Abstract
Purpose
To compare the cytotoxic effects of preservative-free azithromycin on corneal epithelial cells in vivo with those of preservative-free netilmicin and levofloxacin, and the preservative benzalkonium chloride (BAK).
Methods
Rabbit corneal epithelial cells in vitro were incubated for 15 minutes or 6 hours with commercially available ophthalmic preservative-free netilmicin 0.3%, levofloxacin 0.3%, or azithromycin 1.5% preparations or different concentrations of unpreserved azithromycin and different concentrations of BAK. Qualitative analysis was undertaken using phase-contrast optics to examine the morphological aspects of cell cultures and quantitative analysis was undertaken by measuring the release of the cytoplasmic enzyme lactate dehydrogenase into the medium immediately and 24 hours after exposure to drugs. Finally, we observed the wound-healing rate of mechanically injured corneal epithelial cells exposed to each antibiotic ophthalmic preparation for 48 hours.
Results
Our results show that both the commercially available unpreserved mono-dose preparation of azithromycin and ophthalmic preparations of azithromycin up to a concentration of 1.5% were virtually devoid of harmful effects under our experimental conditions. This was not significantly different from the results obtained for the other antibiotic preparations (P > 0.05) tested, but was unlike the results obtained for BAK. Azithromycin 1.5% also showed good recovery properties after a mechanical wound test.
Conclusion
Under our experimental conditions, unpreserved azithromycin 1.5% showed a much lower toxicity than BAK and did not interfere with the wound-healing process.
Introduction
Azithromycin is an azalide, a second-generation 15-C-atom macrolide antibiotic with broad-spectrum activity against Gram-positive, Gram-negative, and atypical bacteria such as Chlamydia trachomatis. It shows a potency higher than erythromycin against Haemophilus influenzae and Neisseria gonorrhoeae in vitro.Citation1,Citation2
Azithromycin has an intracellular action, as it binds to the bacterial ribosomal subunit 50S and inhibits microbial protein synthesis. It is also rapidly distributed in the tissue and has good intracellular penetration and transport into phagocytic cells, reaching high and sustained levels in tissue – especially at infection sites – including in ocular tissue. Thanks to its optimal pharmacokinetic profile, this antibiotic exhibits good bacteriostatic and bactericidal time-dependent action and a long post-antibiotic effect, thus permitting a short oral or topical 3-day administration regimen.Citation3–Citation7
In vitro studies have reported that azithromycin has anti-inflammatory and immunomodulatory actions in addition to its antimicrobial activity, such as the inhibition of cytokine and proinflammatory mediator synthesis and inflammatory cell migration, and the suppression of the nuclear factor kappa B signaling transduction pathway,Citation8–Citation12 thus providing a rationale for clinical investigation into the off-label use of azithromycin eye drops in chronic blepharitis.Citation1
Azithromycin 1.5% ophthalmic solution is approved in several countries in the European Union for the topical treatment of purulent bacterial conjunctivitis caused by susceptible strains and of trachomatous conjunctivitis caused by Chlamydia trachomatis.Citation1,Citation13–Citation16 Azithromycin 1.5% ophthalmic solution proved effective and was well tolerated in patients with these infections.Citation14,Citation15 Two well-designed studies showed that in terms of clinical cure rates, treatment with azithromycin 1.5% ophthalmic solution for 3 days was non-inferior to treatment with tobramycin 0.3% ophthalmic solution for 7 days in pediatric and adult patients with purulent bacterial conjunctivitis and non-inferior to a single dose of azithromycin oral suspension in pediatric patients with trachomatous conjunctivitis.Citation14,Citation15 Moreover, an azithromycin 1.5% ophthalmic solution treatment regimen is shorter and has less frequent dosing requirements than a tobramycin treatment regimen (potentially improving compliance), and has been associated with quicker resolution of clinical signs of bacterial conjunctivitis.Citation14,Citation15
