Abstract
Purpose
To investigate the relationship between dry eye disease (DED) and myopia in Japanese teenagers.
Methods
This clinic-based, retrospective, cross-sectional study assessed DED condition in 10- to 19-year-old teenagers presenting at Japanese eye clinics. They included 106 high myopic patients (HM; mean age, 16.4 ± 2.2 years), 494 mild myopic patients (15.0 ± 2.6 years) and 82 non-myopic teenagers (NM; 13.8 ± 2.6 years). Subjective refraction and anisometropia were measured. Myopia grade was classified as HM (≤ −6.00 D), MM (> −6.00 D, < −0.50 D), or NM (≥ −0.5 D). The presence of DED-related symptoms including dryness, irritation, pain, fatigue, blurring and photophobia were assessed through a questionnaire. Tear film break-up time (BUT) and fluorescein corneal staining were investigated. Comparison among three groups and regression analysis of myopic error and other variables were conducted.
Results
Anisometropia and astigmatic error were greatest in the HM group compared with the other groups (p < 0.001). The HM group reported less photophobia (p < 0.001) and less pain (p = 0.039) compared with the NM group. Regression analysis revealed that myopic error was correlated with astigmatic error (β = −0.231, p <0.001), anisometropia (β = −0.191, p <0.001), short BUT (β = −0.086, p = 0.028) and the presence of diagnosed DED (β = −0.112, p = 0.003). Dryness (β = −0.127 p = 0.004), photophobia (β = 0.117, p = 0.002) and pain (β = 0.084, p = 0.034) correlated with myopic error.
Conclusion
This study associated clinical findings of DED in HM teenagers. The present results suggest DED might be associated with myopia, possibly in a reciprocal relationship.
Introduction
Myopia is defined as a condition in which the spherical equivalent refractive error of an eye is ≤-0.50 D.Citation1 In high myopia (HM), the spherical equivalent refractive error of an eye is ≤-6.00 D when ocular accommodation is relaxed.Citation2 Myopia, particularly HM, not only impacts uncorrected vision in daily life, but it can lead to blindness due to macular degeneration, retinal detachment, glaucoma and cataract.Citation3 The prevalence of myopia is increasing globally, with HM expected to affect 9.8% of the global population by 2050.Citation4 The highest prevalence of myopia is seen in younger adults, particularly in East and Southeast Asian countries.Citation5
Dry eye disease (DED) is a multifactorial disease of the tears and ocular surface resulting in symptoms of discomfort, visual disturbance and tear film instability with potential damage to the ocular surface.Citation6 Increased higher-order aberrations (HOAs), abnormal accommodative microfluctuations, as well as sleep disorders have been reported in DED.Citation7–9 Short tear film break-up time (BUT) type DED is also reported to be associated with visual display terminal (VDT) users.Citation10,Citation11 The increase in myopia prevalence among teenagers is likely due to the widespread use of VDTs, which include smart phones.Citation12 Despite these common findings, the relationship between DED and HM is yet to be investigated. Our group recently showed that BUT was significantly associated with choroidal thickness, which is correlated with axial length (AL).Citation13 In another report, we showed that AL and refraction were associated with DED.Citation14
Hence, the aim of this study was to investigate the relationship between DED and HM in a large case series of Japanese teenagers.
Materials and Methods
Study Design, Ethics Approval and Patient Recruitment
This clinic-based, retrospective, cross-sectional study was conducted at Tsukuba Central Hospital (Ibaraki, Japan) and Otake Eye Clinic (Kanagawa, Japan). Consecutive patients between the ages of 10–19 years were recruited from April 2015 to August 2020. The Institutional Review Boards and Ethics Committees of the Tsukuba Central Hospital (approved 12 December 2014, permission number 141201) and the Kanagawa Medical Association (approved 12 November 2018, permission number krec2059006) approved this study. This study was conducted in accordance with the Declaration of Helsinki. The need for consent was waived by the institutional review boards since the study was conducted in an opt-out fashion. Minors were involved in this study and the need for consent from their parents or guardians was specifically waived. The Institutional Review Board and Ethics Committee of Keio University School of Medicine approved this study (28 June 2021; approval number 20210080) to permit authorship for all authors (O.I., E.Y., H.T., K.N. and M.A.) who have appointments in the Keio University School of Medicine.
