Abstract
Purpose
To report a case of bilateral acute myopia and angle closure glaucoma after ingestion of methazolamide.
Methods
An interventional case report of a 70-year-old male who developed bilateral, acute myopia and angle closure glaucoma after ingesting methazolamide tablets for the treatment of normal tension glaucoma.
Results
Bilateral anterior chamber shallowing associated with ciliary body edema, supraciliary effusions, and shallow posterior choroidal effusions were documented with slit-lamp photography and high-frequency ultrasonography. Near complete resolution of these signs after discontinuation of methazolamide were also documented.
Conclusion
Methazolamide may be associated with secondary myopia and angle closure glaucoma. Discontinuation of methazolamide leads to resolution of this process, as documented by slit-lamp photography and high-frequency ultrasonography.
Introduction
Secondary myopia and angle closure have been associated with a number of sulfonamide-derived medications. Most of these drugs fall into the class of antibiotics (eg, sulfacetamide, sulfadiazine) or antihypertensives (eg, acetazolamide, hydrochlorothiazide). In 2001, topiramate-induced angle closure glaucoma was also described.Citation1 Recently, a single case of methazolamide-induced angle closure glaucoma was described.Citation2 This entity may affect any age and appears to affect each gender with equal incidence.Citation3
The exact pathophysiology of sulfonamide-induced myopia and angle closure glaucoma has yet to be elucidated. In this case, the occurrence and clinical course of induced myopia and bilateral angle closure glaucoma after ingestion of methazolamide is reported. This represents the second report of methazolamide-induced angle closure glaucoma to be described in the literature.
Material and methods
This study presents a case report and literature review. This study was conducted in accordance with the tenets of the Declaration of Helsinki.
Results
A 70-year-old Haitian male presented to the Bascom Palmer Eye Institute (Miami, FL, USA) emergency room complaining of markedly decreased vision and pain in both eyes since the morning of presentation. His past ocular history was significant for medically controlled normal tension glaucoma. The patient’s systemic medical history was significant for hypertension and noninsulin dependent diabetes mellitus. His systemic medications included metformin, amlodipine, olmesartan, and aspirin. On the patient’s most recent clinic visit to Bascom Palmer Eye Institute 2 days prior to presentation, visual acuity measured 20/30 in each eye and intraocular pressure (IOP) measured 8 mmHg in each eye in the setting of advanced optic nerve cupping. The patient was noted to have a follicular conjunctivitis consistent with brimonidine toxicity. Since the patient had been maximized on tolerated topical IOP-lowering therapy, an oral agent was exchanged with brimonidine. Methazolamide, rather than acetazolamide, was instituted in order to decrease the risk of systemic side effects.Citation4 Twice daily frequency was initiated according to the drug’s package insert.Citation5 The patient ingested one tablet the night prior to presentation and one tablet the following morning.
On examination, the patient’s best-corrected visual acuity was reduced to 20/200 in the right eye and counting fingers at 2 feet in the left eye. Manifest refraction revealed an error of −2.00 + 1.00 × 135 in the right eye and −2.00 + 1.00 × 026 in the left eye. IOP by standard Goldmann applanation tonometry (Model AT 900, Haag-Streit USA, Mason, OH, USA) was 35 mmHg in each eye. Slit-lamp examination (B900, Haag-Streit USA, Mason, OH, USA) revealed marked conjunctival chemosis, mild microcystic corneal edema, and shallow anterior chamber in each eye (). High-frequency (35 MHz) ultrasound biomicroscopy (UBM; Model P60, Paradigm Medical Industries, Salt Lake City, UT, USA) confirmed shallow anterior chambers and also revealed anterior displacement of the lens–iris diaphragm associated with ciliary body edema and a prominent ciliochoroidal effusion in each eye (). Posterior B-scan ultrasonography (Eye Cubed, Ellex, Inc., Minneapolis, MN, USA) revealed posterior choroidal effusions ().
Figure 1 Slit-lamp photographs of the right (A) and left (B) eye demonstrating moderate peripheral and central anterior chamber shallowing after recent ingestion of methazolamide. High-frequency (35 MHz) ultrasound biomicroscopy reveals anterior displacement of the lens–iris diaphragm due to ciliary body edema and supraciliary effusion (arrows) in the right (C) and left (D) eye. Anterior chamber depth measured 1.2 mm in each eye and lens thickness measured 4.8 mm and 4.9 mm in the right and left eye, respectively. B-scan ultrasonography revealed shallow posterior choroidal effusions (arrows) in the right (E) and left (F) eye.
