Abstract
Introduction
Previous work has shown that besifloxacin, an 8-chloro-fluoroquinolone, has more potent activity against gram-positive pathogens than moxifloxacin and gatifloxacin, which carry an 8-methoxy group. This study was conducted to determine the contribution of the R7 and R8 substituent to fluoroquinolone antibacterial activity.
Materials and methods
Besifloxacin, moxifloxacin, gatifloxacin, their R8 structural analogs, and ciprofloxacin were tested against representative isolates of various gram-positive and gram-negative species and previously characterized fluoroquinolone-resistant mutants of Staphylococcus aureus. Minimum inhibitory and minimum bactericidal concentrations were determined according to Clinical and Laboratory Standards Institute (CLSI) guidelines. Reserpine was used to determine the effect of efflux pumps on antibacterial activity.
Results
In general, exchanging the R8 residue in besifloxacin slightly reduced the molecule’s potency, while introducing an 8-chloro group in moxifloxacin increased its potency. A similar change in gatifloxacin had little to no effect. Substituting the R8 residues did not increase the susceptibility to the efflux pump inhibitor reserpine or result in a loss of bactericidal activity. In contrast, the positive control, ciprofloxacin, was shown to be a substrate for reserpine and lost bactericidal activity against some fluoroquinolone-resistant isolates of S. aureus.
Conclusion
The data presented here show that, depending on the R7 substituent, replacing an 8-methoxy group with an 8-chloro substituent can improve potency or can have little-to-no effect. These findings highlight the importance of the interplay between the R7 and R8 substituents in determining antibacterial potency.
Introduction
Fluoroquinolones have a broad spectrum of antibacterial activity, which makes them an ideal choice for the empiric treatment of infections of the surface of the eye. Ciprofloxacin, moxifloxacin, and gatifloxacin were approved many years ago for the systemic treatment of bacterial infections and more recently, for the treatment of bacterial conjunctivitis. However, resistance to commonly used fluoroquinolones is becoming more prevalent, even among ocular isolates.Citation1
The potent antibacterial action of the fluoroquinolones is due to their ability to bind to the essential enzymes DNA gyrase and topoisomerase IV, which leads to double strand breaks in the DNA that ultimately results in cell death.Citation2 Bacterial resistance to this class of drugs primarily arises due to spontaneous mutations within the gyrA, gyrB, parC and parE genes that encode DNA gyrase and topoisomerase IV. In each of the four genes, most mutations that confer high-level fluoroquinolone resistance map within “hot spot” regions termed quinolone resistance-determining regions (QRDRs). Other resistance mechanisms, such as efflux pumps, can also play a contributing role in some instances.Citation3
Besifloxacin is a novel chloro-fluoroquinolone that was approved in 2009 exclusively for the topical treatment of bacterial conjunctivitis.Citation4 Compared with the older fluoroquinolones, besifloxacin is unique due to the combination of a 7-azepinyl group and an 8-chloro substituent, making it the first chloro-fluoroquinolone in ophthalmic use (). By comparison, the two fluoroquinolones most similar in structure and potency, moxifloxacin and gatifloxacin, both carry a methoxy group in the R8 position and a pyrrolol-pyridinyl or methyl-piperazinyl substituent, respectively, in the R7 position.Citation5,Citation6
Figure 1 Chemical structure of the fluoroquinolones tested in this study.
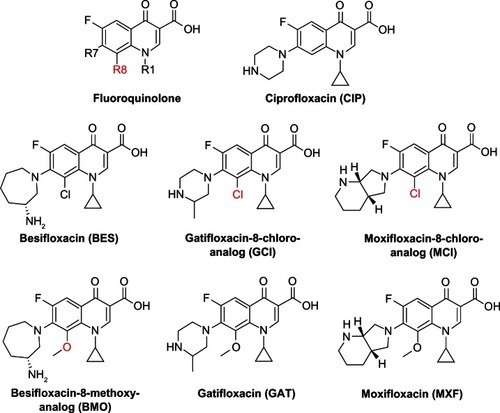
Similar to many classes of antibacterial agents, the fluoroquinolones have undergone many rounds of chemical modifications to optimize their antibacterial, pharmacokinetic, and pharmacodynamic properties. One modification involves altering the substituent in the R8 position. Using a number of analogs to ciprofloxacin, gatifloxacin, and moxifloxacin, Lu et alCitation7 found that molecules with an 8-H atom were less potent than those with an 8-chloro or an 8-methoxy group. Interestingly, the differences in the analogs potency were more pronounced in the fluoroquinolone-resistant isolates of Mycobacterium smegmatis and Staphylococcus aureus when compared to the susceptible strains.