The most common adverse events listed in the summary of product characteristics as occurring during clinical trials and from post-marketing safety data are related to ocular discomfort (ie, pruritus, burning, and stinging) on instillation. Less common adverse events include blurred vision, sticky eye sensation, and foreign body sensation on instillation. To the best of our knowledge, no in vitro studies on the toxicity of an azithromycin ophthalmic solution on corneal epithelial cells has been reported. However, Wingard et al performed an azithromycin toxicity assay on corneal epithelial cells as part of an extensive study designed to investigate the ability of some antibiotics to protect mammalian cells from destruction by bacteria, and showed that azithromycin had low in vitro toxicity.Citation17
The aim of this in vitro study was to evaluate the cytotoxic effects of a commercially available azithromycin 1.5% ophthalmic solution on rabbit corneal epithelial cells and to compare these effects with those of other commonly used antibiotic eye drops. Benzalkonium chloride (BAK) was used as positive control. BAK is a quaternary ammonium compound used as a preservative in over 70% of the existing multi-dose ophthalmic solution bottles, at an average concentration of 0.01%. BAK is known to promote activation of lipoxygenases and the synthesis and secretion of eicosanoids, inflammatory mediators, and many cytokines such as interleukin (IL)-1α, IL-8, IL-10, and tumor necrosis factor-alpha, resulting in delayed hypersensitivity and allergic reactions.Citation18
Three mechanisms of BAK toxicity have been described: (1) a detergent effect causing loss of tear-film stability, (2) direct damage to the corneal and conjunctival epithelium, and (3) an immunoallergic reaction. It acts by denaturing proteins and disrupting cytoplasmic membranes.Citation18
Materials and methods
Incubation with drugs
Statens Serum Institut rabbit corneal (SIRC) cells were purchased from Sigma-Aldrich (St Louis, MO, USA). Cells were resuspended and seeded into 24-well plates (approximately 4 × 10−4 cells/cm2) using a medium containing 90% Eagle’s minimal essential medium, 10% heat-inactivated fetal bovine serum, and 1 mmol/L glutamine. When they reached approximately 70%–80% confluence, the culture medium was removed and cells were exposed to one of the following preservative-free commercially available ophthalmic preparations: azithromycin 1.5% (Azyter; Laboratoires Théa, Clermont-Ferrand, France); caprylic/capric acid triglyceride (Miglyol®, Laboratoires Théa), a vehicle of Azyter, a medium-chain-length triglyceride of saturated fatty acids (Laboratoires Théa); netilmicin 0.3% (Nettacin®, Società Industria Farmaceutica Italiana, Catania, Italy); and levofloxacin 0.3% (Oftaquix, Bausch and Lomb, Milan, Italy). Three different concentrations of BAK (0.0025%, 0.0050%, and 0.0100%) were used as toxicity controls, whereas 0.9% NaCl was used as negative control. Moreover, azithromycin solution (the active principle of Azyter) was applied in five different concentrations ranging from 0.5% to 2.5%.
SIRC cells were incubated with drugs according to three different protocols: (1) short-term (ie, 15 minutes) exposure, (2) short-term exposure followed by the addition of drug-free medium for a 24-hour recovery period, and (3) long-term (ie, 6 hours) exposure.
Phase-contrast microscopy
After incubation with drugs according to the three experimental protocols, the SIRC cells were evaluated qualitatively in a blind fashion and photographed by an inverted phase-contrast microscope (Olympus IX-50; Olympus Europa Holding GmbH, Hamburg, Germany). Morphologic criteria indicating cytotoxic drug effects – “rounding” of cells, loss of processes, increased granularity, isolation of cells, vacuolization, and cell detachment – were assessed semiquantitatively according to criteria originally described by Seitz et al,Citation19 with some modifications: cellular morphology (normal, 0; slight alterations, +; altered, ++), cellular boundaries (not recognizable, 0; mixed shapes, +; good definition, ++), nucleus and cytoplasm (normal, 0; slight alterations, +; altered, ++), nuclear morphology (well-spread chromatin, 0; mixed shapes, +; altered [pycnosis, cariorexis, etc], ++), and vacuolization (absent, 0; rare vacuoles, +; vacuolization present, ++).
