Abstract
Purpose:
We sought to evaluate the visual pathway deficits in schizophrenic patients, compared with their parents and healthy controls, using Matrix frequency doubling technology (FDT) perimetry. Matrix FDT is an ophthalmic test used to detect visual field loss.
Method:
A total of 13 patients, 13 parents, and 12 healthy controls were enrolled in the study. Participants were subjected to Matrix FDT perimetry in a single test session. We analyzed the mean deviation for each eye and used a generalized estimated equation to evaluate differences among the groups and correct the dependency between the eyes.
Results:
The global mean deviation (presented as the mean of both eyes) was significantly lower in the schizophrenic patients than in their parents or controls. Analysis of the general sensitivity of the fibers crossing the optic chiasm showed a difference between the groups (P = 0.006), indicating that the sensitivity of the fibers crossing the optic chiasm was lower than those which did not cross. But when we analyzed the specific groups, the difference between the fibers was not considerable. Comparison of the right and left hemispheres showed that general sensitivity was lower for the left hemisphere, but when we analyzed specific groups, the difference was not significant (P = 0.29).
Conclusion:
These findings are suggestive of a lower global sensitivity in schizophrenic patients and their parents compared with controls. This difference may be an endophenotype of schizophrenia. The present study adds to a growing body of research on early-stage visual processing deficits in schizophrenia.
Introduction
Schizophrenic patients exhibit not only cognitive dysfunctions, such as memory and attention deficits,Citation1,Citation2 but also deficits in basic sensory processing.Citation3–Citation6 In particular, disturbances of the visual system have been documented, in studies employing diverse methodologies, including psychophysical tests (eg, visual masking procedures), contrast sensitivity tests, visually evoked potentials, and smooth pursuit tracking tasks.Citation7–Citation9
Visual processing research in human and nonhuman primates has identified two separate but interacting visual subsystems.Citation10,Citation11 the magnocellular (M) pathway, which is primarily responsible for processing information about location and motion, and the parvocellular pathway, which is primarily responsible for processing information about detail and color.Citation12 There is evidence to suggest that the M pathway is hypoactive in schizophrenia. For example, studies using electroencephalography (EEG) have shown reduced signal amplitudes in the posterior cortical regions along the M pathway in schizophrenic patients, with relatively normal activation along the pathway.Citation13–Citation15 Likewise, Braus et alCitation16 using functional magnetic resonance imaging (fMRI), showed M pathway hypoactivation (particularly in the right hemisphere) in schizophrenic patients but found no evidence of abnormal functioning of the pathway, and Bedwell et alCitation17 reported evidence of right hemisphere M pathway hypoactivity in nonpsychotic first-degree relatives of schizophrenics, using fMRI.Citation17
While visual dysfunctions in schizophrenia have been described in the literature,Citation13,Citation14,Citation18 their potential significance as endophenotypes of the disorder remains unclear. Gottesman and GouldCitation19 defined the endophenotypes of an illness as having the following characteristics: they cosegregate with the illness in affected families; are associated with the illness; are present in unaffected biological relatives at a higher rate than in the general population; are heritable; and are detectable in remitted patients. Deficits in working memory, executive function, sustained attention, sensory gating, smooth pursuit eye movements, verbal memory, and language production all have been identified as endophenotypes of schizophrenia.Citation19
Frequency doubling technology (FDT) is an automated tool that can detect alterations in contrast sensitivity and visual field defects.Citation20 FDT is used in glaucoma disease.Citation21 This technique uses an optical illusion called “frequency doubling,” which occurs when an achromatic, low spatial frequency sinusoidal grating undergoes counter-phased flickering at a high temporal frequency, resulting in an apparent doubling of the spatial frequency of the grating.Citation20,Citation22,Citation23 Processing of the FDT signal is thought to involve the activation in the M pathway and visual association areas.Citation23,Citation24 So, the FDT can be used to assess retinal ganglion cell function and provides a means to study deficits of early visual processing in schizophrenia, and this can have some laterality, as described by Bedwell et al.Citation17
In this study, we aimed to evaluate initial visual processing (perception of a stimulus by ganglion cells) in schizophrenic patients, as compared with their parents and healthy controls, using a Matrix FDT perimetry device to evaluate and follow the evolution of the visual field.