Besifloxacin was shown to be even more potent than moxifloxacin and gatifloxacin against gram-positive pathogens, while maintaining adequate activity against the gram-negatives.Citation8 A recent study by Sanfilippo et alCitation9 showed antibacterial activity to follow the order: besifloxacin > moxifloxacin > gatifloxacin > ciprofloxacin, when tested against 52 ocular clinical isolates of S. aureus.Citation9 Consistent with Lu’s findings, the differences in antibacterial potency were more evident among the resistant isolates and generally increased proportionally to the number of mutations in the QRDRs of DNA gyrase and topoisomerase IV.
This improved activity of besifloxacin could be due to the unique 7-azepinyl substituent, the 8-chloro substituent that is lacking in the comparator drugs, or a combined effect of the R7 and R8 substituents. Since besifloxacin, moxifloxacin (aka BAY 12-8039), and gatifloxacin (aka AM1155, CG5501, or PD135432) differ only by their substituents in the R7 and R8 position, it was of interest to compare those molecules and their corresponding R8 structural analogs.Citation5,Citation7 Therefore, the 8-methoxy structural analog of besifloxacin (BMO), the 8-chloro analog of gatifloxacin (GCl) (aka PD138124), and the 8-chloro analog of moxifloxacin (MCl) (aka BAY y 3118) were obtained and compared with respect to their antibacterial potency ().Citation6,Citation7
Materials and methods
Bacterial strains
The bacterial strains used in this study are listed in . The quality control strains Enterococcus faecalis ATCC 29212, S. aureus ATCC 29213, Streptococcus pneumoniae ATCC 49619, Escherichia coli ATCC 25922, Haemophilus influenzae ATCC 49247, and Pseudomonas aeruginosa ATCC 27853, as well as the wild-type clinical isolate P. aeruginosa PAO1 were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). The P. aeruginosa strains B1181 and D2133 were isolated at the University of Mississippi VA Medical Center (Jackson, MS, USA). The H. influenzae strain Hin1 was obtained from Eurofins Medinet Inc (Chantilly, VA, USA). The 52 S. aureus clinical isolates have been characterized and described previously.Citation9 The isolates were classified as ciprofloxacin-resistant, based on the Clinical and Laboratory Standards Institute (CLSI) interpretative criteria, and placed into different groups according to their QRDR mutations.Citation9,Citation10
Table 1 Bacterial strains and groups of Staphylococcus aureus clinical isolates used in this study
Drugs and analogs
Besifloxacin was obtained from Bausch and Lomb, Inc (Rochester, NY, USA). Moxifloxacin, gatifloxacin, and ciprofloxacin were obtained from LKT Laboratories (St Paul, MN, USA). The 8-methoxy analog of besifloxacin, BMO, was synthesized by Dr Azhwarsamy Jeganathan at Bausch and Lomb, Inc (Rochester, NY, USA). Moxifloxacin and gatifloxacin and their 8-chloro analogs, MCl and GCl, respectively, were synthesized by Alembic Research Centre (Vadodara, India). All molecules made by Alembic were tested and confirmed by high-performance liquid chromatography (HPLC) and mass spectroscopy. The moxifloxacin and gatifloxacin produced by Alembic had the same antibacterial activity as the commercially available reagents. The efflux pump inhibitor reserpine was obtained from SPEX CertiPrep Group LLC (Metuchen, NJ, USA), and ethidium bromide was obtained from EMD Chemicals (Gibbstown, NJ, USA). All antimicrobial agents were solubilized and diluted as recommended by the manufacturers.
Antimicrobial susceptibility testing
All antimicrobial susceptibility tests were performed in triplicate; for each strain, modal or, when modal values could not be defined, central minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values are reported here. MIC testing was performed by the broth microdilution method in accordance with CLSI reference methods (CLSI M07-A8).Citation11 Briefly, 96-well panels containing serial twofold dilutions of antimicrobial agent were inoculated with ~5 × 104 colony forming units per well; the panels were incubated according to CLSI guidelines, and the MIC was reported as the lowest antimicrobial concentration that inhibited the visible growth of bacteria. To test for the contribution of the efflux pump NorA to the fluoroquinolone resistance of S. aureus, the MIC measurements were also performed in the presence of 20 μg/mL of the pump inhibitor reserpine as described elsewhere,Citation9 using ethidium bromide as a positive control.
Bactericidal activities were measured as follows: after overnight incubation to determine MIC values, MBC values were determined by spotting 10 μL from those wells that were at and above the recorded MIC values on drug-free agar medium, in accordance with CLSI reference methods (CLSI M26-A).Citation12 The number of surviving colony forming units after overnight incubation were counted and compared with the inocula. The MBC was defined as the drug concentration that resulted in a ≥3 log decrease in viable bacteria.
Results
Analogs against various species
In order to determine the contribution of the R7 and R8 substituents to the antimicrobial efficacy of the seven fluoroquinolones, we determined the MIC values against various gram-positive and gram-negative species, including fluoroquinolone-resistant isolates ().