Lactate dehydrogenase (LDH) assay
Cell damage in SIRC cells was quantitatively evaluated by measuring the amount of the soluble cytosolic enzyme LDH released from injured cells into the extracellular fluid immediately or 24 hours after exposure to drugs, as previously described for neuronsCitation20 or corneal fibroblasts.Citation21
The LDH level corresponding to complete cell death was determined for each experiment by assaying sister cultures exposed to BAK (0.0025%–0.0100%) for the same incubation period used for experimental drugs. Background LDH release was determined in control cultures not exposed to drugs and was subtracted from all experimental values. The resulting values correlated linearly with the degree of cell loss estimated by observation of cultures under phase-contrast optics or under bright-field optics following 5 minutes incubation with 0.4% trypan blue, which stains debris and nonviable cells. All experiments were carried out in triplicate. The activity of LDH was quantified using a Cytotoxicity Detection Kit (LDH) from Roche Diagnostics (Basel, Switzerland).
Analysis of corneal wound healing
SIRC cell cultures were wounded using a small scalpel in 24-well culture plates.Citation22 The injured SIRC cell cultures were incubated with Azyter, Miglyol, NaCl, or BAK for 24 or 48 hours. Three different experiments (n = 6 per group) were undertaken. The extent of healing was evaluated qualitatively in a blind fashion and photographed by an inverted phase-contrast microscope (Olympus IX-50) at 0 and 48 hours. To quantify wound areas, they were identified and encompassed in a frame using Image-Pro Plus morphometric analysis software (version 5.0 Media Cybernetics, Rockville, MD, USA) and the extent of healing was evaluated quantitatively by determining the ratio of the difference between the wound areas at time 0 and remaining wound areas after 48 hours.
Statistical analysis
Data are presented here as mean ± standard error of the mean of n experiments. The statistical significance of differences for the results displayed in –, and 7 was analyzed using a one-way analysis of variance and Dunnett’s post-hoc test. A P value of 0.05 was considered significant. All statistical calculations were performed using GraphPad Prism (v 4; GraphPad Software, San Diego, CA, USA).
Results
Phase-contrast microscopy
SIRC cell cultures examined by phase-contrast microscopy following their incubation with Azyter, Miglyol, Nettacin, or Oftaquix demonstrated a pattern of cell toxicity that was negligible and comparable to that observed in control cultures ( and ).
Figure 1 Phase-contrast micrographs of untreated (control) and benzalkonium chloride (BAK)-treated cultured rabbit corneal epithelial cells.
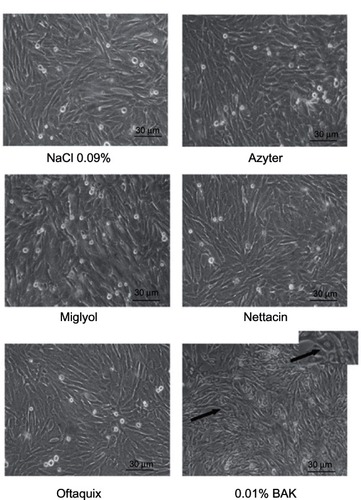
Figure 2 Evaluation of lactate dehydrogenase (LDH) release in Statens Serum Institut rabbit corneal (SIRC) cell cultures after (A) 15 minutes or (B) 6 hours of incubation with commercially available ophthalmic preparations of azithromycin (Azyter; Laboratoires Théa, Clermont-Ferrand, France), netilmicin (nettacin®; Società Industria Farmaceutica Italiana, Catania, Italy), and levofloxacin (Oftaquix; Bausch and Lomb, Milan, Italy) and benzalkonium chloride (BAK; 0.0025%–0.0100%). (C) Evaluation of LDH release in SIRC cell cultures 24 hours after 15 minutes of incubation with all compounds.
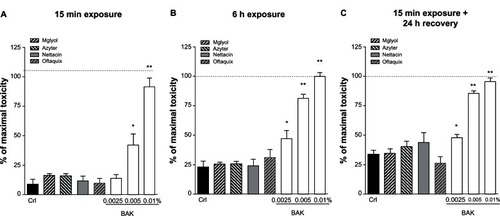
Table 1 Assessment of severity of morphologic criteria
In contrast, the addition of BAK to the incubation medium resulted in a time- and dose-dependent increase in the appearance of cytotoxicity signs, including rounding of the cell bodies, loss of processes, and vacuolization of the cytoplasm, which eventually led to cell detachment and death ( and ).