Methods
Participants
Thirteen schizophrenic patients from the Schizophrenia Program of the Federal University of São Paulo participated in the study. The mean age of study patients was 37.85 ± 8.42 years (46% male and 54% female). All patients were outpatients with a diagnosis of schizophrenia, according to DSM-IV. The diagnosis of schizophrenia was confirmed by a trained psychiatrist, using the Structured Clinical Interview for DSM-IV. All patients were refractory to treatment with antipsychotics, and they were taking clozapine (average dose 300 mg/day). The study also included 13 parents (mother or father) of the patients (mean age was 60.92 ± 9.83 years; 23% male and 77% female) and 12 healthy controls (volunteers recruited from among the hospital staff and students at the Federal University of São Paulo). The mean age of the control group was 38.46 ± 14.79 years; 58% male and 42% female.
Patients included for study had a confirmed diagnosis of schizophrenia for at least 10 years and had been taking antipsychotic drugs or other psychotropic agents (eg, benzodiazepines) for at least 6 months at the time of the study. Other inclusion criteria for the schizophrenic patients were as follows: good vision (visual acuity greater than 20/60 in both eyes, as measured with the Snellen chart and greater than 20/30 in both eyes after refraction correction), normal optic nerve (cup disc ratio less than 0.6), lower intraocular pressure of 20 mmHg, as measured with the Goldmann tonometer, and normal biomicroscopic examination, assessed using a slit lamp. Exclusion criteria for the patients included a history of drug or alcohol abuse or dependence, the presence of any neurological disease that could affect performance on the test (eg, cerebral palsy), or the presence of any disease affecting the visual field (eg, pituitary lesions or diabetic retinopathy).
The inclusion criteria for the parent and control groups consisted of the same ophthalmologic criteria listed above for the patients, as well as a score of less than or equal to seven positive answers on the Self-Reporting Questionnaire-20 mental status assessment. The exclusion criteria for these groups included the presence of psychiatric illness (in the control group), substance abuse, the presence of a disease affecting the visual field (eg, pituitary lesions or diabetic retinopathy), or the presence of any systemic disease potentially affecting the visual system (eg, multiple sclerosis).
All subjects signed a consent form that was approved by the local human research ethics committee. The research was conducted in a manner consistent with the principles outlined in an internationally recognized standard for the ethical conduct of research. The research was adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of the Federal University of São Paulo.
Apparatus
FDT perimetry was measured using the Humphrey® Matrix 800 FDT instrument (Carl Zeiss Meditec, Jena, Germany).Citation25–Citation27
Clinical evaluation
All participants received an ophthalmic examination that included tests of visual acuity, refraction, and biomicroscopy of the anterior chamber, as well as measurement of intraocular pressure and fundus examination.
Participants received instructions about performing the Matrix FDT test. They then were screened for 30 seconds, during which time they learned to recognize the stimuli and adapted to the test conditions. Stimuli were presented on the computer screen and participants were asked to press a button when they saw a gray square. All participants were tested with a standard FDT 24-2 visual field contrast sensitivity threshold exam protocol (programmed into the device), which evaluates 55 visual field regions in the right eye, followed by 55 regions in the left eye.Citation28 This test provides a single measure of the contrast sensitivity threshold (in decibels) at each of the 110 regions, using a maximum likelihood threshold strategy (zippy estimation by sequential testing [ZEST]).Citation29 The Matrix FDT 24-2 exam featured 24-degree coverage with a stimulus size of 5 degrees, a spatial frequency of 0.5 cycles per degree, and a temporal frequency of 18 Hz.Citation28 In addition to the threshold values for each region, two summary measures of general contrast sensitivity across the visual field were also reported, the mean deviation and pattern standard deviation. The mean deviation is a measure of the overall contrast sensitivity in each eye, while the pattern standard deviation indicates how each of the 55 visual field test locations deviates from the expected value from the age-adjusted normative database, after adjustment for any general reduction or enhancement of contrast sensitivity. In addition, because the 24-2 threshold visual field test is iterative, the exam duration also potentially represents a general measure of contrast sensitivity performance. Finally, reliability tests were completed, including estimations of the fixation errors, false positive errors, and false negative errors. Fixation errors were tested by a stimulus of 50% contrast in the location of the blind spot,Citation30 which should not be detected if proper fixation is maintained. False positive errors were tested by presenting stimuli at 0% contrast, with any responses to these stimuli considered false positive errors. False negative errors were tested by presenting stimuli at 100% contrast, so that no response would be a false negative error.Citation20,Citation28 The 24-2 test included ten fixation error trials, ten false positive trials, and six false negative trials. If the test was unreliable at first, it could be repeated a second time.