Table 2 Minimum inhibitory concentrations of besifloxacin and comparators against various species and phenotypes
The MIC values for besifloxacin were identical to or twofold lower than those of BMO, indicating that the R8 substituent did not influence antibacterial activity when the R7 substituent was an azepinyl moiety.
In contrast, the 8-chloro analog of moxifloxacin, MCl, was fourfold more potent than moxifloxacin itself against each of the three gram-positive species, while MCl was eight- to 16-fold more active than moxifloxacin against gram-negative strains. This indicates that a chloro substituent in the R8 position does improve potency if the R7 substituent is a pyrrolol-pyridinyl group.
The activity of the 8-chloro analog of gatifloxacin, GCl, was the same as that of gatifloxacin against S. aureus and was twofold lower against E. faecalis and S. pneumoniae. GCl was twofold more potent than gatifloxacin against E. coli and P. aeruginosa, while both drugs were equally potent against H. influenzae. In this instance, with a methyl-piperazinyl moiety in the R7 position, an 8-chloro group either had no effect on potency or decreased it by twofold.
While the fluoroquinolones besifloxacin and gatifloxacin, with an azepinyl or a methyl-piperazinyl group in the R7 position, respectively, were little affected by the 8-chloro or 8-methoxy substituent, the activity of the 7-pyrrolol-pyridinyl substituent-containing moxifloxacin and MCl were more strongly influenced by the nature of the R8 moiety. Overall, these data show that, depending on the bacterial species and the R7 substituent, replacing the 8-methoxy with an 8-chloro group can improve potency, have no effect, or reduce potency. This finding highlights the importance of the interplay between the R7 and R8 substituents in determining antibacterial potency.
Activity against 53 S. aureus isolates
Previous work with groups of ciprofloxacin-resistant S. aureus mutants has shown that fluoroquinolone MIC values generally increased with the number of mutations in the gyrA, gyrB, parC, and parE genes and that this increase affected older fluoroquinolones more drastically than newer ones.Citation9 This suggested that the R7, the R8, or a combination of the two moieties was able to reduce the impact of resistance-conferring mutations. To address this issue, we tested besifloxacin, moxifloxacin, gatifloxacin, their respective R8 analogs, and ciprofloxacin against six groups of S. aureus strains that differed in their levels of ciprofloxacin-resistance and that contained various QRDR mutations known to contribute to resistance ().
Figure 2 Minimum inhibitory concentrations of various clinical isolates of Staphylococcus aureus against the fluoroquinolones besifloxacin, besifloxacin-8-methoxy analog, moxifloxacin-8-chloro analog, moxifloxacin, gatifloxacin-8-chloro analog, gatifloxacin, and ciprofloxacin.
Abbreviations: BES, besifloxacin; BMO, besifloxacin-8-methoxy analog; CIP, ciprofloxacin; MCI, moxifloxacin-8-chloro analog; MIC, minimum inhibitory concentrations; MXF, moxifloxacin; GAT, gatifloxacin; GCI, gatifloxacin-8-chloro analog.
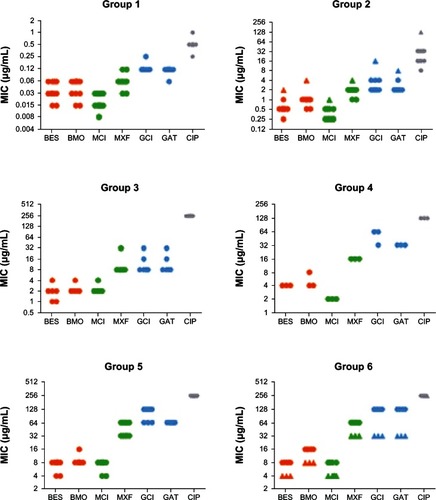
The 14 isolates in group 1, which include 13 clinical isolates and 1 quality control strain, contained no resistance-conferring mutations and had correspondingly low MIC values. MIC50 values, the drug concentrations that inhibit the growth of 50% of isolates, increased in the order MCl (0.015 μg/mL) < besifloxacin (0.03 μg/mL) < BMO (0.06 μg/mL) = moxifloxacin (0.06 μg/mL) < gatifloxacin (0.12 μg/mL) = GCl (0.12 μg/mL) < ciprofloxacin (0.5 μg/mL). Exchanging the 8-chloro for an 8-methoxy group had different effects, depending on the R7 substituent: The MIC values for the besifloxacin analog BMO were either identical (in 64% of isolates) or twofold higher (36%) than the MIC values for besifloxacin. The MIC values for moxifloxacin were twofold (29%) or fourfold (71%) above that of the moxifloxacin analog MCl, while gatifloxacin had either identical (86%) or lower (14%) MIC values than the gatifloxacin analog GCl.