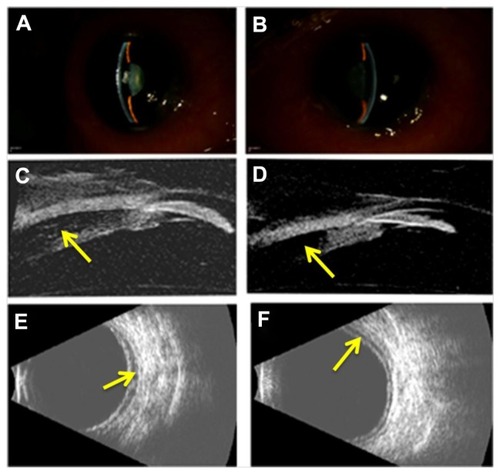
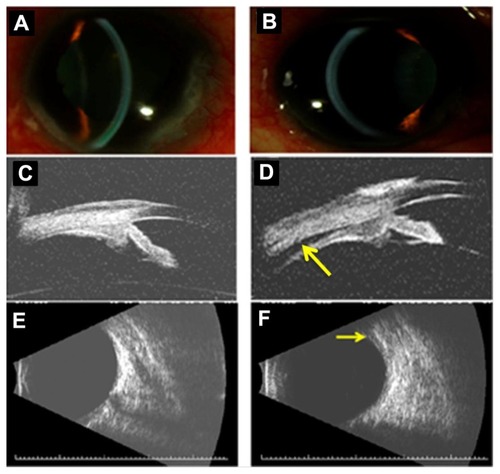
Methazolamide-induced myopia and angle closure glaucoma were diagnosed and the drug was immediately discontinued. The patient’s regular topical ocular hypotensive medical regimen (consisting of 0.1% brimonidine in both eyes twice daily, 2% dorzolamide/0.5% timolol in both eyes twice daily, and 0.005% latanoprost in both eyes every evening) was continued. In addition, the patient was placed on 1% atropine in both eyes twice daily and 1% prednisolone acetate in both eyes four times daily. By the patient’s 1-week follow-up examination, best-corrected visual acuity improved to 20/50 in the right eye and 20/60 in the left eye. Manifest refraction revealed an error of plano +0.75 × 075 D in the right eye and +0.50 + 0.75 × 075 D in the left eye. IOP now measured 4 mmHg in the right eye and 3 mmHg in the left eye. Slit-lamp examination revealed deepening of each anterior chamber () and high-frequency UBM revealed posterior displacement of the lens–iris diaphragm with normal ciliary body architecture (). The supraciliary effusion of the right eye had completely resolved while a small supraciliary effusion remained in the left eye. Posterior B-scan ultrasonography also revealed complete resolution of the posterior choroidal effusion in the right eye with a small choroidal effusion noted in the left eye (). Slow resolution of the patient’s posterior choroidal effusions may have contributed to the patient’s reduction in visual acuity compared to baseline levels of 20/30 in each eye. The patient’s atropine and brimonidine were discontinued and he was continued on all other topical medications. The patient’s topical prednisolone acetate was slowly tapered over several months.
Figure 2 Slit-lamp photographs of the right (A) and left (B) eye demonstrating relative deepening of the anterior chambers after discontinuation of methazolamide. High-frequency (35 MHz) ultrasound biomicroscopy reveals resolution of ciliary body edema and supraciliary effusion in the right eye (C) and near complete resolution (arrow) in the left eye (D). Anterior chamber depth measured 2.7 mm and 2.3 mm in the right and left eye, respectively. Lens thickness measured 4.7 mm and 4.9 mm in the right and left eye, respectively. B-scan ultrasonography reveals complete resolution of the posterior choroidal effusion in the right eye (E) and a small persistent effusion (arrow) in the left eye (F).
Discussion
Sulfonamide-induced myopia and angle closure were first described by Korol in 1962.Citation6 Since then, a number of sulfonamide-derived medications have been associated with secondary myopia and angle closure glaucoma.Citation1 Lee et al describe a 62-year-old female who awakened with bilateral acute vision loss 1 day after ingesting a prophylactic dose of 250 mg acetazolamide following uncomplicated cataract extraction. She was diagnosed with acute bilateral angle closure glaucoma and the episode resolved upon discontinuation of acetazolamide.Citation7
Similar to acetazolamide, methazolamide is a sulfonamide-derived carbonic anhydrase inhibitor. The drug is available as 25 mg and 50 mg tablets, dosed twice daily. Unlike acetazolamide, methazolamide is metabolized by the liver and therefore has a longer, 14-hour half-life. The risk of systemic side effects such as nephrolithiasis and metabolic acidosis is also lessened with methazolamide. Kwon et al recently described the first case of methazolamide-induced angle closure in a 51-year-old male treated with the agent for refractory diabetic macular edema.Citation2 Sulfonamide-induced angle closure and myopia have not been reported in association with topical carbonic anhydrase inhibitors. In the current case, the patient had tolerated topical dorzolamide for several years. Therefore, this agent was deemed to be safe for long-term IOP control once methazolamide was discontinued.