Statistical analysis
Descriptive analysis was used to present the demographic and clinical data. We analyzed global sensitivity as well as cerebral hemisphere sensitivity by comparing the mean deviation (MD) of the schizophrenic patient group, parent group, and control group using analysis of variance (ANOVA). We also analyzed the sensitivity of fibers that cross the optic chiasm (decussating fibers) and those that do not (nondecussating fibers), using the same test.
The MD was used to assess global sensitivity. The right hemisphere analysis included the mean sensitivity of the right temporal and left nasal hemifields. The left hemisphere analysis included the mean sensitivity of the left temporal and right nasal hemifields. The sensitivity analysis for decussating fibers included the nasal hemifields of both sides, and for nondecussating fibers included the temporal hemifields of both sides. Statistical analyses were performed using the SPSS statistical package 17.0 (IBM, Armonk, NY, USA).
Results
The sociodemographic and ophthalmic characteristics of the participants are described in . All the patients were outpatients with diagnosis of schizophrenia according to the DSM-IV. All patients were refractory to treatment with antipsychotics, and they were taking clozapine (average dose 300 mg/day). The majority of the patients were Caucasian (ten out of 13), and three were Asians. The mean duration of disease was 15 years. None in the group was working. The range education was 5 to 10 years. The visual acuity of the whole group was higher than 20/60, as tested by the Snellen chart, and higher than 20/30 after refraction. Intraocular pressure was normal (less than 20 mmHg) for all groups. Group differences in intraocular pressure were not statistically significant and were less than 2 mmHg, which is not clinically relevant and cannot affect the FDT results. Five patients in the schizophrenic group were excluded at the first test, three of whom exhibited >20% loss of fixation and two of whom exhibited both >20% loss of fixation and >33% false negatives. Afterwards, upon retest, they were included once again because the indices were acceptable.
Table 1 Sociodemographic and ophthalmic characteristics of the study participants
presents the global mean sensitivity values in the Matrix FDT test. The MD values in the schizophrenic patients and their parents were significantly lower than those in the control group, but did not differ from one another. shows the distributions of the MD values in the three groups, as box plots.
Figure 1 Distributions of general MD (in dB) in healthy controls, parents, and schizophrenic patients, presented as box plots.
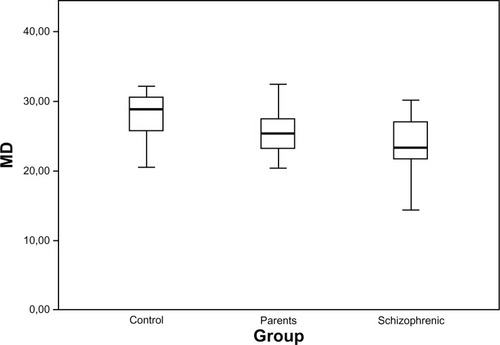
Table 2 Mean sensitivity values (MD) in the FDT test
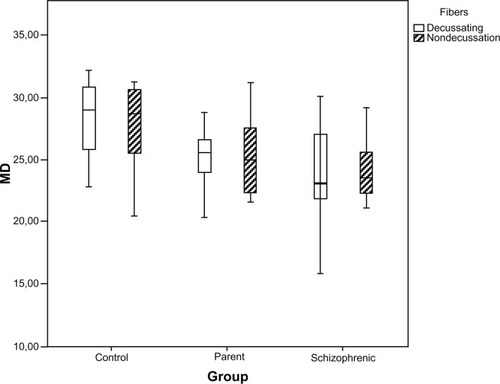
Analysis of the fibers crossing the optic chiasm between the groups (P = 0.006) showed a difference in general sensitivity, indicating that the sensitivity of the fibers crossing the optic chiasm was lower than those which did not cross, but when we analyzed the specific groups, the difference between the fibers was not considerable ().