Against the 39 ciprofloxacin-resistant isolates in groups 2 through 6, the MIC values increased with the number and nature of mutations, but the overall trends remained the same as in the ciprofloxacin-susceptible group: the MIC values for besifloxacin were identical (for 38% of isolates) or twofold (62%) lower than those for BMO, while GCl had identical (69%) or twofold higher (31%) MIC values than gatifloxacin. MCl was fourfold (38%), eightfold (59%), or 16-fold (3%) more potent than moxifloxacin.
These results show that, depending on the R7 moiety, exchanging the R8 substituent can have different effects on antibacterial potency against S. aureus isolates. Replacing the 8-chloro with an 8-methoxy group in besifloxacin either had no effect or resulted in a twofold decrease in potency. In the case of the gatifloxacin analog GCl, the same change either had no effect or resulted in a twofold increase in potency. The moxifloxacin analog MCl was always more potent than moxifloxacin; two- to fourfold against ciprofloxacin-susceptible isolates and four- to 16-fold against ciprofloxacin-resistant isolates.
The effect of reserpine
The staphylococcal efflux pump NorA has been shown to contribute to fluoroquinolone resistance in S. aureus.Citation3 While the pump has a wide spectrum of substrates, some molecules are more susceptible to the action of NorA than others. For example, ethidium bromide is rapidly exported, while the fluoroquinolones show various degrees of susceptibility. In order to determine the impact of the fluoroquinolone’s R7 and R8 substituents on NorA-mediated efflux, we determined the MIC values of the 53 S. aureus strains in the presence and absence of the plant alkaloid reserpine, which is an inhibitor of NorA ().
Table 3 Contribution of reserpine-susceptible efflux pumps to fluoroquinolone MIC values in 52 clinical ophthalmic Staphylococcus aureus isolates and control strain ATCC29213
For 69.8% or more of S. aureus isolates, MIC values for moxifloxacin, gatifloxacin, and their 8-chloro analogs did not change in the presence of reserpine, suggesting that NorA has no impact on fluoroquinolone resistance in these strains. The remaining strains exhibited either a twofold increase or a twofold decrease in MIC values in the presence of reserpine, which could be attributed to experimental fluctuation. In the case of besifloxacin, reserpine did not change the besifloxacin MIC values for 64.2% of the isolates and resulted in twofold lower MIC values for 34.0% of isolates. For BMO, following reserpine treatment, no change in the MIC values was determined in 43.4% of isolates, and a twofold lower MIC was exhibited in 54.7% of the isolates. These data suggest that NorA-mediated export plays little to no role in the antistaphy-lococcal potency of besifloxacin, moxifloxacin, gatifloxacin, and their R8 analogs. In contrast, the ciprofloxacin MIC values decreased by twofold for 17.0% of isolates and by fourfold for 30.2% of isolates. Even more noticeably, MIC values for the ethidium bromide positive control increased by fourfold for 20.8% of isolates, by eightfold for 15.1% of isolates, and by 16-fold for 9.4% of isolates, confirming that ciprofloxacin and especially ethidium bromide are good substrates for NorA.
The contribution of the R8 substituent to bactericidal activity
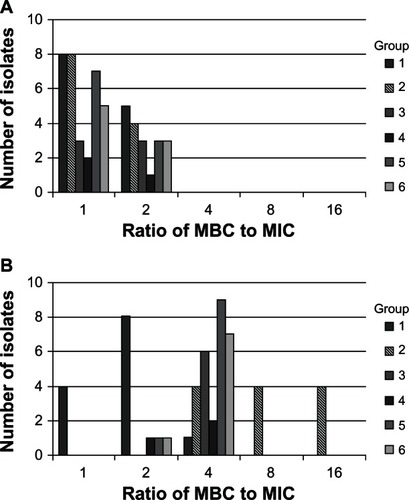
Previous work has shown that ciprofloxacin loses its bactericidal activity against fluoroquinolone-resistant strains of S. aureus.Citation13 In order to determine whether the fluoroquinolone analogs maintain the same bactericidal activity as their commercially available counterparts, we measured the MBC for each strain listed in and one representative isolate per S. aureus mutant group depicted in . The MBC-to-MIC ratios for besifloxacin, BMO, moxifloxacin, MCl, gatifloxacin, GCl, and ciprofloxacin were ≤4:1 and usually 1:1 or 2:1 (data not shown). This indicates that all analogs maintained potent bactericidal activity, even against fluoroquinolone-resistant isolates. The only exception was a S. aureus isolate in mutant group 2, which had a MBC:MIC ratio of 16:1 for ciprofloxacin. An MBC:MIC ratio ≥8:1 is considered bacteriostatic, which was in good agreement with earlier time-kill experiments.Citation13 Overall, our results show that the presence of a chloro or a methoxy group in the R8 position does not notably alter bactericidal activity.