Inclusion and Exclusion Criteria
Inclusion criteria was a best-corrected visual acuity of greater than 20/30 and participants were enrolled at first visit. Individuals were excluded if they had contact lens use, orthokeratology, topical atropine medication, vitreoretinal disease, diabetic mellitus, Sjögren’s syndrome, any ocular surgery in the previous month, or acute ocular disease in the previous two weeks.
Ophthalmological Examinations
Refractive status was determined with subjective refraction to achieve best-corrected visual acuity using a standard Landolt optotype chart (CSV-1000 chart (VectorVision) Ohio, USA). Test strips containing fluorescein sodium (Fluores Ocular Examination Test Paper; Ayumi Pharmaceutical Co., Tokyo, Japan) were used to evaluate BUT and vital corneal staining. After applying two drops of saline solution to the test strip, we gently touched its edge to the inferior temporal lid margin. Participants were instructed to gently close their eyes then quickly open them. We measured the interval between the last complete blink and the appearance of the first dark corneal spot and regarded the average of three measurements as the BUT. Corneal epithelial damage was evaluated using fluorescein vital staining and viewed through a blue-free filter.Citation15 A BUT ≤5 s and any corneal staining were considered as positive clinical DED signs.Citation16
Questionnaire
All participants completed a DED-related symptoms questionnaire that assessed the presence of dryness, irritation, pain, fatigue, blurring and photophobia symptoms (yes/no). These symptoms were retrieved from DEQS that is a validated questionnaire and selected as the most prevalent subjective symptoms in the dry eye clinic of Keio University Hospital in Tokyo, Japan, in 2012.Citation17
Statistical Analysis
Data are expressed as the mean ± standard deviation. Data from the right eye were analyzed. Myopia grade was classified with spherical equivalent as HM (≤-6.00 D), mild myopia (MM) (>-6.00 D, <-0.50 D), or no myopia (NM) (≥-0.50 D). Patients with short BUT (≤5 s) and any one of the six DED-related symptoms were diagnosed as having DED according to the criteria of the Asia Dry Eye Society.Citation18
The prevalence of symptoms, diagnosed DED, short BUT and positive corneal staining was compared among the three groups using a chi-square test. Regression analyses of myopic error and other variables were conducted using a standardized partial regression coefficient. StatFlex (Atech, Osaka, Japan) software was used for statistical analysis. A p-value <0.05 was considered to indicate statistical significance.
Results
Patient Demographics
A total of 106 HM teenagers, 494 MM teenagers and 82 NM teenagers were eligible for analysis (). The mean age of the HM group was significantly higher than the MM and NM groups. The mean refractive error in the HM group was significantly higher than in the MM and NM groups. Astigmatic error and anisometropia were significantly higher in the HM group compared to the MM and NM groups (). There were 51 participants using anti-allergic eyedrop; 2 in HM, 49 in MM, and none in NM.
Table 1 Participant Characteristics and Comparison of Parameters Among Myopic Groups
Prevalence of DED-Related Signs and Symptoms
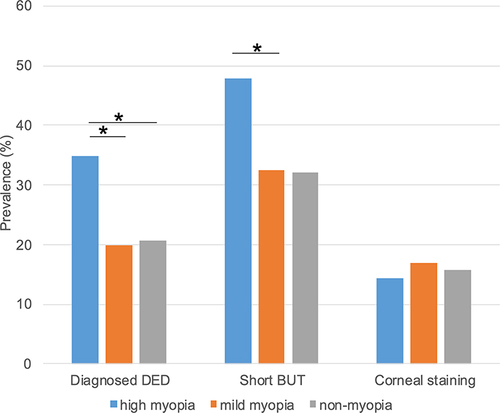
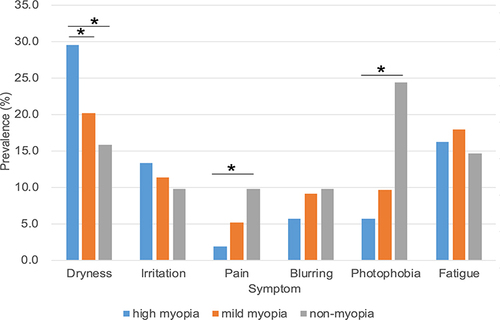
The prevalence of DED-related signs in the three groups is shown in and . Diagnosed DED (p <0.001, HM vs MM and HM vs NM, chi-squared test) and short BUT (p <0.001, HM vs MM; p = 0.132, HM vs NM, chi-squared test) were predominantly prevalent in the HM group. No significant differences were observed regarding corneal staining among the three groups. Dryness was significantly more prevalent in the HM compared to the MM and NM groups (p = 0.036, HM vs MM; p = 0.034, HM vs NM, chi-squared test), while the prevalence of pain (p = 0.039, HM vs NM, chi-squared test) and photophobia (p <0.001, HM vs NM, chi-squared test) were significantly lower in the HM group compared to the NM group (). There was no significant difference regarding irritation, blurring and fatigue among the three groups.
