Dr Mitch J Ibach and Dr Justin A Schweitzer report grants from Alcon, during the conduct of the study. The authors report no other conflicts of interest in this work.
Acknowledgments
Results from this study were presented virtually at the American Society of Cataract and Refractive Surgery May 16–17, 2020 and live in Las Vegas, Nevada at the American Society for Cataract and Refractive Surgeons on July 25, 2021.
Additional information
Funding
References
- Balgos MJTD, Vargas V, Alió JL. Correction of presbyopia: an integrated update for the practical surgeon. Taiwan J Ophthalmol. 2018;8(3):121–140. doi:10.4103/tjo.tjo_53_18
- Berdahl J, Bala C, Dhariwal M, Lemp-Hull J, Thakker D, Jawla S. Patient and economic burden of presbyopia: a systematic literature review. Clin Ophthalmol. 2020;14:3439–3450. doi:10.2147/OPTH.S269597
- Gibbons A, Ali TK, Waren DP, Donaldson KE. Causes and correction of dissatisfaction after implantation of presbyopia-correcting intraocular lenses. Clin Ophthalmol. 2016;10:1965–1970. doi:10.2147/OPTH.S114890
- Akella SS, Juthani VV. Extended depth of focus intraocular lenses for presbyopia. Curr Opin Ophthalmol. 2018;29(4):318–322. doi:10.1097/ICU.0000000000000490
- Dick HB. Small-aperture strategies for the correction of presbyopia. Curr Opin Ophthalmol. 2019;30(4):236–242. doi:10.1097/ICU.0000000000000576
- Greenwood M, Bafna S, Thompson V. Surgical correction of presbyopia: lenticular, corneal, and scleral approaches. Int Ophthalmol Clin. 2016;56(3):149–166. doi:10.1097/IIO.0000000000000124
- Kelava L, Barić H, Bušić M, Čima I, Trkulja V. Monovision versus multifocality for presbyopia: systematic review and meta-analysis of randomized controlled trials. Adv Ther. 2017;34(8):1815–1839. doi:10.1007/s12325-017-0579-7
- Monaco G, Gari M, Di Censo F, Poscia A, Ruggi G, Scialdone A. Visual performance after bilateral implantation of 2 new presbyopia-correcting intraocular lenses: trifocal versus extended range of vision. J Cataract Refract Surg. 2017;43(6):737–747. doi:10.1016/j.jcrs.2017.03.037
- Bianchi GR. Spectacle independence after cataract surgery: a prospective study with a multifocal intraocular lens. Med Hypothesis Discov Innov Ophthalmol. 2020;9(1):38–46.
- Hogarty DT, Russell DJ, Ward BM, Dewhurst N, Burt P. Comparing visual acuity, range of vision and spectacle independence in the extended range of vision and monofocal intraocular lens. Clin Experiment Ophthalmol. 2018;46(8):854–860. doi:10.1111/ceo.13310
- Sezgin Asena B. Visual and refractive outcomes, spectacle independence, and visual disturbances after cataract or refractive lens exchange surgery: comparison of 2 trifocal intraocular lenses. J Cataract Refract Surg. 2019;45(11):1539–1546. doi:10.1016/j.jcrs.2019.06.005
- Böhm M, Petermann K, Hemkeppler E, Kohnen T. Defocus curves of 4 presbyopia-correcting IOL designs: diffractive panfocal, diffractive trifocal, segmental refractive, and extended-depth-of-focus. J Cataract Refract Surg. 2019;45(11):1625–1636. doi:10.1016/j.jcrs.2019.07.014
- Gil MA, Varón C, Cardona G, Buil JA. Visual acuity and defocus curves with six multifocal intraocular lenses. Int Ophthalmol. 2019;40:393–401. doi:10.1007/s10792-019-01196-4
- Shafer BM, Greenwood M. Presbyopia correction at the time of cataract surgery. Curr Ophthalmol Rep. 2020;8(3):79–87. doi:10.1007/s40135-020-00236-y
- Sieburth R, Chen M. Intraocular lens correction of presbyopia. Taiwan J Ophthalmol. 2019;9(1):4–17. doi:10.4103/tjo.tjo_136_18