To test whether the loss of bactericidal action was linked to a specific resistance genotype, such as the one found in mutant group 2, we determined the ciprofloxacin MBC:MIC ratio for all of the 53 S. aureus isolates. Besifloxacin was used as a positive control. For most S. aureus isolates, the ciprofloxacin MBC:MIC ratios were 1:1 or 2:1 and did not exceed 4:1, indicating that these isolates were rapidly killed by the drug despite high levels of fluoroquinolone resistance (). The exceptions to this were eight of the 12 isolates in mutant group 2, which had MBC:MIC ratios of 8:1 or 16:1, demonstrating that these isolates were no longer effectively killed by ciprofloxacin. In contrast, besifloxacin maintained its high bactericidal potency with MBC:MIC ratios of 1:1 or 2:1 against those strains. Further studies are required to determine why those particular isolates, and not others, lost their susceptibility to be killed by ciprofloxacin.
Figure 3 Ratio of minimum bactericidal concentrations to minimum inhibitory concentrations against 52 Staphylococcus aureus strains, including the fluoroquinolone-resistant isolates in groups 2–6, for (A) besifloxacin and (B) ciprofloxacin.
Abbreviations: MBC, minimum bactericidal concentrations; MIC, minimum inhibitory concentrations.
Discussion
The MIC data presented here for various species demonstrated that all of the seven fluoroquinolones had broad spectrum activity against various gram-positive and gram-negative species. Comparing besifloxacin, moxifloxacin, and gatifloxacin with their R8 analogs showed that it is the combination of the R7 and R8 substituents that determines the potency of the fluoroquinolone. For example, against the gram-positive pathogens, antibacterial potency followed the order: MCl > besifloxacin > GCl, when the R7 substituent was a chloro group; but it was: BMO > moxifloxacin > gatifloxacin when the R7 substituent was a methoxy group. When the R7 substituent was constant and the R8 moiety was changed from a chloro to a methoxy group, MIC values for the besifloxacin/BMO pair remained constant or increased by twofold, increased by four- to eightfold for the MCl/moxifloxacin pair, and remained constant or decreased by twofold for the GCl/gatifloxacin pair. Therefore, replacing the 8-chloro with an 8-methoxy group can have either no or little effect, as in the case of besifloxacin and the gatifloxacin analog GCl, or it can make a big difference in antibacterial potency, as exemplified by the moxifloxacin analog MCl.
MIC values for besifloxacin, moxifloxacin, gatifloxacin, and ciprofloxacin against S. aureus, E. faecalis, E. coli, and P. aeruginosa were within the quality control ranges suggested by CLSI.Citation10 The MIC data for the ATCC quality control strains of E. faecalis, S. aureus, S. pneumoniae, E. coli, and P. aeruginosa were similar to previously published MIC values for besifloxacin, moxifloxacin, MCl, and gatifloxacin.Citation5,Citation8,Citation14–Citation18 Little has been published about the antibacterial potency of GCl, and no manuscripts that describe the antibacterial potency of BMO have been identified in the literature.Citation7
For ciprofloxacin-resistant strains of S. aureus, the MIC values presented here were remarkably similar to those published by others, especially if one considers the differences in genetic background and testing methods. Fukuda et alCitation19 tested gatifloxacin, ciprofloxacin, and other fluoroquinolones against sequentially obtained quinolone-resistant mutants of S. aureus that were similar to mutant groups 2, 3, and 6 in our study.Citation19
Lu et alCitation7 tested the activity of gatifloxacin, GCl, and comparator molecules that differed only in their R8 substituent against two strains of S. aureus that were fluoroquinolone-susceptible and -resistant, respectively. Similar to the data presented here, gatifloxacin and GCl had virtually identical activities against these two strains, while moxifloxacin was more potent than both drugs.Citation7 For the gatifloxacin analogs with various R8 substitutions, antistaphylococcal potency increased in the order: H < F < Br < Cl < methoxy (MO), for the fluoroquinolone-susceptible isolate, and H < F < Br < MO < Cl, for the fluoroquinolone-resistant isolate.Citation7 The enhancement in potency due to the Br, MO, or Cl R8 substituent was especially notable in the case of the fluoroquinolone-resistant strain, which is consistent with the findings presented here.
The 53 strains of S. aureus were of interest because their susceptibility sheds light on the nature of the fluoroquinolone–target interaction. Strains were grouped based on their mutations in the QRDR of gyrA and gyrB (encoding DNA gyrase) and parC and parE (encoding topoisomerase IV), which confer high-level fluoroquinolone resistance.