Figure 2 Distributions of MD (in dB) for the decussating and nondecussating fibers, in healthy controls, parents, and schizophrenic patients, presented as box plots.
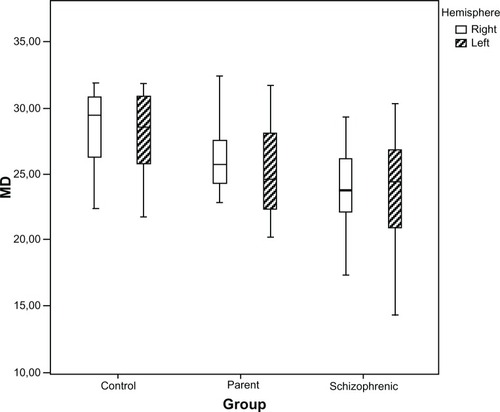
Analysis of the right and left hemispheres showed the general sensitivity was lower for the left hemisphere, but when we analyzed specific groups, this difference was not significant (P = 0.29) ().
Figure 3 Distributions of the MD (in dB) for the right and left hemispheres, in healthy controls, parents, and schizophrenic patients, presented as box plots.
Global MD values were not impacted by age, gender, length of time with a diagnosis of schizophrenia, or the use of antipsychotics.
Discussion
Few physiological studies have examined M pathway functioning in schizophrenia. Three studies using EEG reported reduced signal amplitude in the posterior cortical regions along the M pathway in persons with schizophrenia, with normal activation in the P pathway.Citation13–Citation15 One study used fMRI and reported hypoactivation of the M pathway (particularly in the right hemisphere) in persons with schizophrenia, but no evidence of abnormal functioning in the pathway.Citation16 This is the first article using FDT to detect this loss. Our findings coincide with the literature showing a decrease of M pathway function in schizophrenic patients (MD worse than relatives and control patients).
Another important point is that it has been suggested that early visual processing deficits are a risk factor for, or are an endophenotype of, schizophrenia, as opposed to an effect of the illness.Citation31 Examination of the parents of schizophrenics allows for the identification of the genetic markers of schizophrenia in the absence of confounds that come with the study of the patients themselves, such as neuroleptic exposure, chronic hospitalization, or active symptom effects.Citation2,Citation3 In the present study, we found lower global sensitivity values in both schizophrenic patients and their parents compared with healthy controls. These could be a signal that there is a genetic correspondence to this visual field loss.
There is a relationship between the deficits in the parents and individuals with schizophrenia that can be inferred. Two articles have shown that schizophrenic patients and their first-degree relatives have deficits in the gating of the P50 electrophysiological response to repeated stimuli.Citation32,Citation33 Deficits in the gating of the P50 response also appear in adolescents showing symptoms consistent with a heightened risk for imminent onset of psychosis.Citation34 P50 suppression is assessed using a “paired-click” paradigm, in which two identical stimuli are separated by a brief interstimulus interval. P50 suppression refers to the reduction in P50 amplitude to the second stimuli relative to the first and is considered an index of the strength of “sensory gating.”Citation35 P50 suppression has been linked to chromosome loci, neurotransmitter systems, and anatomical structures implicated in the neuropathology of schizophrenia.Citation36 This suppression deficit has been reported in at-risk individualsCitation37 and unaffected first-degree relatives of schizophrenia probands,Citation38 though heritability is evidenced to be lower than other endophenotype candidates.Citation39
A hemispheric difference in schizophrenic patients has also been suggested. Hellige and CumberlandCitation41 reported that there is some psychophysical evidence that the right hemisphere is more involved with processing M pathway information, such as spatial relationships, than is the left hemisphere. However, visual field researchers have tested this possibility using psychophysical methods and concluded that M pathway function is similar in both hemispheres.Citation40 We found a difference between the right and left hemispheres in the present study when the general MD was considered, but when we analyzed the MD in each group, we did not find any difference. Furthermore, analysis of the sensitivity of the fibers crossing the optic chiasm showed a difference, and this was the first time this has been seen in the literature.
The main clinical implication of our findings is that the M pathway deficit could affect the motion perception, for example. Of course, schizophrenic patients can have negative symptoms but these could be worsened by the visual field loss.