LDH assay
Using LDH as a quantitative cytotoxicity index, we examined the extent of SIRC cell death (). The maximal degree of cell death was produced in our culture system by incubation for 6 hours with 0.01% BAK, which produced a release of LDH (115 units/L) that was approximately threefold higher than the basal levels detected at the same time point (32 units/L) (). Incubation with 0.01% BAK for only 15 minutes produced a similar increase in LDH release (105 units/L) that was sixfold higher than the basal levels at the same time point (16 units/L) (). Incubation with 0.0025%–0.0050% BAK evoked a similar time-dependent but less pronounced increase in the release of LDH from SIRC cell cultures ().
After 15 minutes of incubation, all compounds displayed negligible SIRC cell death (). A similar pattern supporting the nontoxic effects of Azyter and Miglyol was observed after 6 hours (). To examine whether these compounds could exert their toxic effects at more distant time intervals following exposure, we exposed SIRC cell cultures to all compounds for 15 minutes and measured LDH release 24 hours later (). Our results showed that Azyter was devoid of toxicity even when cell death was assessed at 24 hours after its application to SIRC cell cultures.
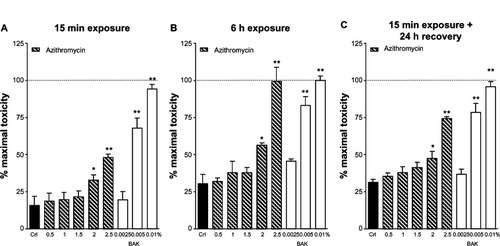
Finally, we tested different concentrations of azithromycin (0.5%–2.5%) according to our three different protocols (). Azithromycin displayed no signs of toxicity at concentrations below 2.0% but displayed signs of toxicity at 2.0% and 2.5% that were comparable to those displayed by BAK 0.005% (after 15 minutes of exposure) or 0.01% (after 6 hours of exposure).
Figure 3 Evaluation of lactate dehydrogenase (LDH) release in Statens Serum Institut rabbit corneal (SIRC) cell cultures after (A) 15 minutes or (B) 6 hours of incubation with azithromycin 0.5%–2.5%. (C) Evaluation of LDH release in SIRC cultures 24 hours after 15 minutes incubation with all different concentrations of azithromycin.
Corneal wound healing
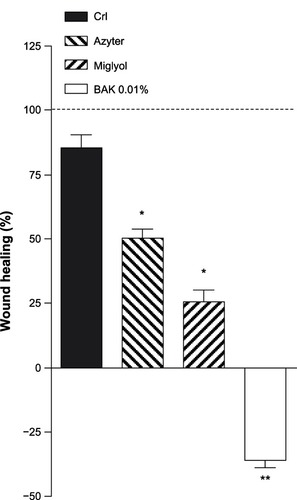
In control SIRC cell cultures (NaCl 0.9%), analysis of the corneal wound surface revealed that the wound was almost completely covered and repaired after 48 hours (87.5%). Treatment with Azyter or Miglyol for 48 hours somewhat reduced wound healing (47.3% and 25.6%, respectively), whereas incubation with 0.01% BAK for 48 hours severely inhibited epithelial wound healing (−36%) ( and ).
Figure 4 Phase-contrast micrographs of wounded cultured Statens Serum Institut rabbit corneal (SIRC) cells.
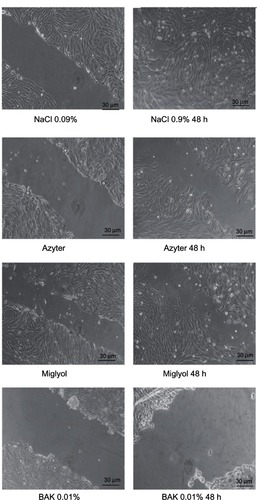
Figure 5 The wound-healing rates in Statens Serum Institut rabbit corneal (SIRC) cells incubated with different agents.