Strains in group 1 contained no mutations, while isolates in groups 2 and 3 contained two or three mutations, respectively. All strains in groups 4–6 contained four mutations each, but the mutated amino acid was different in each group. It could be expected that, if a particular amino acid in the quinolone-binding site of the target protein was interacting with the R8 substituent of the fluoroquinolone, then a change in that amino acid or in the R8 substituent might be expected to change the MIC value. However, this does not seem to be the case based on the data presented here. Regardless of the group of S. aureus mutants, besifloxacin had the same potency or was twofold more potent than BMO, while GCl had the same potency or was twofold less potent than gatifloxacin. Similarly, MCl was more potent than moxifloxacin: two- to fourfold more potent against the ciprofloxacin-susceptible strains in group 1 and four- to eightfold (and in one case 16-fold) more potent against the ciprofloxacin-resistant strains. Moreover, there was some natural fluctuation in the MIC data, which was, at least to some degree, mitigated by taking MIC readings from three independent susceptibility tests. Despite this, MIC values were rather consistent within the mutant groups and rarely varied by more than a twofold dilution. In some instances, one strain in group 2 and three strains in group 6, this fluctuation seemed to be linked to specific mutations that caused those strains to be slightly different from the other strains in the group. The one strain in group 2 carries a Pro585-Ser mutation in parE that is absent in all the other strains, which might increase fluoroquinolone resistance.Citation9 The three strains in group 6 carry Glu88-Ala mutations in GyrA instead of the Glu88-Lys mutations found in the other strains in this group. Strains with the Glu88-Ala mutation showed consistently lower resistance levels than strains with a Glu88-Lys. Surprisingly, other mutations that were previously presumed to result in differences in fluoroquinolone resistance, such as the ParC-80 and ParE-432 mutations in the three strains of group 3, did not have the expected effect.Citation9
These results show that a twofold difference in MIC is likely not to be biologically meaningful. Therefore, since the slight variations in MIC values within the pairs of R8 analogs is most likely due to natural variation and the overall trend remains the same from one mutant group to another, it is reasonable to assume that the R8 substituent does not interact with residues 84, 85, and 88 of GyrA, 80 and 84 of ParC, or 432 and 585 of ParE, at least not to the extent that it would alter the MIC value measurably. More sophisticated methods, such as X-ray crystallography, might shed a better light on these interactions.
Bax et alCitation20 investigated the three-dimensional structure of S. aureus DNA gyrase in a complex with DNA and ciprofloxacin.Citation20 Based on that model (NCBI [National Center for Biotechnology Information] Protein database code 2XCT), the R7 substituent of ciprofloxacin appears adjacent to Asn476, which is located at the end of an α-helix. The R8 moiety of the fluoroquinolone lies opposite of Arg458, which is located between a β-sheet and an α-helix. Both amino acids are part of the Toprim domain of the GyrB subunit. How Asn476 and Arg458 interact with the fluoroquinolones is currently unknown, and no S. aureus strain investigated in this study contained a mutation in these amino acids. However, Pan and Fisher,Citation21 using clinafloxacin selection in S. pneumoniae, obtained strains containing mutations in the corresponding amino acids, Glu474 and Pro454, respectively.Citation21 Both mutations resulted in minor increases in fluoroquinolone MIC values. Additional evidence for the importance of GyrB Arg458 in fluoroquinolone resistance comes from work done in E. coli, where Arg458 corresponds to Lys447. Strains with Lys447-Glu mutations in GyrB were found to be resistant to some quinolones, but hypersusceptible to others.Citation22 Unfortunately, the quinolones tested in this study were structurally very diverse, so no conclusions about possible interactions between the R8 substituent of the quinolone and the amino acids Lys447 or Glu447 of GyrB can be drawn. A better understanding of the interactions between Asn476 and Arg458 and the fluoroquinolones will have to await further mutational analysis.
Previous results by Shinabarger et al,Citation3 using genetically defined mutants and various pump inhibitors, have shown that moxifloxacin is not a substrate for NorA-mediated efflux. The data presented here confirm these results and further show that gatifloxacin, MCl, and GCl MIC values also remain virtually unchanged in the presence of the pump inhibitor reserpine. Therefore, the presence of a chloro or a methoxy group in the R8 position appears to have little impact on NorA-mediated efflux. The observation that some strains exhibited either a twofold increase or a twofold decrease in MIC values in the presence of reserpine might be due to natural variation in a biological system.
Shinabarger et al also showed that besifloxacin is a poor substrate for NorA, which was also confirmed in this study. Changing the 8-chloro to an 8-methoxy resulted in 11 (20.7%) additional strains that exhibited a reserpine-induced twofold decrease in MIC values, a change that could be due to natural fluctuations or due to an increased ability of NorA to export BMO when compared to besifloxacin. The latter hypothesis is consistent with work by Takenouchi et al, who proposed a correlation between the activity of efflux pumps and the bulkiness of the R7 substituent and the bulkiness and hydrophobicity of the R8 substituent.Citation23 However, even if replacement of the 8-chloro with an 8-methoxy group made BMO a better substrate for NorA, the effect is rather subtle and probably not biologically significant. In contrast, MIC values for ciprofloxacin and ethidium bromide changed more drastically in the presence of reserpine, confirming that they are good or very good substrates for NorA.