Regression Analysis of Refractive Error and Variables
Regression analysis revealed a significant association between myopic error and astigmatic error (ß = −0.231, p <0.001) and anisometropia (ß = −0.191, p <0.001) (). A similar tendency was observed when the data were adjusted for age and sex. DED-related parameters including short BUT and diagnosed DED also showed a significant association with myopic error (ß = −0.086, p = 0.028; and ß = −0.112, p = 0.003, respectively). The prevalence of dryness was positively correlated with the magnitude of myopic error, whilst the prevalence of pain and photophobia were inversely correlated. Stepwise regression analysis further confirmed these correlations.
Table 2 Regression Analysis of Myopic Error and Variables
Discussion
This study demonstrated distinct differences between HM and less myopic groups in relation to DED-related signs and symptoms and refractive parameters. Contact lenses, orthokeratology and LASIK treatment in HM patients are blamed for inducing DED; however, in the current study, the HM group had significantly worse symptoms and signs of DED than the NM group despite the exclusion of contact lens users.Citation19 The prevalence of diagnosed DED, short BUT and dryness was significantly higher in HM group. This was in accordance without previous report that showed that the AL increased as the BUT became shorter.Citation13 Those who perform more near work have a greater increased risk of both evaporate DED, which affects the BUT, and choroidal thickness, which in turn affects the axial length. Indeed, fluorescein instillation could induce reflex tearing and require subjective assessment, which is a limitation of our study.Citation20 Therefore, future studies should consider noninvasive assessment of tear stability using methods such as tear film lipid layer interferometry, the xeroscope, and the tearscope.Citation21–23
We did not observe significant differences regarding the corneal staining among the three groups, while the prevalence of pain and photophobia was significantly lower in the HM group compared to the NM group. The current results suggest a contradictory relationship between a greater magnitude of myopic error and less prevalent ocular pain and photophobia, despite both being typical symptoms of DED. It could be postulated that a decreased intrinsically photosensitive retinal ganglion cell (ipRGC) number or function might lead to less pain and photophobia in HM. IpRGCs are a subtype of RGCs and are partly associated with photophobia and ocular pain in response to blue-light in the presence of melanopsin.Citation24–26 Thinning of the ganglion cell layer is likely in a myopic retina,Citation27 presumably leading to less ipRGC-driven photogenic pain and photophobia, although this needs to be confirmed through additional studies.Citation27 This association between HM and DED could suggest a common mechanism for the deterioration of DED and myopia as described in our previous studies.Citation13,Citation14
The present study also indicated anisometropia and astigmatic error were greatest in the HM group compared to less myopic groups and they were significantly correlated with DED and myopic error. DED may induce disturbed vision leading to the inability to correctly focus the light on the retina. A degrading quality of image being formed on the retina may initiate a signaling cascade resulting in abnormal eye growth and development of refractive errors as shown by animal experiments.Citation28
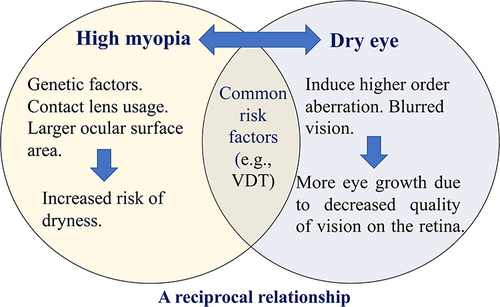
In many cases, HM could be initially induced by genetic factors.Citation29 Contact lens usage and an enlarged ocular surface area due to an elongated AL may increase the risk of developing DED.Citation19 DED in turn induces HOAs, corneal backward light scattering and blurred vision, which decreases the quality of vision on the retina.Citation30,Citation31 It is likely that this visual disturbance might consequently prompt more growth of the globe and facilitate myopia progression.Citation28 There are additional common risk factors linked to the simultaneous development of myopia and DED, such as VDT and smart phone usage. It is possible that the deterioration of DED may affect myopic status and vice versa, hence a reciprocal relationship might exist ().Citation32,Citation33
Figure 3 Schematic representation of the proposed relationship between myopia and dry eye syndrome. In many cases, high myopia could be initially induced by genetic factors. Contact lens usage and a larger ocular surface area increase the risk of developing dry eye. Dry eye in turn induces astigmatism and blurred vision, which decreases vision quality on the retina and consequently prompts more growth of the eye axis and increased myopia. There are also common risk factors that develop high myopia and dry eye simultaneously, such as visual display terminal (VDT) and smart phone usage. It is possible that the deterioration of dry eye condition could affect the myopic status and vice versa, hence a reciprocal relationship might exist.