Abbreviation: BAK, benzalkonium chloride.
Discussion
While the safety of azithromycin ophthalmic solutions has been largely studied in vivo in rabbits, to our knowledge, this is the first in vitro study on the toxicity of an azithromycin ophthalmic solution in corneal epithelial cells. The aim of our study was to evaluate the cytotoxic effects of a commercially available azithromycin 1.5% ophthalmic solution in rabbit corneal epithelial cells and to compare these with those of other commonly used antibiotic eye drops.
The current literature supports the efficacy and safety of topical azithromycin ophthalmic 1.5% solution in the treatment of various ocular surface disorders, namely bacterial conjunctivitis, blepharitis, and dry eye. Azithromycin has been shown to be efficacious against the most common bacterial pathogens of infective conjunctivitis (ie, Staphylococcus aureus, Haemophilus influenzae, Streptococcus pneumoniae)Citation23 and is indicated for the treatment of bacterial conjunctivitis, with the recommended dosing regimen being one drop in the affected eye twice daily (8–12 hours apart) for the first 2 days, then one drop once daily for the next 5 days.
The combination of anti-inflammatory properties along with high and prolonged tissue concentrations, particularly in the lid margin and ocular surface, suggests that an azithromycin ophthalmic solution may serve as a treatment option for patients experiencing a wide range of conditions associated with the lid margin and ocular surface.Citation1 The treatment of trachoma with topical azithromycin in addition to an oral dose is also encouraging.Citation14
In the present study, we incubated corneal epithelial cells for 15 minutes or 6 hours with preservative-free netilmicin or levofloxacin and different concentrations of unpreserved azithromycin. Qualitative analysis was undertaken using phase-contrast optics and examination of the morphological aspects of the cell cultures. Quantitative analysis was performed by measuring the release of cytoplasmic enzyme LDH into the medium immediately and 24 hours after exposure to the study drugs. We also studied the wound-healing rates of mechanically injured corneal epithelial cells cultured for 48 hours. According to our results, unpreserved preparation of azithromycin up to a concentration of 1.5% showed a low cell toxicity, which was not significantly different from the other antibiotic preparations (P > 0.05), representative of the major antibiotic families of aminoglycosides and fluoroquinolones and available in preservative-free formulations.
Moreover, azithromycin did not inhibit the wound-healing process after the mechanical injury. The unpreserved preparation of azithromycin was virtually devoid of harmful effects under our experimental conditions, up to a concentration of 1.5% and also showed good recovery properties after a mechanical wound test. In control SIRC cell cultures (NaCl 0.9%), qualitative analysis of the corneal wound surface demonstrated that the wound was almost completely covered and repaired after 48 hours. Treatment with Azyter or Miglyol for 48 hours slightly reduced cell wound healing, whereas incubation with 0.01% BAK for 48 hours severely delayed epithelial wound healing. This evidence supports our hypothesis concerning the cytotoxic role of preservatives in commercial preparations of eye drops and the relative non-toxicity of mono-dose unpreserved antibiotics already demonstrated by a previous study.Citation24 The low toxic effects of azithromycin may work synergistically with the previously described anti-inflammatory effects on the ocular surface.
Conclusion
This cell-culture study provides remarkable information about the in vitro toxicity profile of an unpreserved 1.5% azithromycin ophthalmic solution. Even though this study has the limitations of an in vitro analysis, the available clinical data on tolerability appear in line with our results.
Disclosure
The authors declare no conflicts of interest in this work.