Work by Lu et al showed that the effect of the R8 substituent on the ability to kill cells was dependent on the R7 substituent, since changing the R8-H in ciprofloxacin to a 8-chloro group improved bactericidal activity, while the kill rates of moxifloxacin or gatifloxacin did not notably change when the 8-methoxy group was replaced with an 8-H or an 8-chloro group.Citation7 The results presented here show that, although their absolute potency varied, the ophthalmic fluoroquinolones besifloxacin, moxifloxacin, gatifloxacin, and their R8 analogs had potent bactericidal activity, even against ciprofloxacin-resistant isolates. Replacing the 8-chloro with an 8-methoxy substituent, or vice versa, did not alter the MBC:MIC ratios, suggesting that the two substituents contribute equally (or not at all) to the lethal activity of the agents tested.
In contrast, ciprofloxacin was bactericidal for some isolates of S. aureus but only bacteriostatic against others, particularly against strains in mutant group 2. These findings are consistent with previous time kill experiments that had shown that ciprofloxacin was unable to reduce the number of ciprofloxacin-resistant S. aureus and Staphylococcus epidermidis cells below the levels of the initial inocula within 2 hours.Citation13 Isolates in mutant group 2 all contain a Ser84-Leu mutation in GyrA and a Ser80-Tyr or -Phe mutation in ParC. However, there was no obvious correlation between the genotype of the mutants and the lack of bactericidal activity of ciprofloxacin, requiring further investigation. While ciprofloxacin had low potency and only bacteriostatic activity against ciprofloxacin-resistant S. aureus isolates, the more modern fluoroquinolones besifloxacin, moxifloxacin, and gatifloxacin that are in ophthalmic use today, have more potent activity and retain their bactericidal action.
The data presented here show that MCl is more potent than moxifloxacin, begging the question why moxifloxacin is commercially available while MCl is not. Development of MCl (aka BAY y 3118) has been discontinued because it is photochemically labile, producing radicals in the presence of ultraviolet A (UVA) light and oxygen.Citation6,Citation24,Citation25 While toxicity issues might have prevented the development of certain fluoroquinolones for systemic use, the ophthalmic fluoroquinolones available today for the treatment of ocular infections have been shown to be safe and effective.Citation26–Citation29
Previous work had shown that besifloxacin, an 8-chloro-fluoroquinolone, had more potent activity against grampositive pathogens than moxifloxacin and gatifloxacin, which carry an 8-methoxy group. The data presented here show that, depending on the R7 substituent, replacing an 8-methoxy group with an 8-chloro substituent can improve potency or can have little-to-no effect. However, there was no difference between the 8-chloro and the 8-methoxy group with respect to NorA-mediated efflux or bactericidal activity. These findings highlight the importance of the interplay between and contributions from both the R7 and R8 substituents in determining antibacterial potency.
Acknowledgments
The authors would like to thank Dr Mary Marquart and Andrea Swiatlo (University of Mississippi VA Medical Center, Jackson, MS, USA) for providing bacterial strains and Dr Azhwarsamy Jeganathan (Bausch and Lomb, Rochester, NY, USA) for synthesizing the 8-methoxy analog of besifloxacin, BMO.
Disclosure
All authors work for Bausch & Lomb Incorporated. The authors report no other conflicts of interest in this work.
References
- HaasWPillarCMTorresMMorrisTWSahmDFMonitoring antibiotic resistance in ocular microorganisms: results from the Antibiotic Resistance Monitoring in Ocular micRorganisms (ARMOR) 2009 surveillance studyAm J Ophthalmol2011152456757421652021
- DrlicaKMalikMKernsRJZhaoXQuinolone-mediated bacterial deathAntimicrob Agents Chemother200852238539217724149
- ShinabargerDLZurenkoGEHesjeCSanfilippoCMMorrisTWHaasWEvaluation of the effect of bacterial efflux pumps on the antibacterial activity of the novel fluoroquinolone besifloxacinJ Chemother201123808621571623
- ComstockTLKarpeckiPMMorrisTWZhangJZBesifloxacin: a novel anti-infective for the treatment of bacterial conjunctivitisClin Ophthalmol2010421522520463787
- BauernfeindAComparison of the antibacterial activities of the quinolones Bay 12-8039, gatifloxacin (AM 1155), trovafloxacin, clinafloxacin, levofloxacin and ciprofloxacinJ Antimicrob Chemother19974056396519421311