There are differing hypotheses regarding the relationship between types of HOAs and AL. Some studies have showed that asymmetric HOAs, such as coma, inhibit axial elongation.Citation34 Nevertheless, not all the published studies agree that HOAs lead to elongation of the AL.Citation7,Citation35 Several reports showed that corneal HOAs and total ocular HOAs are elevated in DED in adults.Citation30,Citation36 While the current study did not investigate HOAs, our group previously reported corneal HOAs and total ocular HOAs are elevated in children with DED.Citation13 Therefore, we presume that there might be a relationship between DED patients with HOAs and AL.
A previous report showed that infantile astigmatism is associated with increased myopia during school years due to a disruption in focusing mechanisms.Citation37 Another study suggested that myopia development could be due to irregular astigmatism in the tear film.Citation31 The HM group had higher astigmatic error than the MM and NM groups. In addition, regression analysis showed a negative significant association between astigmatic error and myopia indicating that astigmatic error might have a role in myopia development. However, these reported studies have differences in sample size, ethnicity and age, so further investigations are required.
Future research should investigate the effect of DED treatment on the course of myopia progression in individuals with HM, as image quality could be ameliorated with topical DED medication by improving BUT.Citation38 In general, regular checks and treatment of DED could avoid development of DED-related signs and symptoms and provide a higher quality of vision.
This study has several limitations. First, we did not measure AL in participants, which is closely correlated with parental myopia in elementary school students.Citation39 Further research with AL measurement would confirm the present results. Second, we did not assess cycloplegic autorefraction, which is the preferred method for determining the degree of myopia.Citation1 Therefore, there is a possibility that the degree of myopia obtained in the current study might be less accurate, although the participants were older than 10 years and measured subjective refraction could be acceptable for analysis. Third, this is a cross-sectional retrospective study, and longitudinal studies on how myopic status can change with time and its association with DED conditions would provide useful information. Furthermore, age difference among the groups is another major limitation of the current study in terms of the precise evaluation of the conditions, although we recognize older subjects naturally develop high myopia, and observed only teenagers to minimize age differences.
In conclusion, a high prevalence of DED was found in high myopic teenagers and a distinct association between myopic error and BUT was identified. Short BUT, astigmatism and anisometropia in HM patients may indicate considerable changes in ocular surface and play a role in the development of HM through a reciprocal relationship.
Abbreviations
AL, axial length; BUT, tear break-up time; DED, dry eye disease; HM, high myopia; HOA, higher order aberration; ipRGC, intrinsically photosensitive retinal ganglion cell; MM, mild myopia; NM, no myopia; VDT, visual display terminal.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgment
Poster presentation in ARVO 2022 and paper presentation at the 46th Japan Cornea Conference 2022.