References
- Bremond-GignacDChiambrettaFMilazzoSA European perspective on topical ophthalmic antibiotics: current and evolving optionsOphthalmol Eye Dis201132943
- RetsemaJGirardASchelklyWSpectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organismsAntimicrob Agents Chemother19873112193919472449865
- AmsdenGWAdvanced-generation macrolides: tissue-directed antibioticsInt J Antimicrob Agents200118 Suppl 1S11S1511574189
- GladueRPSniderMEIntracellular accumulation of azithromycin by cultured human fibroblastsAntimicrob Agents Chemother1999346105610602168141
- GladueRPBrightGMIsaacsonRENewborgMFIn vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infectionAntimicrob Agents Chemother19893332772822543276
- LiuPAllaudeenHChandraRComparative pharmacokinetics of azithromycin in serum and white blood cells of healthy subjects receiving a single-dose extended-release regimen versus a 3-day immediate-release regimenAntimicrob Agents Chemother200751110310917060516
- SchentangJJBallowCHTissue-directed pharmacokineticsAm J Med1991913A5S11S
- AmsdenGWAnti-inflammatory effects of macrolides – an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions?J Antimicrob Chemother2005551102115590715
- IanaroAIalentiAMaffiaPAnti-inflammatory activity of macrolide antibioticsJ Pharmacol Exp Ther2000292115616310604943
- Fernandez-RobredoPRecaldeSMoreno-OrduñaMGarcía-GarcíaLZarranz-VenturaJGarcía-LayanaAAzithromycin reduces inflammation in a rat model of acute conjunctivitisMol Vis20131915316523378729
- HoffmannNLeeBHentzerMAzithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates chronic P. aeruginosa lung infection in Cftr(−/−) miceAntimicrob Agents Chemother200751103677368717620382
- HoweRASpencerRCMacrolides for the treatment of Pseudomonas aeruginosa infections?J Antimicrob Chemother19974021531559301978
- Bremond-GignacDMariani-KurkdjianPBeresniakAEfficacy and safety of azithromycin 1.5% eye drops for purulent bacterial conjunctivitis in pediatric patientsPediatr Infect Dis J201029322222619935122
- CochereauIGoldschmidtPGoepoguiAEfficacy and safety of short duration azithromycin eye drops versus azithromycin single oral dose for the treatment of trachoma in children: a randomised, controlled, double-masked clinical trialBr J Ophthalmol200791566767217005549
- CochereauIMeddeb-OuertaniAKhairallahM3-day treatment with azithromycin 1.5% eye drops versus 7-day treatment with tobramycin 0.3% for purulent bacterial conjunctivitis: multicentre, randomised and controlled trial in adults and childrenBr J Ophthalmol200791446546917050578
- Garnock-JonesKPAzithromycin 1.5% ophthalmic solution: in purulent bacterial or trachomatous conjunctivitisDrugs201272336137322316352
- WingardJBRomanowskiEGKowalskiRPA novel cell-associated protection assay demonstrates the ability of certain antibiotics to protect ocular surface cell lines from subsequent clinical Staphylococcus aureus challengeAntimicrob Agents Chemother20115583788379421628536
- ValenteCLesterMImpact of glaucoma medication on ocular tissueExpert Rev Ophthalmol201053405412
- SeitzBHayashiSWeeWRLa BreeLMc DonnellPJIn vitro effects of aminoglycosides and fluoroquinolones on keratocytesInvest Ophthalmol Vis Sci1996376566658595966
- Pellegrini-GiampietroDECozziAPeruginelliF1-Aminoindan-1,5-dicarboxylic acid and (S)-(+)-2-(3′-carboxybicyclo[1.1.1] pentyl)-glycine, two mGlu1 receptor-preferring antagonists, reduce neuronal death in in vitro and in vivo models of cerebral ischaemiaEur J Neurosci199911103637364710564371
- KimTITchahHLeeSASungKChoBJKookMSApoptosis in keratocytes caused by mitomycin CInvest Ophthalmol Vis Sci20034451912191712714623
- KimSYLimJAChoiJSChoiECJooCKComparison of antibiotic effect and corneal epithelial toxicity of levofloxacin and moxilfloxacin in vitroCornea200726672072517592324
- SealDVBarrettSPMcGillJIAetiology and treatment of acute bacterial infection of the external eyeBr J Ophthalmol19826663573607082605
- MencucciRPaladiniIPellegrini-GiampietroDEIn vitro comparison of the cytotoxic effects of clinically available ophthalmic solutions of fluoroquinolones on human keratocytesCan J Ophthalmol201146651352022153639