- DalhoffAComparative in vitro and in vivo activity of the C-8 methoxy quinolone moxifloxacin and the C-8 chlorine quinolone BAY y 3118Clin Infect Dis200132Suppl 1S16S2211249824
- LuTZhaoXLiXEnhancement of fluoroquinolone activity by C-8 halogen and methoxy moieties: action against a gyrase resistance mutant of Mycobacterium smegmatis and a gyrase-topoisomerase IV double mutant of Staphylococcus aureusAntimicrob Agents Chemother200145102703270911557458
- HaasWPillarCPZurenkoGELeeJCBrunnerLSMorrisTWBesifloxacin, a novel fluoroquinolone, has broad-spectrum in vitro activity against aerobic and anaerobic bacteriaAntimicrob Agents Chemother20095383552356019506065
- SanfilippoCMHesjeCKHaasWMorrisTWTopoisomerase mutations that are associated with high-level resistance to earlier fluoroquinolones in Staphylococcus aureus have less effect on the antibacterial activity of besifloxacinChemotherapy201157536337121996946
- Clinical and Laboratory Standards InstitutePerformance Standards for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement M100-S212011Wayne, PAClinical and Laboratory Standards Institute2011
- Clinical and Laboratory Standards InstituteMethods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard, Eighth Edition. CLSI Document M7-A8Wayne, PAClinical and Laboratory Standards Institute2009
- Clinical and Laboratory Standards InstituteMethods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline. CLSI Document M26-AWayne, PAClinical and Laboratory Standards Institute1999
- HaasWPillarCMHesjeCKSanfilippoCMMorrisTWBactericidal activity of besifloxacin against staphylococci, Streptococcus pneumoniae and Haemophilus influenzaeJ Antimicrob Chemother20106571441144720435780
- WiseRBrenwaldNPAndrewsJMBoswellFThe activity of the methylpiperazinyl fluoroquinolone CG 5501: a comparison with other fluoroquinolonesJ Antimicrob Chemother19973944474529145816
- HosakaMYasueTFukudaHTomizawaHAoyamaHHiraiKIn vitro and in vivo antibacterial activities of AM-1155, a new 6-fluoro-8-methoxy quinoloneAntimicrob Agents Chemother19923610210821171332587
- WakabayashiEMitsuhashiSIn vitro antibacterial activity of AM-1155, a novel 6-fluoro-8-methoxy quinoloneAntimicrob Agents Chemother19943835946018203860
- FassRJIn vitro activity of Bay y 3118, a new quinoloneAntimicrob Agents Chemother19933711234823578285618
- MolinariGSchitoGCComparative in vitro activity of BAY Y 3118 with other fluoroquinolonesDrugs199549Suppl 22222258549310
- FukudaHHoriSHiramatsuKAntibacterial activity of gatifloxacin (AM-1155, CG5501, BMS-206584), a newly developed fluoroquinolone, against sequentially acquired quinolone-resistant mutants and the norA transformant of Staphylococcus aureusAntimicrob Agents Chemother1998428191719229687384
- BaxBDChanPFEgglestonDSType IIA topoisomerase inhibition by a new class of antibacterial agentsNature2010466730993594020686482
- PanXSFisherLMDNA gyrase and topoisomerase IV are dual targets of clinafloxacin action in Streptococcus pneumoniaeAntimicrob Agents Chemother19984211281028169797208
- YoshidaHBogakiMNakamuraMYamanakaLMNakamuraSQuinolone resistance-determining region in the DNA gyrase gyrB gene of Escherichia coliAntimicrob Agents Chemother1991358164716501656869
- TakenouchiTTabataFIwataYHanzawaHSugawaraMOhyaSHydrophilicity of quinolones is not an exclusive factor for decreased activity in efflux-mediated resistant mutants of Staphylococcus aureusAntimicrob Agents Chemother1996408183518428843290
- SchmidtUSchlüterGStudies on the mechanism of phototoxicity of BAY y 3118 and other quinolonesAdv Exp Med Biol19963871171208794202
- BallPMandellLNikiYTillotsonGComparative tolerability of the newer fluoroquinolone antibacterialsDrug Saf199921540742110554054
- SilverLHWoodsideAMMontgomeryDBClinical safety of moxifloxacin ophthalmic solution 0.5% (VIGAMOX) in pediatric and nonpediatric patients with bacterial conjunctivitisSurv Ophthalmol200550Suppl 1S55S6316257311
- McDonaldMBProtzkoEEBrunnerLSEfficacy and safety of besifloxacin ophthalmic suspension 0.6% compared with moxifloxacin ophthalmic solution 0.5% for treating bacterial conjunctivitisOphthalmology200911691615162319643483
- ComstockTLPaternoMRUsnerDWPichicheroMEEfficacy and safety of besifloxacin ophthalmic suspension 0.6% in children and adolescents with bacterial conjunctivitis: a post hoc, subgroup analysis of three randomized, double-masked, parallel-group, multicenter clinical trialsPaediatr Drugs201012210511220218747
- ComstockTLPaternoMRDecoryHHUsnerDWSafety and tolerability of besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis: data from six clinical and phase I safety studiesClin Drug Investig20103010675685