Additional information
Funding
References
- Flitcroft DI, He M, Jonas JB, et al. IMI - defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci. 2019;60(3):M20–M30. doi:10.1167/iovs.18-25957
- Lin F, Chen S, Song Y, et al. Classification of visual field abnormalities in highly myopic eyes without pathologic change. Ophthalmology. 2022;129(7):803–812. doi:10.1016/j.ophtha.2022.03.001
- Ikuno Y. OVERVIEW OF THE COMPLICATIONS OF HIGH MYOPIA. Retina. 2017;37(12):2347–2351. doi:10.1097/iae.0000000000001489
- Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012;32(1):3–16. doi:10.1111/j.1475-1313.2011.00884.x
- Dolgin E. The myopia boom. Nature. 2015;519(7543):2768. doi:10.1038/519276a
- Lemp MA, Foulks GN. The definition and classification of dry eye disease: report of the Definition and classification subcommittee of the international dry eye workshop (2007). Ocul Surf. 2007;5(2):75–92. doi:10.1016/s1542-0124(12)70081-2
- Little JA, McCullough SJ, Breslin KM, Saunders KJ. Higher order ocular aberrations and their relation to refractive error and ocular biometry in children. Invest Ophthalmol Vis Sci. 2014;55(8):4791–4800. doi:10.1167/iovs.13-13533
- Huang HM, Chang DS, Wu PC. The association between near work activities and myopia in children-A systematic review and meta-analysis. PLoS One. 2015;10(10):e0140419. doi:10.1371/journal.pone.0140419
- Ayaki M, Tsubota K, Kawashima M, Kishimoto T, Mimura M, Negishi K. Sleep disorders are a prevalent and serious comorbidity in dry eye. Invest Ophthalmol Vis Sci. 2018;59(14):DES143–DES150. doi:10.1167/iovs.17-23467
- Nakamura S. Approach to dry eye in video display terminal workers (Basic Science). Invest Ophthalmol Vis Sci. 2018;59(14):Des130–Des137. doi:10.1167/iovs.17-23762
- Uchino M, Kawashima M, Uchino Y, Tsubota K, Yokoi N. Association between tear film break up time and blink interval in visual display terminal users. Int J Ophthalmol. 2018;11(10):1691–1697. doi:10.18240/ijo.2018.10.18
- Moon JH, Lee MY, Moon NJ. Association between video display terminal use and dry eye disease in school children. J Pediatr Ophthalmol Strabismus. 2014;51(2):87–92. doi:10.3928/01913913-20140128-01
- Hazra D, Yotsukura E, Torii H, et al. Relation between dry eye and myopia based on tear film breakup time, higher order aberration, choroidal thickness, and axial length. Sci Rep. 2022;12(1):10891. doi:10.1038/s41598-022-15023-x
- Yotsukura E, Torii H, Inokuchi M, et al. Current prevalence of myopia and association of myopia with environmental factors among schoolchildren in Japan. JAMA Ophthalmol. 2019;137(11):1233–1239. doi:10.1001/jamaophthalmol.2019.3103
- Whitcher JP, Shiboski CH, Shiboski SC, et al. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjögren’s Syndrome International Registry. Am J Ophthalmol. 2010;149(3):405–415. doi:10.1016/j.ajo.2009.09.013
- Bron AJ, Abelson MB, Ousler G, et al. Methodologies to diagnose and monitor dry eye disease: report of the diagnostic methodology subcommittee of the international dry eye workshop (2007). Ocul Surf. 2007;5(2):108–152. doi:10.1016/s1542-0124(12)70083-6
- Sakane Y, Yamaguchi M, Yokoi N, et al. Development and validation of the dry eye-related quality-of-life score questionnaire. JAMA Ophthalmol. 2013;131(10):1331–1338. doi:10.1001/jamaophthalmol.2013.4503
- Tsubota K, Yokoi N, Shimazaki J, et al. New perspectives on dry eye definition and diagnosis: a consensus report by the Asia Dry Eye Society. Ocul Surf. 2017;15(1):65–76. doi:10.1016/j.jtos.2016.09.003
- Nichols JJ, Sinnott LT. Tear film, contact lens, and patient-related factors associated with contact lens-related dry eye. Invest Ophthalmol Vis Sci. 2006;47(4):1319–1328. doi:10.1167/iovs.05-1392
- Kojima T, Ishida R, Dogru M, et al. A new noninvasive tear stability analysis system for the assessment of dry eyes. Invest Ophthalmol Vis Sci. 2004;45(5):1369–1374. doi:10.1167/iovs.03-0712