Some specific characteristics of our study should be considered. First, all schizophrenic patients were on antipsychotic treatment at the time of testing. In other words, all these patients were medicated and chronically ill. But no patient was acutely psychotic at the time of testing. Some visual functions, such as spatial contrast sensitivity, temporal sensitivity, and color vision, are controlled in part by dopamine.Citation41 Since antipsychotic medications affect the dopaminergic system, we cannot ignore the possibility of a medication effect in our test results. Second, our sample was small because we had difficulty in identifying clinically controlled patients who were treated with the same drugs and who had available first-degree relatives. Third, there was a difference between the ages of the patients and controls and relatives. For obvious reasons, we cannot pair this variable and this may also have affected the outcome of the FDT. Lastly, the only exam used to detect visual field loss was FDT, but other exams such as optical coherence tomography (OCT) or Humphrey Visual Field analysis could also have been correlated; however, these could not have been used to isolate the M pathway.
In conclusion, we have found that schizophrenic patients and their parents exhibit lower global sensitivity as compared with healthy controls. This effect may be an endophenotype of schizophrenia and may be useful in future studies of the genetic markers of schizophrenia. The present study adds to a growing body of research on early-stage visual processing deficits in schizophrenia.
Acknowledgements
The authors express thanks to Aurea Garibaldi who provided a review of the grammar in the text.
Disclosure
The authors report no conflicts of interest in this work.
References
- Goldman-RakicPSWorking memory dysfunction in schizophreniaJ Neuropsychiatry Clin Neurosci1994643483577841806
- WeinbergerDRGallhoferBCognitive function in schizophreniaInt Clin Psychopharmacol199712Suppl 4S29S369352344
- AdlerLEFreedmanRRossRGOlincyAWaldoMCElementary phenotypes in the neurobiological and genetic study of schizophreniaBiol Psychiatry199946181810394470
- BraffDLSaccuzzoDPGeyerMAInformation processing dysfunctions in schizophrenia: studies of visual backward masking, sensorimotor gating, and habituationZubinJSteinhauerSGruzelierJHHandbook of Schizophrenia; Neuropsychology, Psychophysiology and Information ProcessingAmsterdamElsevier20104303334
- JavittDCLiedermanECienfuegosAShelleyAMPanmodal processing imprecision as a basis for dysfunction of transient memory storage systems in schizophreniaSchizophr Bull199925476377510667746
- TuretskyBIMobergPJOwzarKJohnsonSCDotyRLGurREPhysiologic impairment of olfactory stimulus processing in schizophreniaBiol Psychiatry200353540341112614993
- GreenMFNuechterleinKHBreitmeyerBBackward masking performance in unaffected siblings of schizophrenic patients. Evidence for a vulnerability indicatorArch Gen Psychiatry19975454654729152100
- SkottunBCSkoylesJROn identifying magnocellular and parvocellular responses on the basis of contrast-response functionsSchizophr Bull2011371232620929967
- GreenMFButlerPDChenYPerception measurement in clinical trials of schizophrenia: promising paradigms from CNTRICSSchizophr Bull200935116318119023123
- BreitmeyerBGGanzLImplications of sustained and transient channels for theories of visual pattern masking, saccadic suppression, and information processingPsychol Rev1976831136766038
- LivingstoneMSHubelDHPsychophysical evidence for separate channels for the perception of form, color, movement, and depthJ Neurosci1987711341634683316524
- BedwellJSMillerLSBrownJMYanasakNESchizophrenia and red light: fMRI evidence for a novel biobehavioral markerInt J Neurosci2006116888189416861153
- ButlerPDSchechterIZemonVDysfunction of early-stage visual processing in schizophreniaAm J Psychiatry200115871126113311431235
- DonigerGMFoxeJJMurrayMMHigginsBAJavittDCImpaired visual object recognition and dorsal/ventral stream interaction in schizophreniaArch Gen Psychiatry200259111011102012418934
- FoxeJJDonigerGMJavittDCEarly visual processing deficits in schizophrenia: impaired P1 generation revealed by high-density electrical mappingNeuroreport200112173815382011726801