Two theories prevail regarding the pathophysiology of sulfonamide-induced angle closure glaucoma. Sen et al postulate that an osmotic disturbance within the lens leads to lens hydration, thickening, and anterior displacement of the lens–iris diaphragm.Citation8 Craig et al proposes a theory of sulfonamide-induced ciliary body edema with supraciliary effusion leading to forward rotation of the ciliary body and mechanical angle closure.Citation9 Although both an osmotic disturbance within the lens and ciliary body edema are likely to contribute to this process, the current case supports the latter theory as UBM-measured lens thickness did not change appreciably before and after institution of treatment and subsequent anterior chamber deepening. The entity may be dose dependent as prior cases of topiramate-induced angle closure occurred only upon doubling the dose of the drug.Citation3 The patient described herein may have indeed tolerated a 25 mg dose of methazolamide, but given his reaction, he was not rechallenged with the agent.
The diagnosis of sulfonamide-induced angle closure glaucoma remains a clinical one, which is aided by the temporal relationship to sulfonamide ingestion. In the current case, the patient’s history and UBM findings allowed a Naranjo score of seven to be assigned, indicating a probable adverse drug reaction.Citation10 Although the magnitude of the patient’s induced myopia may have been blunted by corneal edema, resolution of this refractive error with discontinuation of the drug also supported the diagnosis.
The differential diagnosis of acute, bilateral angle closure glaucoma can be divided into pupillary block (eg, anatomically narrow angles, anticholinergic or sympathomimetic use, inflammatory membrane occluding the pupil) and nonpupillary block mechanisms (eg, sulfonamide-induced, plateau iris syndrome, annular choroidal effusion). As demonstrated in the current case, high-frequency UBM is helpful in differentiating between these causes and typically reveals bilateral ciliary body edema and supraciliary effusion with forward displacement of the lens–iris diaphragm.
The mainstay of treatment of sulfonamide-induced angle closure glaucoma is consultation with the prescribing physician and discontinuation of the offending agent as soon as possible. Maximum IOP-lowering therapy (with exception to systemic sulfonamide derivatives and miotic agents) should be instituted. The uveal effusions associated with this process are thought to have an underlying inflammatory mechanism, thus anti-inflammatory treatment is also recommended. Laser iridotomy is not indicated as sulfonamide-induced angle closure does not occur secondary to pupillary block. Topical miotic agents are contraindicated as these may lead to further anteriorization of the lens–iris diaphragm, aggravating the episode.Citation3
Conclusion
In conclusion, sulfonamide ingestion is an important cause of acute, bilateral angle closure. The patient’s medication list should be reviewed carefully in these cases and methazolamide should be considered as a potential cause of this condition.
Acknowledgments
RKL is supported by NIH NEI grant EY016775. The Bascom Palmer Eye Institute is supported by an unrestricted research grant from Research to Prevent Blindness and NIH center grant EY014801.
Disclosure
The authors report no conflicts of interest in this work.
References
- PandayVARheeDJReview of sulfonamide-induced acute myopia and bilateral angle-closure glaucomaCompr Ophthalmol Update20078527127618201514
- KwonSJParkDHShinJPBilateral transient myopia, angle-closure glaucoma, and choroidal detachment induced by methazolamideJpn J Ophthalmol201256551551722782646
- FraunfelderFWFraunfelderFTKeatesEUTopiramate-associated acute, bilateral, secondary angle-closure glaucomaOphthalmology2004111110911114711721
- LichterPRReducing side effects of carbonic anhydrase inhibitorsOphthalmology19818832662697231916
- Fera PharmaceuticalsNeptazane® (methazolamide tablets) tablet [package insert]Locust Valley, NYFera Pharmaceuticals2010
- KorolEATransitory myopia in combination with transitory glaucomaZdravookhr Beloruss196286667 Belorussian14034847
- LeeGCTamCPDanesh-MeyerHVMyersJSKatzLJBilateral angle closure glaucoma induced by sulphonamide-derived medicationsClin Experiment Ophthalmol2007351555817300572
- SenHAO’HalloranHSLeeWBCase reports and small case series: topiramate-induced acute myopia and retinal striaeArch Ophthalmol2001119577577711346412
- CraigJEOngTJLouisDLWellsJMMechanism of topiramate-induced acute-onset myopia and angle closure glaucomaAm J Ophthalmol2004137119319514700673
- NaranjoCABustoUSellersEMA method for estimating the probability of adverse drug reactionsClin Pharmacol Ther19813022392457249508