- Goto E, Tseng SC. Kinetic analysis of tear interference images in aqueous tear deficiency dry eye before and after punctal occlusion. Invest Ophthalmol Vis Sci. 2003;44(5):1897–1905. doi:10.1167/iovs.02-0818
- Mengher LS, Bron AJ, Tonge SR, Gilbert DJ. A non-invasive instrument for clinical assessment of the pre-corneal tear film stability. Curr Eye Res. 1985;4(1):1–7. doi:10.3109/02713688508999960
- Goto E, Tseng SC. Differentiation of lipid tear deficiency dry eye by kinetic analysis of tear interference images. Arch Ophthalmol. 2003;121(2):173–180. doi:10.1001/archopht.121.2.173
- Schmidt TM, Do MT, Dacey D, Lucas R, Hattar S, Matynia A. Melanopsin-positive intrinsically photosensitive retinal ganglion cells: from form to function. J Neurosci. 2011;31(45):16094–16101. doi:10.1523/jneurosci.4132-11.2011
- Elenberger J, Kim B, de Castro-Abeger A, Rex TS. Connections between intrinsically photosensitive retinal ganglion cells and TBI symptoms. Neurology. 2020;95(18):826–833. doi:10.1212/wnl.0000000000010830
- Matynia A, Parikh S, Deot N, et al. Light aversion and corneal mechanical sensitivity are altered by intrinsically photosensitive retinal ganglion cells in a mouse model of corneal surface damage. Exp Eye Res. 2015;137:57–62. doi:10.1016/j.exer.2015.05.025
- Salehi MA, Nowroozi A, Gouravani M, Mohammadi S, Arevalo JF. Associations of refractive errors and retinal changes measured by optical coherence tomography: a systematic review and meta-analysis. Surv Ophthalmol. 2022;67(2):591–607. doi:10.1016/j.survophthal.2021.07.007
- Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35(9):1175–1194. doi:10.1016/0042-6989(94)00233-c
- Mutti DO, Mitchell GL, Moeschberger ML, Jones LA, Zadnik K. Parental myopia, near work, school achievement, and children’s refractive error. Invest Ophthalmol Vis Sci. 2002;43(12):3633–3640.
- Koh S. Mechanisms of visual disturbance in dry eye. Cornea. 2016;35(Suppl 1):S83–s88. doi:10.1097/ico.0000000000000998
- Koh S, Maeda N, Kuroda T, et al. Effect of tear film break-up on higher-order aberrations measured with wavefront sensor. Am J Ophthalmol. 2002;134(1):115–117. doi:10.1016/s0002-9394(02)01430-7
- Al-Mohtaseb Z, Schachter S, Shen Lee B, Garlich J, Trattler W. The relationship between dry eye disease and digital screen use. Clin Ophthalmol. 2021;15:3811–3820. doi:10.2147/opth.S321591
- McCrann S, Loughman J, Butler JS, Paudel N, Flitcroft DI. Smartphone use as a possible risk factor for myopia. Clin Exp Optom. 2021;104(1):35–41. doi:10.1111/cxo.13092
- Hiraoka T, Kakita T, Okamoto F, Oshika T. Influence of ocular wavefront aberrations on axial length elongation in myopic children treated with overnight orthokeratology. Ophthalmology. 2015;122(1):93–100. doi:10.1016/j.ophtha.2014.07.042
- Cheng X, Bradley A, Hong X, Thibos LN. Relationship between refractive error and monochromatic aberrations of the eye. Optom Vis Sci. 2003;80(1):43–49. doi:10.1097/00006324-200301000-00007
- Montés-Micó R, Cáliz A, Alió JL. Wavefront analysis of higher order aberrations in dry eye patients. J Refract Surg. 2004;20(3):243–247. doi:10.3928/1081-597x-20040501-08
- Gwiazda J, Grice K, Held R, McLellan J, Thorn F. Astigmatism and the development of myopia in children. Vision Res. 2000;40(8):1019–1026. doi:10.1016/s0042-6989(99)00237-0
- Ohashi Y, Munesue M, Shimazaki J, et al. Long-term safety and effectiveness of diquafosol for the treatment of dry eye in a real-world setting: a prospective observational study. Adv Ther. 2020;37(2):707–717. doi:10.1007/s12325-019-01188-x
- Terasaki H, Yamashita T, Yoshihara N, Kii Y, Sakamoto T. Association of lifestyle and body structure to ocular axial length in Japanese elementary school children. BMC Ophthalmol. 2017;17(1):123. doi:10.1186/s12886-017-0519-y