- BrausDFWeber-FahrWTostHRufMHennFASensory information processing in neuroleptic-naive first-episode schizophrenic patients: a functional magnetic resonance imaging studyArch Gen Psychiatry200259869670112150645
- BedwellJSMillerLSBrownJMMcDowellJEYanasakNEFunctional magnetic resonance imaging examination of the magnocellular visual pathway in nonpsychotic relatives of persons with schizophreniaSchizophr Res2004712–350951015474923
- KériSKelemenOJankaZBenedekGVisual-perceptual dysfunctions are possible endophenotypes of schizophrenia: evidence from the psychophysical investigation of magnocellular and parvocellular pathwaysNeuropsychology200519564965616187883
- GottesmanIIGouldTDThe endophenotype concept in psychiatry: etymology and strategic intentionsAm J Psychiatry2003160463664512668349
- AndersonAJJohnsonCAFrequency-doubling technology perimetryOphthalmol Clin North Am200316221322512809159
- CelloKENelson-QuiggJMJohnsonCAFrequency doubling technology perimetry for detection of glaucomatous visual field lossAm J Ophthalmol2000129331432210704546
- KellyDHNonlinear visual responses to flickering sinusoidal gratingsJ Opt Soc Am1981719105110557277060
- RichardsWFeltonTBSpatial frequency doubling: retinal or central?Vision Res19731311212921374763526
- WhiteAJSunHSwansonWHLeeBBAn examination of physiological mechanisms underlying the frequency-doubling illusionInvest Ophthalmol Vis Sci200243113590359912407172
- MaddessTHemmiJMJamesACEvidence for spatial aliasing effects in the Y-like cells of the magnocellular visual pathwayVision Res19983812184318599797962
- MaddessTJamesACGoldbergIWineSDobinsonJA spatial frequency-doubling illusion-based pattern electroretinogram for glaucomaInvest Ophthalmol Vis Sci200041123818382611053281
- SamplePABosworthCFBlumenthalEZGirkinCWeinrebRNVisual function-specific perimetry for indirect comparison of different ganglion cell populations in glaucomaInvest Ophthalmol Vis Sci20004171783179010845599
- ZeppieriMDemirelSKentKJohnsonCAPerceived spatial frequency of sinusoidal gratingsOptom Vis Sci200885531832918451736
- McKendrickAMTurpinAAdvantages of terminating Zippy Estimation by Sequential Testing (ZEST) with dynamic criteria for white-on-white perimetryOptom Vis Sci2005821198198716317375
- AsmanPFingeretMRobinAKinetic and static fixation methods in automated threshold perimetryJ Glaucoma19998529029610529927
- GreenMFNuechterleinKHBackward masking performance as an indicator of vulnerability to schizophreniaActa Psychiatr Scand Suppl1999395344010225331
- FreedmanROlincyARossRGThe genetics of sensory gating deficits in schizophreniaCurr Psychiatry Rep20035215516112685995
- WintererGEganMFRaedlerTP300 and genetic risk for schizophreniaArch Gen Psychiatry200360111158116714609891
- Myles-WorsleyMOrdLBlailesFNgiralmauHFreedmanRP50 sensory gating in adolescents from a pacific island isolate with elevated risk for schizophreniaBiol Psychiatry200455766366715038993
- FreedmanRAdlerLEWaldoMCPachtmanEFranksRDNeurophysiological evidence for a defect in inhibitory pathways in schizophrenia: comparison of medicated and drug-free patientsBiol Psychiatry19831855375516134559
- LeonardSGaultJHopkinsJAssociation of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophreniaArch Gen Psychiatry200259121085109612470124
- CadenheadKSLightGAGeyerMABraffDLSensory gating deficits assessed by the P50 event-related potential in subjects with schizotypal personality disorderAm J Psychiatry20001571555910618013
- ClementzBAGeyerMABraffDLPoor P50 suppression among schizophrenia patients and their first-degree biological relativesAm J Psychiatry199815512169116949842777
- GreenwoodTABraffDLLightGAInitial heritability analyses of endophenotypic measures for schizophrenia: the consortium on the genetics of schizophreniaArch Gen Psychiatry200764111242125017984393
- HelligeJBCumberlandNCategorical and coordinate spatial processing: more on contributions of the transient/magnocellular visual systemBrain Cogn200145215516311237364
- DjamgozMBHankinsMWHiranoJArcherSNNeurobiology of retinal dopamine in relation to degenerative states of the tissueVision Res19973724350935299425527